The Effect of Statins on the Incidence and Prognosis of Bladder Cancer: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Data Extraction
2.5. Assessment of Bias
2.6. Quantitative Analysis
3. Results
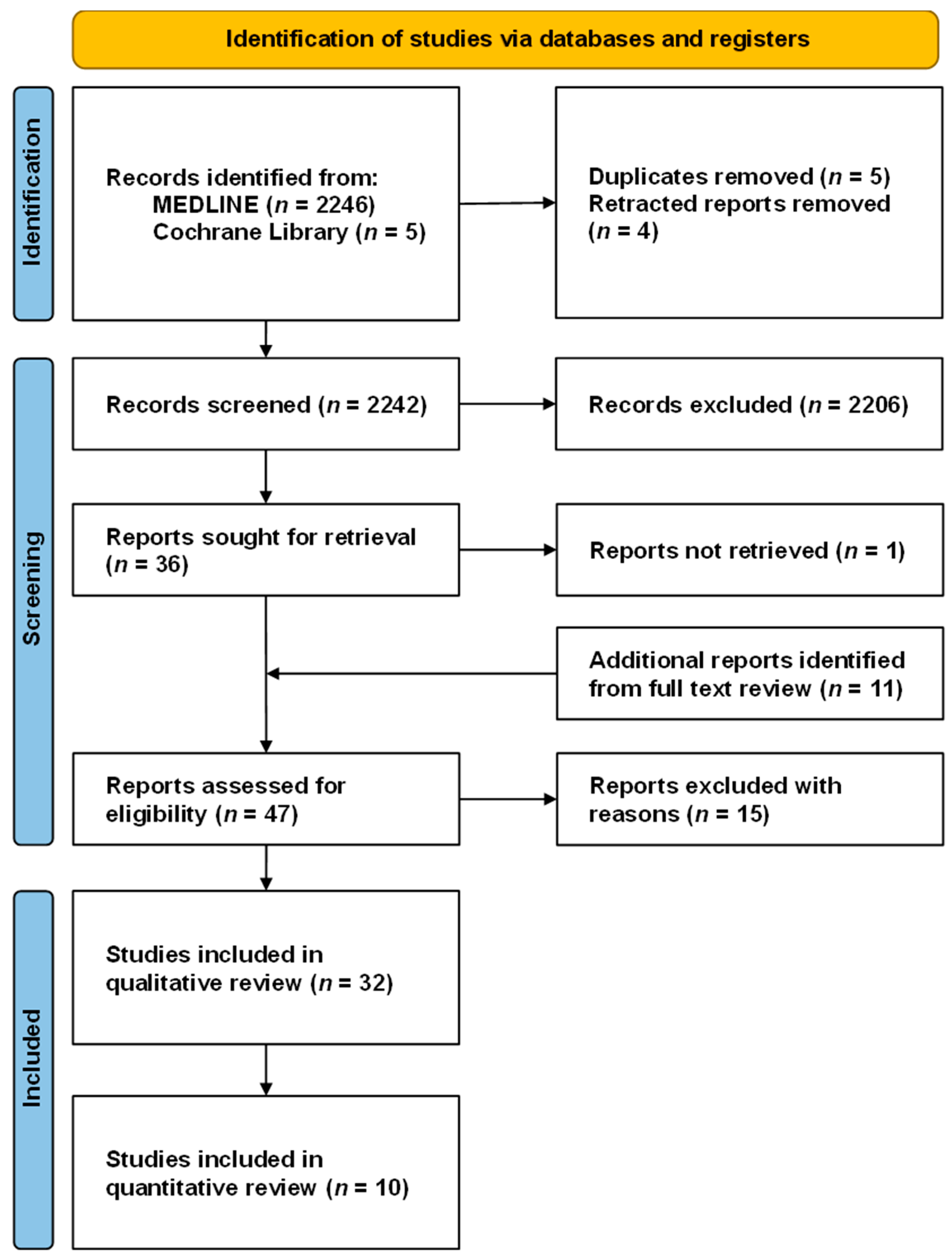
3.1. Study Selection
3.2. Study Characteristics
3.3. Outcomes of the Included Studies
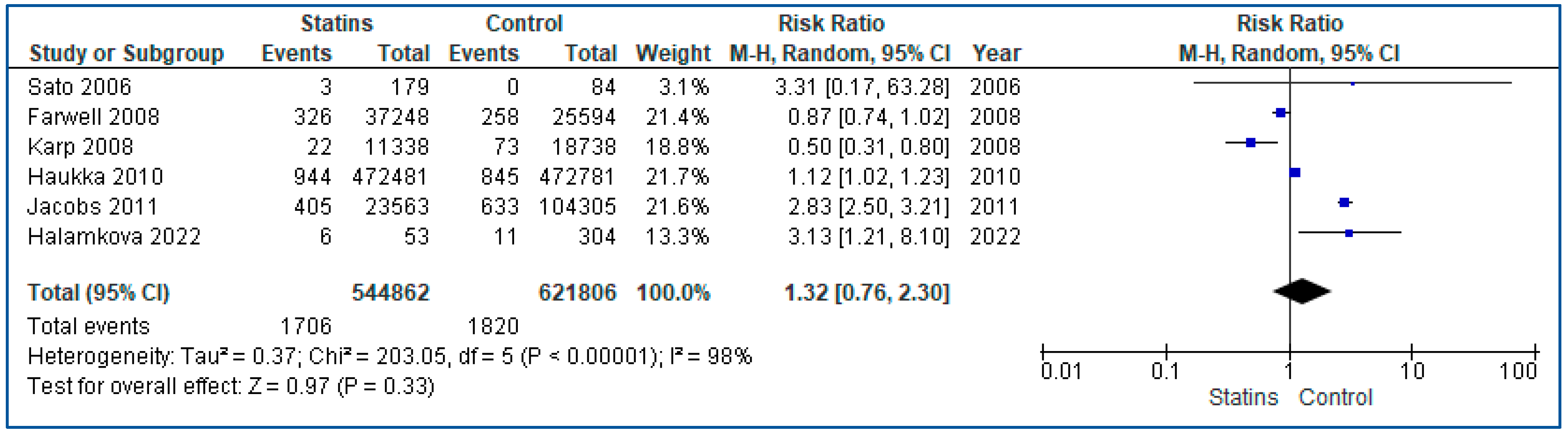
3.4. Results from the Quantitative Synthesis
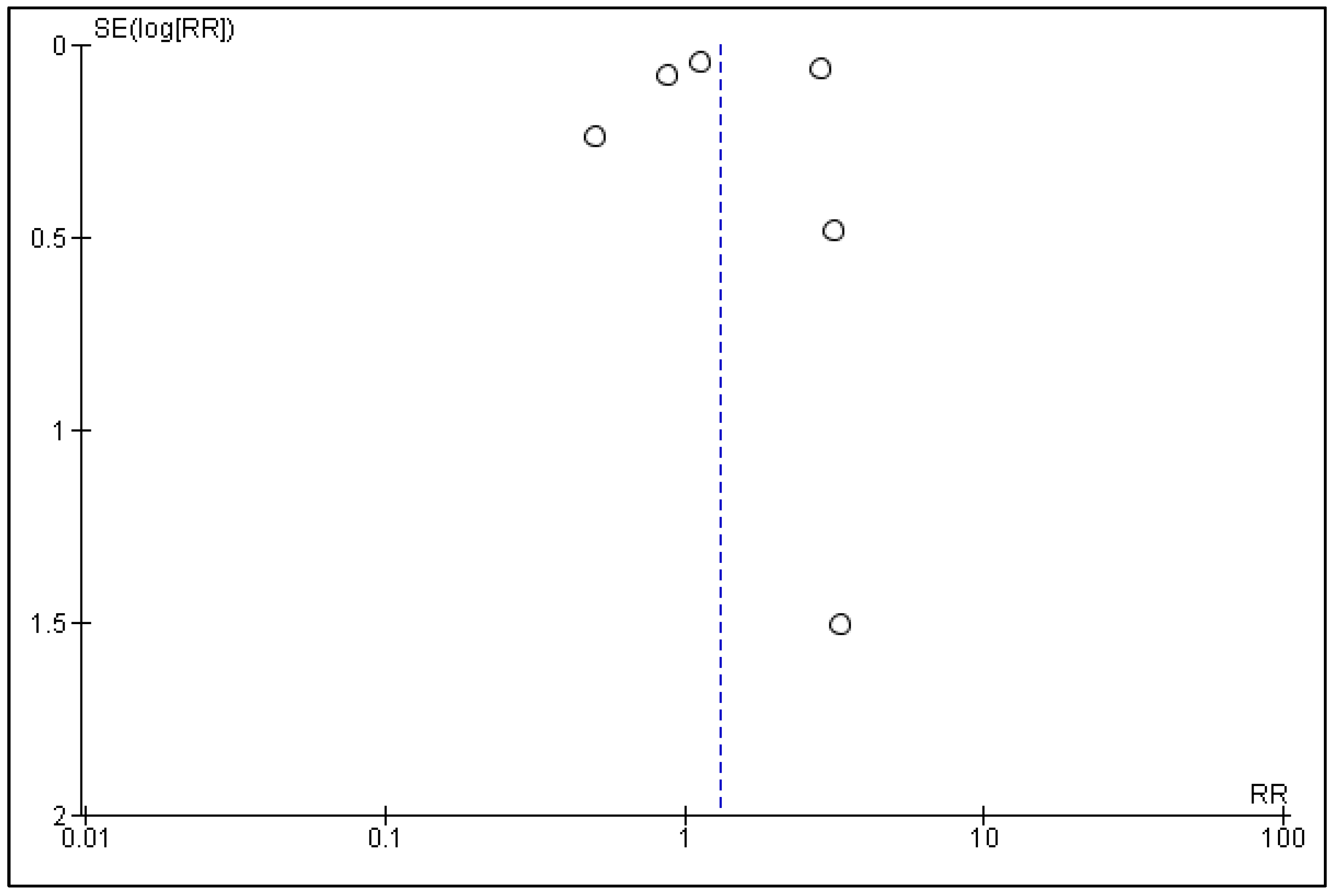
3.5. Publication Bias
3.6. Quality Appraisal
4. Discussion
4.1. Incidence
4.2. Local Control and Recurrence
4.3. Progression to Cystectomy
4.4. Survival and Mortality
4.5. Bacille Calmette–Guérin Immunotherapy
4.6. Role of Statins in Bladder Cancer
4.7. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-Muscle-Invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef]
- Agouridis, A.P.; Elisaf, M.S.; Nair, D.R.; Mikhailidis, D.P. All for Statins and Statins for All; An Update. Curr. Pharm. Des. 2016, 22, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Sizar, O.; Khare, S.; Jamil, R.T.; Talati, R. Statin Medications. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Newman, C.B.; Preiss, D.; Tobert, J.A.; Jacobson, T.A.; Page, R.L.; Goldstein, L.B.; Chin, C.; Tannock, L.R.; Miller, M.; Raghuveer, G.; et al. Statin Safety and Associated Adverse Events: A Scientific Statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e38–e81. [Google Scholar] [CrossRef] [PubMed]
- Kostapanos, M.S.; Agouridis, A.P.; Elisaf, M.S. Variable Effects of Statins on Glucose Homeostasis Parameters and Their Diabetogenic Role. Diabetologia 2015, 58, 1960–1961. [Google Scholar] [CrossRef]
- Dale, K.M.; Coleman, C.I.; Henyan, N.N.; Kluger, J.; White, C.M. Statins and Cancer Risk: A Meta-Analysis. JAMA 2006, 295, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Bedi, O.; Dhawan, V.; Sharma, P.L.; Kumar, P. Pleiotropic Effects of Statins: New Therapeutic Targets in Drug Design. Naunyn. Schmiedebergs Arch. Pharmacol. 2016, 389, 695–712. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-Bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-Bias Assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P.; Ga, S.W. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2014. [Google Scholar]
- Ford, I.; Murray, H.; Packard, C.J.; Shepherd, J.; Macfarlane, P.W.; Cobbe, S.M. Long-Term Follow-up of the West of Scotland Coronary Prevention Study. N. Engl. J. Med. 2007, 357, 1477–1486. [Google Scholar] [CrossRef]
- Tseng, C.-H. Diabetes and Risk of Bladder Cancer: A Study Using the National Health Insurance Database in Taiwan. Diabetologia 2011, 54, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.D.; Xylinas, E.; Kluth, L.; Crivelli, J.J.; Chrystal, J.; Chade, D.; Guglielmetti, G.B.; Pycha, A.; Lotan, Y.; Karakiewicz, P.I.; et al. Impact of Statin Use on Oncologic Outcomes in Patients with Urothelial Carcinoma of the Bladder Treated with Radical Cystectomy. J. Urol. 2014, 190, 487–492, Erratum in J. Urol. 2014, 191, 273. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, B.C.; Sarici, H.; Yuceturk, C.N. Re: Impact of Statin Use on Oncologic Outcomes in Patients with Urothelial Carcinoma of the Bladder Treated with Radical Cystectomy: R. D. Da Silva, E. Xylinas, L. Kluth, J. J. Crivelli, J. Chrystal, D. Chade, G. B. Guglielmetti, A. Pycha, Y. Lotan, P. I. Karakiewicz, M. Sun, H. Fajkovic, M. Zerbib, D. S. Scherr and S. F. Shariat J Urol 2013;190:487-492. J. Urol. 2014, 192, 618–619. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Higuchi, T.; Hosomi, K.; Takada, M. Association between Statin Use and Cancer: Data Mining of a Spontaneous Reporting Database and a Claims Database. Int. J. Med. Sci. 2015, 12, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.M.; Gislason, G.; Moore, L.L.; Andersson, C.; Torp-Pedersen, C.; Denis, G.V.; Schmiegelow, M.D. Associations between Metabolic Disorders and Risk of Cancer in Danish Men and Women—A Nationwide Cohort Study. BMC Cancer 2016, 16, 133. [Google Scholar] [CrossRef]
- Hu, Y.-B.; Hu, E.-D.; Fu, R.-Q. Statin Use and Cancer Incidence in Patients with Type 2 Diabetes Mellitus: A Network Meta-Analysis. Gastroenterol. Res. Pract. 2018, 2018, 8620682. [Google Scholar] [CrossRef]
- Roy, S.; Vallepu, S.; Barrios, C.; Hunter, K. Comparison of Comorbid Conditions between Cancer Survivors and Age-Matched Patients without Cancer. J. Clin. Med. Res. 2018, 10, 911–919. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Gencer, B.; Wiviott, S.D.; Park, J.-G.; Fuchs, C.S.; Goessling, W.; Musliner, T.A.; Tershakovec, A.M.; Blazing, M.A.; Califf, R.; et al. Prospective Evaluation of Malignancy in 17,708 Patients Randomized to Ezetimibe Versus Placebo: Analysis from IMPROVE-IT. JACC Cardio Oncol. 2020, 2, 385–396. [Google Scholar] [CrossRef]
- Bedimo, R.J.; Park, L.S.; Shebl, F.; Sigel, K.; Rentsch, C.T.; Crothers, K.; Rodriguez-Barradas, M.C.; Goetz, M.B.; Butt, A.A.; Brown, S.T.; et al. Statin Exposure and Risk of Cancer in People with and without HIV Infection. AIDS 2021, 35, 325–334. [Google Scholar] [CrossRef]
- Okada, S.; Morimoto, T.; Ogawa, H.; Soejima, H.; Matsumoto, C.; Sakuma, M.; Nakayama, M.; Doi, N.; Jinnouchi, H.; Waki, M.; et al. Association Between Statins and Cancer Incidence in Diabetes: A Cohort Study of Japanese Patients with Type 2 Diabetes. J. Gen. Intern. Med. 2021, 36, 632–639. [Google Scholar] [CrossRef]
- Marrone, M.T.; Mondul, A.M.; Prizment, A.E.; Couper, D.; Barber, J.R.; Chappidi, M.R.; Joshu, C.E.; Platz, E.A. Lipid-Lowering Drug Use and Cancer Incidence and Mortality in the ARIC Study. JNCI Cancer Spectr. 2021, 5, pkab080. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.-J.; Lai, J.-N.; Lin, C.-L.; Hsu, C.-Y.; Kao, C.-H. Time-Dependent Propensity-Matched General Population Study of the Effects of Statin Use on Cancer Risk in an Interstitial Lung Disease and Pulmonary Fibrosis Cohort. BMJ Open 2021, 11, e047039. [Google Scholar] [CrossRef]
- Ahn, J.; Won, S.; Lee, S. Impact of Statins on the Survival of Patients with Cancer: A Nationwide Population-Based Cohort Study in South Korea. Cancer Commun. 2022, 42, 184–187. [Google Scholar] [CrossRef]
- Stepien, K.; Nowak, K.; Kachnic, N.; Horosin, G.; Walczak, P.; Karcinska, A.; Schwarz, T.; Wojtas, M.; Zalewska, M.; Pastuszak, M.; et al. Statin Use in Cancer Patients with Acute Myocardial Infarction and Its Impact on Long-Term Mortality. Pharmaceuticals 2022, 15, 919. [Google Scholar] [CrossRef]
- Clearfield, M.; Downs, J.R.; Weis, S.; Whitney, E.J.; Kruyer, W.; Shapiro, D.R.; Stein, E.A.; Langendorfer, A.; Beere, P.A.; Gotto, A.M. Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS): Efficacy and Tolerability of Long-Term Treatment with Lovastatin in Women. J. Womens Health Gend. Based Med. 2001, 10, 971–981. [Google Scholar] [CrossRef]
- Strandberg, T.E.; Pyörälä, K.; Cook, T.J.; Wilhelmsen, L.; Faergeman, O.; Thorgeirsson, G.; Pedersen, T.R.; Kjekshus, J. 4S Group Mortality and Incidence of Cancer during 10-Year Follow-up of the Scandinavian Simvastatin Survival Study (4S). Lancet 2004, 364, 771–777. [Google Scholar] [CrossRef]
- Heart Protection Study Collaborative Group. The Effects of Cholesterol Lowering with Simvastatin on Cause-Specific Mortality and on Cancer Incidence in 20,536 High-Risk People: A Randomised Placebo-Controlled Trial [ISRCTN48489393]. BMC Med. 2005, 3, 6. [Google Scholar] [CrossRef]
- Rossebø, A.B.; Pedersen, T.R.; Boman, K.; Brudi, P.; Chambers, J.B.; Egstrup, K.; Gerdts, E.; Gohlke-Bärwolf, C.; Holme, I.; Kesäniemi, Y.A.; et al. Intensive Lipid Lowering with Simvastatin and Ezetimibe in Aortic Stenosis. N. Engl. J. Med. 2008, 359, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Coogan, P.F.; Rosenberg, L.; Strom, B.L. Statin Use and the Risk of 10 Cancers. Epidemiol. Camb. Mass 2007, 18, 213–219. [Google Scholar] [CrossRef]
- Graaf, M.R.; Beiderbeck, A.B.; Egberts, A.C.G.; Richel, D.J.; Guchelaar, H.-J. The Risk of Cancer in Users of Statins. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004, 22, 2388–2394. [Google Scholar] [CrossRef]
- Kaye, J.A.; Jick, H. Statin Use and Cancer Risk in the General Practice Research Database. Br. J. Cancer 2004, 90, 635–637. [Google Scholar] [CrossRef]
- Vinogradova, Y.; Coupland, C.; Hippisley-Cox, J. Exposure to Statins and Risk of Common Cancers: A Series of Nested Case-Control Studies. BMC Cancer 2011, 11, 409. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Chiu, H.-F.; Lee, I.-M.; Kuo, H.-W.; Lee, C.-T.; Yang, C.-Y. Statin Use and the Risk of Bladder Cancer: A Population-Based Case-Control Study. Expert Opin. Drug Saf. 2012, 11, 733–738. [Google Scholar] [CrossRef]
- Sato, S.; Ajiki, W.; Kobayashi, T.; Awata, N. Pravastatin Use and the Five-Year Incidence of Cancer in Coronary Heart Disease Patients: From the Prevention of Coronary Sclerosis Study. J. Epidemiol. 2006, 16, 201–206. [Google Scholar] [CrossRef]
- Farwell, W.R.; Scranton, R.E.; Lawler, E.V.; Lew, R.A.; Brophy, M.T.; Fiore, L.D.; Gaziano, J.M. The Association between Statins and Cancer Incidence in a Veterans Population. J. Natl. Cancer Inst. 2008, 100, 134–139. [Google Scholar] [CrossRef]
- Friedman, G.D.; Flick, E.D.; Udaltsova, N.; Chan, J.; Quesenberry, C.P.; Habel, L.A. Screening Statins for Possible Carcinogenic Risk: Up to 9 Years of Follow-up of 361,859 Recipients. Pharmacoepidemiol. Drug Saf. 2008, 17, 27–36. [Google Scholar] [CrossRef]
- Karp, I.; Behlouli, H.; Lelorier, J.; Pilote, L. Statins and Cancer Risk. Am. J. Med. 2008, 121, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Haukka, J.; Sankila, R.; Klaukka, T.; Lonnqvist, J.; Niskanen, L.; Tanskanen, A.; Wahlbeck, K.; Tiihonen, J. Incidence of Cancer and Statin Usage–Record Linkage Study. Int. J. Cancer 2010, 126, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, E.J.; Newton, C.C.; Thun, M.J.; Gapstur, S.M. Long-Term Use of Cholesterol-Lowering Drugs and Cancer Incidence in a Large United States Cohort. Cancer Res. 2011, 71, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Halámková, J.; Bohovicová, L.; Pehalová, L.; Goněc, R.; Staněk, T.; Kazda, T.; Mouková, L.; Krákorová, D.A.; Kozáková, Š.; Svoboda, M.; et al. Use of Hypolipidemic Drugs and the Risk of Second Primary Malignancy in Colorectal Cancer Patients. Cancers 2022, 14, 1699. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.K.; Katz, M.S.; Coen, J.J.; Zietman, A.L.; Kaufman, D.S.; Shipley, W.U. Association of Statin Use with Improved Local Control in Patients Treated with Selective Bladder Preservation for Muscle-Invasive Bladder Cancer. Urology 2006, 68, 1188–1192. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.M.; Wu, X. Statins and the Effect of BCG on Bladder Cancer. N. Engl. J. Med. 2007, 356, 1276–1277. [Google Scholar] [CrossRef] [PubMed]
- Berglund, R.K.; Savage, C.J.; Vora, K.C.; Kurta, J.M.; Cronin, A.M. An Analysis of the Effect of Statin Use on the Efficacy of Bacillus Calmette-Guerin Treatment for Transitional Cell Carcinoma of the Bladder. J. Urol. 2008, 180, 1297–1300. [Google Scholar] [CrossRef]
- Crivelli, J.J.; Xylinas, E.; Kluth, L.A.; da Silva, R.D.; Chrystal, J.; Novara, G.; Karakiewicz, P.I.; David, S.G.; Scherr, D.S.; Lotan, Y.; et al. Effect of Statin Use on Outcomes of Non-Muscle-Invasive Bladder Cancer. BJU Int. 2013, 112, E4–E12. [Google Scholar] [CrossRef]
- Pastore, A.; Palleschi, G.; Fuschi, A.; Silvestri, L.; Al Salhi, Y.; Costantini, E.; Zucchi, A.; Petrozza, V.; de Nunzio, C.; Carbone, A. Can Daily Intake of Aspirin and/or Statins Influence the Behavior of Non-Muscle Invasive Bladder Cancer? A Retrospective Study on a Cohort of Patients Undergoing Transurethral Bladder Resection. BMC Cancer 2015, 15, 120. [Google Scholar] [CrossRef]
- Ferro, M.; Marchioni, M.; Lucarelli, G.; Vartolomei, M.D.; Soria, F.; Terracciano, D.; Mistretta, F.A.; Luzzago, S.; Buonerba, C.; Cantiello, F.; et al. Association of Statin Use and Oncological Outcomes in Patients with First Diagnosis of T1 High Grade Non-Muscle Invasive Urothelial Bladder Cancer: Results from a Multicenter Study. Minerva Urol. Nephrol. 2021, 73, 796–802. [Google Scholar] [CrossRef]
- Hoffmann, P.; Roumeguère, T.; Schulman, C.; van Velthoven, R. Use of Statins and Outcome of BCG Treatment for Bladder Cancer. N. Engl. J. Med. 2006, 355, 2705–2707. [Google Scholar] [CrossRef] [PubMed]
- Singla, N.; Haddad, A.Q.; Passoni, N.M.; Meissner, M.; Lotan, Y. Anti-Inflammatory Use May Not Negatively Impact Oncologic Outcomes Following Intravesical BCG for High-Grade Non-Muscle-Invasive Bladder Cancer. World J. Urol. 2017, 35, 105–111. [Google Scholar] [CrossRef]
- Skolarus, T.A.; Lee, E.W.; Virgo, K.S.; Katz, M.D.; Hudson, M.A.; Kibel, A.S.; Grubb, R.L., 3rd. Intravesical Bacille Calmette-Guérin Therapy for Non-Muscle-Invasive Bladder Cancer: Effects of Concurrent Statin Therapy. J. Am. Coll. Surg. 2009, 209, 248–253. [Google Scholar] [CrossRef]
- Brooks, N.A.; Kokorovic, A.; Xiao, L.; Matulay, J.T.; Li, R.; Ranasinghe, W.K.B.; Nagaraju, S.; Shen, Y.; Gao, J.; Navai, N.; et al. The Obesity Paradox: Defining the Impact of Body Mass Index and Diabetes Mellitus for Patients with Non-Muscle-Invasive Bladder Cancer Treated with Bacillus Calmette-Guérin. BJU Int. 2021, 128, 65–71. [Google Scholar] [CrossRef]
- Segal, R.; Yafi, F.A.; Brimo, F.; Tanguay, S.; Aprikian, A.; Kassouf, W. Prognostic Factors and Outcome in Patients with T1 High-Grade Bladder Cancer: Can We Identify Patients for Early Cystectomy? BJU Int. 2012, 109, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Richard, P.O.; Ahmad, A.E.; Bashir, S.; Hamilton, R.J.; Nam, R.K.; Leao, R.; Jeldres, C.; Kulkarni, G.S. Effect of Statins as a Secondary Chemopreventive Agent among Individuals with Non-Muscle-Invasive Bladder Cancer: A Population-Based Analysis. Urol. Oncol. 2017, 35, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Guercio, V.; Turati, F.; Bosetti, C.; Polesel, J.; Serraino, D.; Montella, M.; Libra, M.; Galfano, A.; La Vecchia, C.; Tavani, A. Bladder Cancer Risk in Users of Selected Drugs for Cardiovascular Disease Prevention. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP 2019, 28, 76–80. [Google Scholar] [CrossRef]
- Lundberg, E.; Hagberg, O.; Jahnson, S.; Ljungberg, B. Association between Occurrence of Urinary Bladder Cancer and Treatment with Statin Medication. Turk. J. Urol. 2019, 45, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Haimerl, L.; Strobach, D.; Mannell, H.; Stief, C.G.; Buchner, A.; Karl, A.; Grimm, T. Retrospective Evaluation of the Impact of Non-Oncologic Chronic Drug Therapy on the Survival in Patients with Bladder Cancer. Int. J. Clin. Pharm. 2022, 44, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Geng, J.; Zhang, X.; Peng, B.; Che, J.; Yan, Y.; Wang, G.; Xia, S.; Wu, Y.; Zheng, J. Statin Use and Risk of Bladder Cancer: A Meta-Analysis. Cancer Causes Control 2013, 24, 769–776. [Google Scholar] [CrossRef]
- Emilsson, L.; García-Albéniz, X.; Logan, R.W.; Caniglia, E.C.; Kalager, M.; Hernán, M.A. Examining Bias in Studies of Statin Treatment and Survival in Patients with Cancer. JAMA Oncol. 2018, 4, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Hu, J.; Othmane, B.; Li, H.; Qiu, D.; Yi, Z.; Chen, J.; Zu, X. Effects of Fibrin Clot Inhibitors and Statins on the Intravesical Bacille Calmette-Guérin Therapy for Bladder Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 614041. [Google Scholar] [CrossRef]
- Clendening, J.W.; Pandyra, A.; Boutros, P.C.; El Ghamrasni, S.; Khosravi, F.; Trentin, G.A.; Martirosyan, A.; Hakem, A.; Hakem, R.; Jurisica, I.; et al. Dysregulation of the Mevalonate Pathway Promotes Transformation. Proc. Natl. Acad. Sci. USA 2010, 107, 15051–15056. [Google Scholar] [CrossRef]
- Kamat, A.M.; Nelkin, G.M. Atorvastatin: A Potential Chemopreventive Agent in Bladder Cancer. Urology 2005, 66, 1209–1212. [Google Scholar] [CrossRef]
- Peng, T.; Wang, G.; Cheng, S.; Xiong, Y.; Cao, R.; Qian, K.; Ju, L.; Wang, X.; Xiao, Y. The Role and Function of PPARγ in Bladder Cancer. J. Cancer 2020, 11, 3965–3975. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Miyai, K.; Kato, K.; Asano, T.; Sato, A. Simvastatin-Romidepsin Combination Kills Bladder Cancer Cells Synergistically. Transl. Oncol. 2021, 14, 101154. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cao, R.; Wang, Y.; Qian, G.; Dan, H.C.; Jiang, W.; Ju, L.; Wu, M.; Xiao, Y.; Wang, X. Simvastatin Induces Cell Cycle Arrest and Inhibits Proliferation of Bladder Cancer Cells via PPARγ Signalling Pathway. Sci. Rep. 2016, 6, 35783. [Google Scholar] [CrossRef] [PubMed]
- Tilija Pun, N.; Jeong, C.-H. Statin as a Potential Chemotherapeutic Agent: Current Updates as a Monotherapy, Combination Therapy, and Treatment for Anti-Cancer Drug Resistance. Pharmaceuticals 2021, 14, 470. [Google Scholar] [CrossRef]




| First Author | Study Type | Country | Exposure and Comparator | Population (Exposure/Controls) | Outcome(s) Reported (Exposure/ Controls) | Characteristics and Comorbidities | Study Conclusion |
|---|---|---|---|---|---|---|---|
| Clearfield, M. et al., 2001 [27] | RCT | USA | Lovastatin vs. Placebo | 499/498 | Incidence 1/0 | Women 55–73 years. | Incidence similar in both groups. |
| Graaf, M.R. et al., 2004 [32] | Case-Control | Netherlands | Any statin vs. No statin | 1130/18,661 | Incidence 249/986 | Patients with prescriptions of cardiovascular drugs. | Suggested protective effect of statins against cancer. |
| Kaye, J.A. et al., 2004 [33] | Case-Control | UK | Any statin vs. No statin | 3244/14,844 | Incidence 19/74 | Patients 50–89 years old who used antihyperlipidemic drugs or had a recorded diagnosis of untreated hyperlipidemia and age-matched controls. | Statin use does not have a substantial effect on cancer risk. |
| Strandberg, T.E. et al., 2004 [28] | RCT | Nordic countries § | Simvastatin vs. Placebo | 2221/2223 | Incidence 19/17 | CHD patients. | Incidence similar in both groups. |
| Heart Protection Study Collaborative Group, 2005 [29] | RCT | UK | Simvastatin vs. Placebo | 10,269/10,267 | Incidence 74/90 | Patients 40–80 years with non-fasting blood total cholesterol concentrations of at least 135 mg/dL with history of occlusive arterial disease; diabetes mellitus; or treated hypertension. | No adverse effects of statins on cancer incidence. |
| Hoffmann, P. et al., 2006 [49] | Cohort | USA | Any statin vs. No statin | 19/65 | Progression 10/12 Cystectomy 8/9 | Patients receiving BCG for NMIBC. | Discontinuation of statins during BCG therapy might be beneficial. |
| Sato, S. et al., 2006 [36] | Cohort | Japan | Pravastatin vs. No statin | 179/84 | Incidence 3/0 | CHD patients aged 70 years or younger. | Significantly elevated risk of bladder cancer. |
| Tsai, H.K. et al., 2006 [43] | Cohort | USA | Any statin vs. No statin | 35/251 | Local control (UVA) 73%/52% | Patients with MIBC with maximal transurethral resection followed by chemoradiotherapy. | Statin use associated with improved LC on UVA but not after controlling for known prognostic factors. |
| Coogan, P.F. et al., 2007 [31] | Case-Control | USA | Any statin vs. No statin | 190/3652 | Incidence 20/216 | Patients 40–79 years with a primary cancer of a site and regular users of statins. | No support for an association between statin use and cancer. |
| Kamat, A. et al., 2007 [44] | Cohort | USA | Any statin vs. No statin | 39/117 | Recurrence 23/69 Progression 12/33 | Cohort of 156 patients receiving BCG immunotherapy. | No effect of statin use on recurrence, progression, or number of deaths during BCG therapy. |
| Rossebø, A.B. et al., 2008 [30] | RCT | USA | Simvastatin/Ezetimibe vs. Placebo | 944/929 | Incidence 7/7 | Patients with mild-to-moderate asymptomatic aortic stenosis. | Similar incidence and risk between the two groups. |
| Berglund, R.K. et al., 2008 [45] | Cohort | USA | Any statin vs. No statin | Total 952, 245/707 | Recurrence 214/582 Progression to surgery 78/287 | Cohort of 952 patients treated with BCG immunotherapy. | No statistical difference in recurrence or progression to surgery. |
| Farwell, W.R. et al., 2008 [37] | Cohort | UK | Any statin vs. No statin | 37,248/25,594 | Incidence 326/258 | Patients using antihypertensive medications but no cholesterol-lowering medications. | Incidence rate lower among statin users. Lower incidence during entire follow-up for users. |
| Friedman, G.D. et al., 2008 [38] | Cohort | USA | Any statin: any duration vs. ≥5 years | Total 353,199 | Incidence 498 (418 men, 80 women)/111 (94 men, 17 women) | Statin users. | No strong evidence but observed increased risk for bladder cancer in both men and women. |
| Karp, I. et al., 2008 [39] | Cohort | Canada | High-dose statin vs. Low-dose statin vs. No statin † | High-dose 6015 Low-dose 5323 None 18,738 | Incidence (per group) 9/13/73 | Patients aged ≥ 45 years discharged with a history of MI. | Suggested dose-response effect of lipophilic statins on cancer occurrence. |
| Skolarus, T.A. et al., 2009 [51] | Cohort | USA | Any statin vs. No statin | 43/47 | Progression 6/6 Mortality 3/2 Mortality 11/16 | Patients diagnosed with UBC and treated with BCG immunotherapy. | No association of treatment outcomes with statin use. |
| Haukka, J. et al., 2010 [40] | Cohort | Finland | Any statin vs. No statin | 472,481/472,781 | Incidence 944/845 | Individuals who purchased statins with no history of cancer. | Weak association between statin use and incidence. |
| Jacobs, E.J. et al., 2011 [41] | Cohort | USA | Any statin (subdivided into former use, current use < 5 y, current use > 5 y) vs. No statin | Former 5387/ Current (<5 y) 13,313/ Current (>5 y) 10,250/ No use 104,305 | Incidence 1081/ Former 43/ Current (<5 y) 202/ Current (>5 y) 203/ No use 633 | Participants of the CPS-II nutrition cohort. | Long-term use does not increase cancer risk. |
| Vinogradova, Y. et al., 2011 [34] | Case-Control | UK | Any statin (subdivided with treatment duration) vs. No statin | 4227/17,559 | Incidence 856/3125 | Open cohort. Identified patients aged 30–100 with a history of cancer. Allocated 5 controls per case. | Non-significant increased risk observed. |
| Kuo, C.C. et al., 2012 [35] | Case-Control | Taiwan | Any statin vs. No statin | 268/1032 | Incidence 64/261 | National health insurance (NHI). Patients aged ≥ 50 years, first-time diagnosed with UBC. Controls, patients with admission unrelated to statin use. | No association between statin use and UBC risk. |
| Segal, R. et al., 2012 [53] | Cohort | Canada | NA | NR/NR | Total 278 HR: 0.784 (95% CI 0.453–1.341, p = 0.375) | Cohort of 2570 patient records with T1HG UBC. | Statin use was not associated with worse prognosis. |
| Crivelli, J.J. et al., 2013 [46] | Cohort | USA | Any statin vs. No statin | 341/776 | NA | Patients with NMIBC treated with TURB. | Statin use was not associated with disease recurrence, progression, cancer-specific mortality, or any-cause mortality. Similar results in subgroup analyses. |
| da Silva, R.D. et al., 2013 [14] | Cohort | USA | Any statin vs. No statin | 642/860 | Disease recurrence (UVA, MVA) HR 1.22 (95% CI: 1.03–1.46, p = 0.02) HR 1.04 (95% CI: 0.86–1.24, p = 0.66) Ca specific mortality (UVA, MVA) HR 1.26 (95% CI: 1.04–1.54, p = 0.02) HR 1.04 (95% CI: 0.84–1.28, p = 0.68) | Patients treated with radical cystectomy and pelvic lymphadenectomy without neoadjuvant therapy. | Statin use was associated with disease recurrence and cancer specific mortality on UVA, but not on MVA. |
| Pastore, A.L. et al., 2015 [47] | Cohort | Italy | Statin (±Aspirin) vs. None or Aspirin only | 189/385 | Recurrence UVA OR 1.853 (95% CI: 1.144–3.1, p = 0.012) OR 1.886 (95% CI: 1.095–3.247, p = 0.022) | Patients with NMIBC treated with TURB. | Aspirin and statins are able to modify the behavior of NMIBC. Statins and combination treatment with aspirin groups showed increased recurrence rates and progression. |
| Richard, O.P. et al., 2017 [54] | Cohort | Canada | Any statin vs. No statin | 4748/9063 | CSS Before diagnosis: HR 1.04 (95% CI: 0.99–1.09, p = 0.43) After diagnosis: HR 1.04 (95% CI: 0.99–1.09, p = 0.10) OS Before diagnosis: HR 1.01 (95% CI: 0.99–1.03, p = 0.10) After diagnosis: HR 0.93 (95% CI: 0.91–0.96, p < 0.001) | Patients ≥ 66 years diagnosed with NMIBC with no record of statin use before that age. | Cumulative statin use was associated with an improvement in OS but not CSS. |
| Singla, N. et al., 2017 [50] | Cohort | USA | Any statin | 64/35 | Recurrence HR 0.93 (95% CI: 0.56–1.54, p = 0.764) Stage progression HR 0.72 (95% CI: 0.23–2.27, p = 0.574) Cystectomy HR 1.40 (95% CI: 0.58–3.37, p = 0.449) Overall mortality HR 0.76 (95% CI: 0.31–1.88, p = 0.554) Cancer-specific mortality HR 0.27 (95% CI: 0.05–1.49, p = 0.133) | Patients receiving intravesical BCG therapy for high-grade NMIBC. | No effect of statins on any of the oncologic outcomes. |
| Guercio, V. et al., 2019 [55] | Case-Control | Italy | Any statin vs. No statin | 71/618 for cases 121/1233 for both groups | Total 690 71/0 * | UBC case-control study patients and hospital controls. Patients (cases) diagnosed with UBC. | Statin use does not increase cancer risk. |
| Lundberg, E. et al., 2019 [56] | Cohort | Sweden | Any statin vs. No statin | 7754 of 22,936/68,247 of 229,326 | Occurrence UBC OR 1.23 (95% CI: 1.19–1.27, p < 0.001) NMIBC OR 1.31 (95% CI: 1.26–1.35, p < 0.0001) MIBC OR 1.02 (95% CI: 0.96–1.08, p = 0.6) | Patients with diagnosed UBC and matched controls. | Statins were significantly associated with an increased risk of UBC. |
| Brooks, N.A. et al., 2021 [52] | Cohort | USA | Any statin vs. No statin | 244/334 (1 unknown) | NA | Patients with NMIBC treated with BCG immunotherapy at least once. | No data for statins, but BMI (of ≥25 kg/m2) was significantly associated with improved PFS, OS, and CSS. |
| Ferro, M. et al., 2021 [48] | Cohort | Italy | Any statin vs. No statin | 402/1108 | MVA Recurrence HR 0.80 (95% CI: 0.67–0.95, p = 0.009) Progression HR 0.97 (95% CI: 0.79–1.19, p = 0.753) Overall mortality HR 0.71 (95% CI: 0.50–1.03, p = 0.068) | Patients with first diagnosis of T1 HG NMIBC after TURB. | Statin users exhibited lower disease rates, disease progression, and similar overall mortality compared to non-users. No adverse effect of statins on BCG efficacy. |
| Haimerl, L. et al., 2022 [57] | Cohort | Germany | Any statin vs. No statin | 972 | UVA RFS 174/685, p = 0.653 CSS 203/769, p = 0.296 OS 203/769, p = 0.482 | Database of UBC patients who underwent radical cystectomy. | No correlation between statin use and RFS, CSS, or OS. |
| Halámková, J. et al., 2022 [42] | Cohort | Czech Republic | Any statin vs. No statin | 53/304 | Incidence 6/11 | Adult patients with a histologically confirmed colorectal cancer diagnosis. | Use of hypolipidemic agents was associated with a lower incidence of an SPM, where the protective effect was most prominent in statin users. |
| Study | Selection | Comparability | Exposure | Total Score |
|---|---|---|---|---|
| Graaf, M.R. et al., 2004 [32] | ★★★☆ | ★☆ | ★★☆ | 6 |
| Kaye, J.A. et al., 2004 [33] | ★★★★ | ★★ | ★★☆ | 8 |
| Coogan, P.F. et al., 2007 [31] | ★★★☆ | ★★ | ★★☆ | 7 |
| Vinogradova, Y. et al., 2011 [34] | ★★★★ | ★★ | ★★☆ | 8 |
| Kuo, C.C. et al., 2012 [35] | ★★★☆ | ★☆ | ★★☆ | 6 |
| Guercio, V. et al., 2019 [55] | ★★★☆ | ★★ | ★★☆ | 7 |
| Mean | 7 |
| Study | Selection | Comparability | Outcome | Total Score |
|---|---|---|---|---|
| Hoffmann, P. et al., 2006 [49] | ★★☆☆ | ★☆ | ★★☆ | 5 |
| Sato, S. et al., 2006 [36] | ★★★☆ | ★★ | ★★★ | 8 |
| Tsai, H.K. et al., 2006 [43] | ★★★☆ | ★☆ | ★★★ | 6 |
| Kamat, A. et al., 2007 [44] | ★★★☆ | ★★ | ★★★ | 8 |
| Berglund, R.K. et al., 2008 [45] | ★★★★ | ★☆ | ★★★ | 8 |
| Farwell, W.R. et al., 2008 [37] | ★★★☆ | ★★ | ★★★ | 8 |
| Friedman, G.D. et al., 2008 [38] | ★★★★ | ★★ | ★★★ | 9 |
| Karp, I. et al., 2008 [39] | ★★★★ | ★☆ | ★★☆ | 7 |
| Skolarus, T.A. et al., 2009 [51] | ★★★☆ | ★★ | ★★★ | 8 |
| Haukka, J. et al., 2010 [40] | ★★☆☆ | ★☆ | ★★★ | 6 |
| Jacobs, E.J. et al., 2011 [41] | ★★☆☆ | ★★ | ★★★ | 7 |
| Segal, R. et al., 2012 [53] | ★★★★ | ★★ | ★★★ | 9 |
| Crivelli, J.J. et al., 2013 [46] | ★★★★ | ★★ | ★★☆ | 8 |
| da Silva, R.D. et al., 2013 [14] | ★★★☆ | ★★ | ★★★ | 8 |
| Pastore, A.L. et al., 2015 [47] | ★★★★ | ★★ | ★★★ | 9 |
| Richard, O.P. et al., 2017 [54] | ★★★☆ | ★☆ | ★★★ | 7 |
| Singla, N. et al., 2017 [50] | ★★★★ | ★★ | ★★★ | 9 |
| Lundberg, E. et al., 2019 [56] | ★★★★ | ★☆ | ★★★ | 8 |
| Brooks, N.A. et al., 2021 [52] | ★★★★ | ★★ | ★★★ | 9 |
| Ferro, M. et al., 2021 [48] | ★★★★ | ★☆ | ★★★ | 8 |
| Haimerl, L. et al., 2022 [57] | ★★★★ | ★★ | ★★★ | 9 |
| Halámková, J. et al., 2022 [42] | ★★★★ | ★☆ | ★★★ | 8 |
| Mean | 7.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Symvoulidis, P.; Tsioutis, C.; Zamboglou, C.; Agouridis, A.P. The Effect of Statins on the Incidence and Prognosis of Bladder Cancer: A Systematic Review and Meta-Analysis. Curr. Oncol. 2023, 30, 6648-6665. https://doi.org/10.3390/curroncol30070488
Symvoulidis P, Tsioutis C, Zamboglou C, Agouridis AP. The Effect of Statins on the Incidence and Prognosis of Bladder Cancer: A Systematic Review and Meta-Analysis. Current Oncology. 2023; 30(7):6648-6665. https://doi.org/10.3390/curroncol30070488
Chicago/Turabian StyleSymvoulidis, Panagiotis, Constantinos Tsioutis, Constantinos Zamboglou, and Aris P. Agouridis. 2023. "The Effect of Statins on the Incidence and Prognosis of Bladder Cancer: A Systematic Review and Meta-Analysis" Current Oncology 30, no. 7: 6648-6665. https://doi.org/10.3390/curroncol30070488
APA StyleSymvoulidis, P., Tsioutis, C., Zamboglou, C., & Agouridis, A. P. (2023). The Effect of Statins on the Incidence and Prognosis of Bladder Cancer: A Systematic Review and Meta-Analysis. Current Oncology, 30(7), 6648-6665. https://doi.org/10.3390/curroncol30070488







