The Use of Salvage Chemotherapy for Patients with Relapsed Testicular Germ Cell Tumor (GCT) in Canada: A National Survey
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canadian Cancer Society and National Cancer Institute of Canada; Advisory Committee on Records and Statistics Canada. Canadian Cancer Statistics 2021. Available online: https://cancer.ca/Canadian-Cancer-Statistics-2021-EN (accessed on 15 December 2022).
- Abughanimeh, O.; Teply, B.A. Current Management of Refractory Germ Cell Tumors. Curr. Oncol. Rep. 2021, 23, 101. [Google Scholar] [CrossRef] [PubMed]
- Oing, C.; Lorch, A. The Role of Salvage High-Dose Chemotherapy in Relapsed Male Germ Cell Tumors. Oncol. Res. Treat. 2018, 41, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Lorch, A. Management of Refractory Germ Cell Cancer. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 324–329. [Google Scholar] [CrossRef]
- Pico, J.-L.; Rosti, G.; Kramar, A.; Wandt, H.; Koza, V.; Salvioni, R.; Theodore, C.; Lelli, G.; Siegert, W.; Horwich, A. A randomised trial of high-dose chemotherapy in the salvage treatment of patients failing first-line platinum chemotherapy for advanced germ cell tumours. Ann. Oncol. 2005, 16, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Lorch, A.; Kleinhans, A.; Kramar, A.; Kollmannsberger, C.K.; Hartmann, J.T.; Bokemeyer, C.; Rick, O.; Beyer, J. Sequential Versus Single High-Dose Chemotherapy in Patients With Relapsed or Refractory Germ Cell Tumors: Long-Term Results of a Prospective Randomized Trial. J. Clin. Oncol. 2012, 30, 800–805. [Google Scholar] [CrossRef]
- Beyer, J.; Stenning, S.; Gerl, A.; Fossa, S.; Siegert, W. High-dose versus conventional-dose chemotherapy asfirst-salvage treatment in patients with non-seminomatousgerm-cell tumors: A matched-pair analysis. Ann. Oncol. 2002, 13, 599–605. [Google Scholar] [CrossRef]
- Lorch, A.; Beyer, J.; Bascoul-Mollevi, C.; Kramar, A.; Einhorn, L.H.; Necchi, A.; Massard, C.; De Giorgi, U.; Fléchon, A.; Margolin, K.A.; et al. Prognostic factors in patients with metastatic germ cell tumors who experienced treatment failure with cisplatin-based first-line chemotherapy. J. Clin. Oncol. 2010, 28, 4906–4911. [Google Scholar] [CrossRef]
- Seftel, M.D.; Paulson, K.; Doocey, R.; Song, K.; Czaykowski, P.; Coppin, C.; Forrest, D.; Hogge, D.; Kollmansberger, C.; Smith, C.A.; et al. Long-term follow-up of patients undergoing auto-SCT for advanced germ cell tumour: A multicentre cohort study. Bone Marrow Transpl. 2011, 46, 852–857. [Google Scholar] [CrossRef]
- Rashdan, S.; Einhorn, L.H. Salvage Therapy for Patients with Germ Cell Tumor. J. Oncol. Pract. 2016, 12, 437–443. [Google Scholar] [CrossRef]
- Zschäbitz, S.; Distler, F.A.; Krieger, B.; Wuchter, P.; Schäfer-Eckart, K.; Jenzer, M.; Hohenfellner, M.; Dreger, P.; Haag, G.M.; Jäger, D.; et al. Survival outcomes of patients with germ cell tumors treated with high-dose chemotherapy for refractory or relapsing disease. Oncotarget 2018, 9, 22537–22545. [Google Scholar] [CrossRef]
- Miller, M.I.; Feifer, A.; Feldman, D.R.; Carver, B.S.; Bosl, G.J.; Motzer, R.J.; Bajorin, D.F.; Sheinfeld, J. Surgical Management of Patients with Advanced Germ Cell Tumors following Salvage Chemotherapy: Memorial Sloan Kettering Cancer Center (MSKCC) Experience. Urology 2019, 124, 174–178. [Google Scholar] [CrossRef]
- Shamash, J.; Ansell, W.; Alifrangis, C.; Thomas, B.; Wilson, P.; Stoneham, S.; Mazhar, D.; Warren, A.; Barrett, T.; Alexander, S.; et al. The impact of a supranetwork multidisciplinary team (SMDT) on decision-making in testicular cancers: A 10-year overview of the Anglian Germ Cell Cancer Collaborative Group (AGCCCG). Br. J. Cancer 2021, 124, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, L.H.; Williams, S.D.; Chamness, A.; Brames, M.J.; Perkins, S.M.; Abonour, R. High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. N. Engl. J. Med. 2007, 357, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.; Kollmannsberger, C.; Jewett, M.; Chung, P.; Hotte, S.; O’Malley, M.; Sweet, J.; Anson-Cartwright, L.; Winquist, E.; North, S.; et al. Canadian consensus guidelines for the management of testicular germ cell cancer. Can. Urol. Assoc. J. 2010, 4, e19–e38. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.J.; Canil, C.; Shrem, N.S.; Kuhathaas, K.; Jiang, M.D.; Chung, P.; North, S.; Czaykowski, P.; Hotte, S.; Winquist, E.; et al. Canadian Urological Association consensus guideline: Management of testicular germ cell cancer. Can. Urol. Assoc. J. 2022, 16, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.R.; Sheinfeld, J.; Bajorin, D.F.; Fischer, P.; Turkula, S.; Ishill, N.; Patil, S.; Bains, M.; Reich, L.M.; Bosl, G.J.; et al. TI-CE High-Dose Chemotherapy for Patients With Previously Treated Germ Cell Tumors: Results and Prognostic Factor Analysis. J. Clin. Oncol. 2010, 28, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Adra, N.; Abonour, R.; Althouse, S.K.; Albany, C.; Hanna, N.H.; Einhorn, L.H. High-Dose Chemotherapy and Autologous Peripheral-Blood Stem-Cell Transplantation for Relapsed Metastatic Germ Cell Tumors: The Indiana University Experience. J. Clin. Oncol. 2017, 35, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Lorch, A.; Neubauer, A.; Hackenthal, M.; Dieing, A.; Hartmann, J.T.; Rick, O.; Bokemeyer, C.; Beyer, J. High-dose chemotherapy (HDCT) as second-salvage treatment in patients with multiple relapsed or refractory germ-cell tumors. Ann. Oncol. 2010, 21, 820–825. [Google Scholar] [CrossRef]
- Pectasides, D.; Pectasides, M.; Farmakis, D.; Aravantinos, G.; Nikolaou, M.; Koumpou, M.; Gaglia, A.; Kostopoulou, V.; Mylonakis, N.; Skarlos, D. Gemcitabine and oxaliplatin (GEMOX) in patients with cisplatin-refractory germ cell tumors: A phase II study. Ann. Oncol. 2004, 15, 493–497. [Google Scholar] [CrossRef]
- Kondagunta, G.V.; Bacik, J.; Donadio, A.; Bajorin, D.; Marion, S.; Sheinfeld, J.; Bosl, G.J.; Motzer, R.J. Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. J. Clin. Oncol. 2005, 23, 6549–6555. [Google Scholar] [CrossRef]
- Mead, G.M.; Cullen, M.H.; Huddart, R.; Harper, P.; Rustin, G.J.; Cook, P.A.; Stenning, S.P.; Mason, M. A phase II trial of TIP (paclitaxel, ifosfamide and cisplatin) given as second-line (post-BEP) salvage chemotherapy for patients with metastatic germ cell cancer: A medical research council trial. Br. J. Cancer 2005, 93, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Mardiak, J.; Sálek, T.; Sycová-Milá, Z.; Obertová, J.; Hlavatá, Z.; Mego, M.; Recková, M.; Koza, I. Paclitaxel plus ifosfamide and cisplatin in second-line treatment of germ cell tumors: A phase II study. Neoplasma 2005, 52, 497–501. [Google Scholar] [PubMed]
- Pizzocaro, G.; Salvioni, R.; Piva, L.; Faustini, M.; Nicolai, N.; Gianni, L. Modified cisplatin, etoposide (or vinblastine) and ifosfamide salvage therapy for male germ-cell tumors. Long-term results. Ann. Oncol. 1992, 3, 211–216. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, J.A.; Mazumdar, M.; Bajorin, D.F.; Bosl, G.J.; Vlamis, V.; Motzer, R.J. Ifosfamide- and cisplatin-containing chemotherapy as first-line salvage therapy in germ cell tumors: Response and survival. J. Clin. Oncol. 1997, 15, 2559–2563. [Google Scholar] [CrossRef] [PubMed]
- Loehrer, P.J., Sr.; Gonin, R.; Nichols, C.R.; Weathers, T.; Einhorn, L.H. Vinblastine plus ifosfamide plus cisplatin as initial salvage therapy in recurrent germ cell tumor. J. Clin. Oncol. 1998, 16, 2500–2504. [Google Scholar] [CrossRef]
- Motzer, R.J.; Cooper, K.; Geller, N.L.; Bajorin, D.F.; Dmitrovsky, E.; Herr, H.; Morse, M.; Fair, W.; Sogani, P.; Russo, P.; et al. The role of ifosfamide plus cisplatin-based chemotherapy as salvage therapy for patients with refractory germ cell tumors. Cancer 1990, 66, 2476–2481. [Google Scholar] [CrossRef]
- Loehrer, P.J., Sr.; Einhorn, L.H.; Williams, S.D. VP-16 plus ifosfamide plus cisplatin as salvage therapy in refractory germ cell cancer. J. Clin. Oncol. 1986, 4, 528–536. [Google Scholar] [CrossRef]
- Farhat, F.; Culine, S.; Théodore, C.; Békradda, M.; Terrier-Lacombe, M.J.; Droz, J.P. Cisplatin and ifosfamide with either vinblastine or etoposide as salvage therapy for refractory or relapsing germ cell tumor patients: The Institut Gustave Roussy experience. Cancer 1996, 77, 1193–1197. [Google Scholar] [CrossRef]
- Ferraro, S.; Trevisiol, C.; Gion, M.; Panteghini, M. Human Chorionic Gonadotropin Assays for Testicular Tumors: Closing the Gap between Clinical and Laboratory Practice. Clin. Chem. 2018, 64, 270–278. [Google Scholar] [CrossRef]
- Ferraro, S.; Incarbone, G.P.; Rossi, R.S.; Dolci, A.; Panteghini, M. Human chorionic gonadotropin in oncology: A matter of tight (bio)marking. Clin. Chem. Lab. Med. 2020, 58, 57–60. [Google Scholar] [CrossRef]
- Cary, C.; Pedrosa, J.A.; Jacob, J.; Beck, S.D.; Rice, K.R.; Einhorn, L.H.; Foster, R.S. Outcomes of postchemotherapy retroperitoneal lymph node dissection following high-dose chemotherapy with stem cell transplantation. Cancer 2015, 121, 4369–4375. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Einhorn, L.H. Maintenance chemotherapy with daily oral etoposide following salvage therapy in patients with germ cell tumors. J. Clin. Oncol. 1995, 13, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
- Taza, F.; Abonour, R.; Zaid, M.A.; Althouse, S.K.; Anouti, B.; Akel, R.; Hanna, N.H.; Adra, N.; Einhorn, L.H. Maintenance Oral Etoposide after High-Dose Chemotherapy (HDCT) for Patients with Relapsed Metastatic Germ-Cell Tumors (mGCT). Clin. Genitourin. Cancer 2023, 21, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Ashkar, R.; Adra, N.; Abonour, R.; Zaid, M.I.A.; Snow, C.I.; Hanna, N.H.; Einhorn, L.H. Randomized phase 2 trial of maintenance oral etoposide or observation following high-dose chemotherapy for relapsed metastatic germ cell tumor. J. Clin. Oncol. 2022, 40, TPS429. [Google Scholar] [CrossRef]

| Question | Answers | n = 30 | Total Response | % |
|---|---|---|---|---|
| Specialty | Medical Oncology | 25 | 30 | 83.3 |
| Malignant Hematology | 3 | 10 | ||
| Both | 2 | 6.7 | ||
| Staff | 30 | 100 | ||
| Cancer Center location | Ontario | 16 | 30 | 53.3 |
| Quebec | 6 | 20 | ||
| Alberta | 2 | 6.6 | ||
| BC | 1 | 3.3 | ||
| Manitoba | 2 | 6.6 | ||
| New Brunswick | 1 | 3.3 | ||
| Nova Scotia | 1 | 3.3 | ||
| PEI | 1 | 3.3 | ||
| Number of patients receiving HDCT + ASCT for relapsed GCT at your center | <1 cases/year | 3 | 20 | 15 |
| 1 case/year | 3 | 15 | ||
| 1–5 cases/year | 11 | 55 | ||
| 6–10 cases/year | 3 | 15 |
| Questions | Responses | n | Total Response | % |
|---|---|---|---|---|
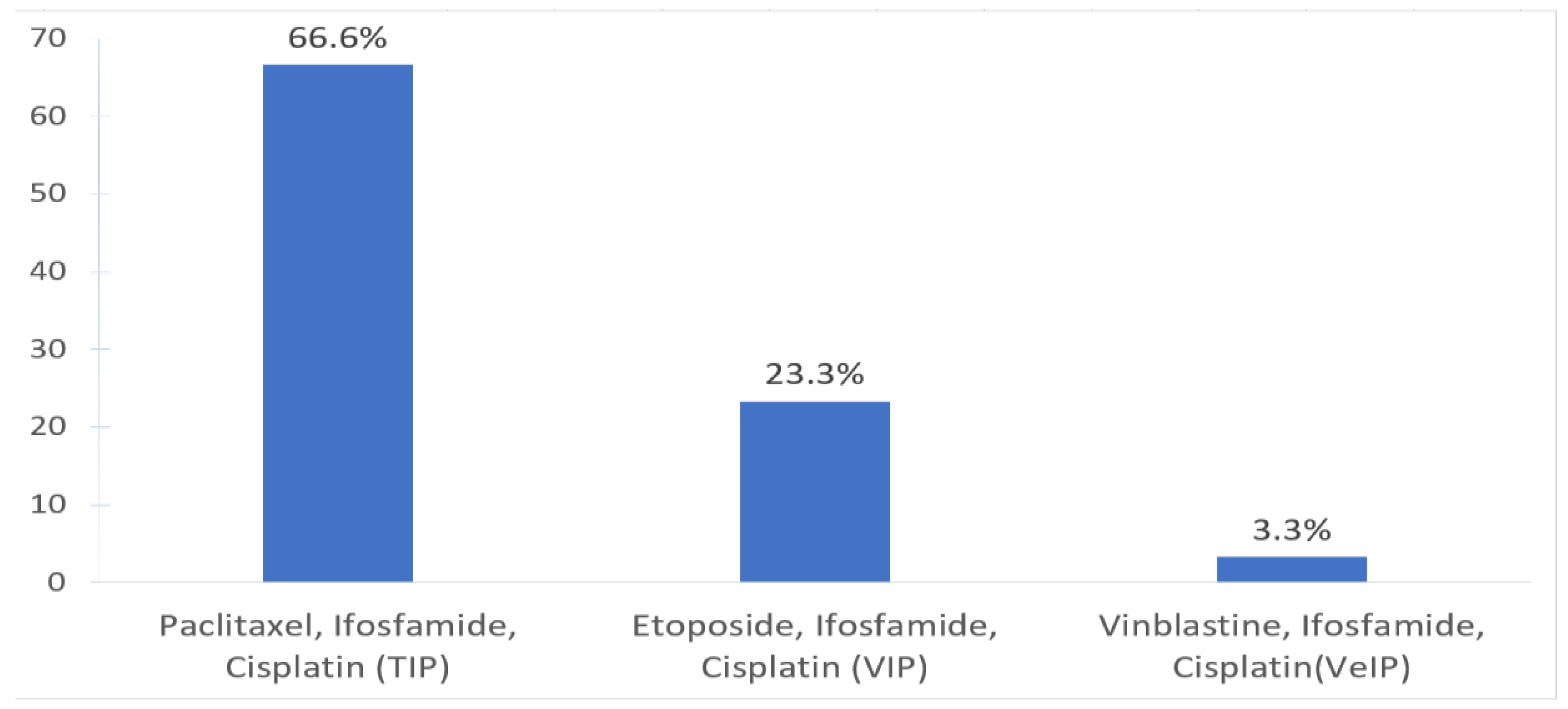
| 1. Which of the following salvage chemotherapy treatments do you oversee at your center? | Use of CDCT only | 14 | 30 | 46.6 |
| Use both CDCT and HDCT | 12 | 40 | ||
| Use of HDCT & ASCT only | 4 | 13.3 | ||
| 2. Number of patients receiving salvage chemotherapy (CDCT, or HDCT) for relapsed GCT at your center | <1 cases/year | 5 | 30 | 16.6 |
| 1 case/year | 7 | 23.3 | ||
| 1–5 cases/year | 12 | 40 | ||
| 6–10 cases/year | 6 | 20 | ||
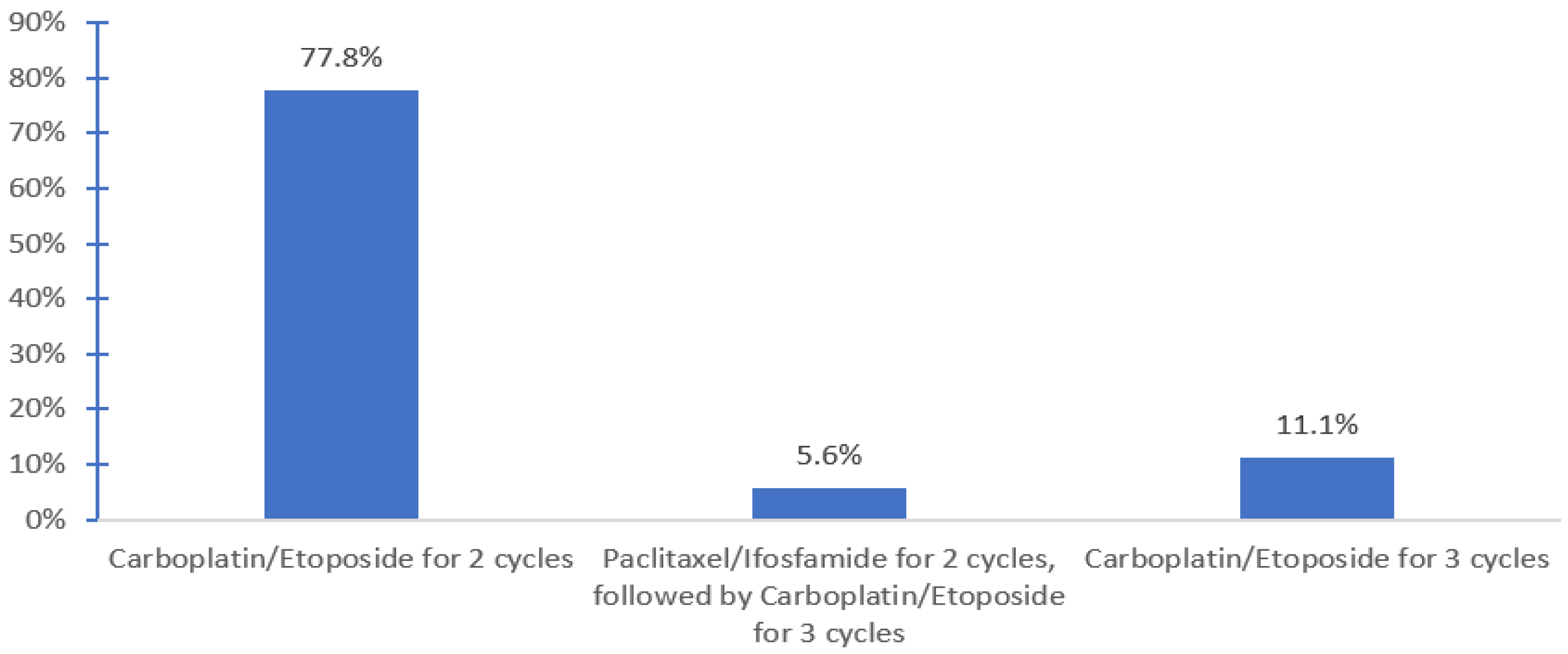
| 3. Percentage of salvage HDCT + ASCT given in the following treatment settings (% reflect averages of responses for each) (range) | First-line setting | 69% (0–100) | ||
| Second-line salvage setting | 33% (0–100) | |||
| Third-line salvage setting or beyond | 4% (0–20) | |||
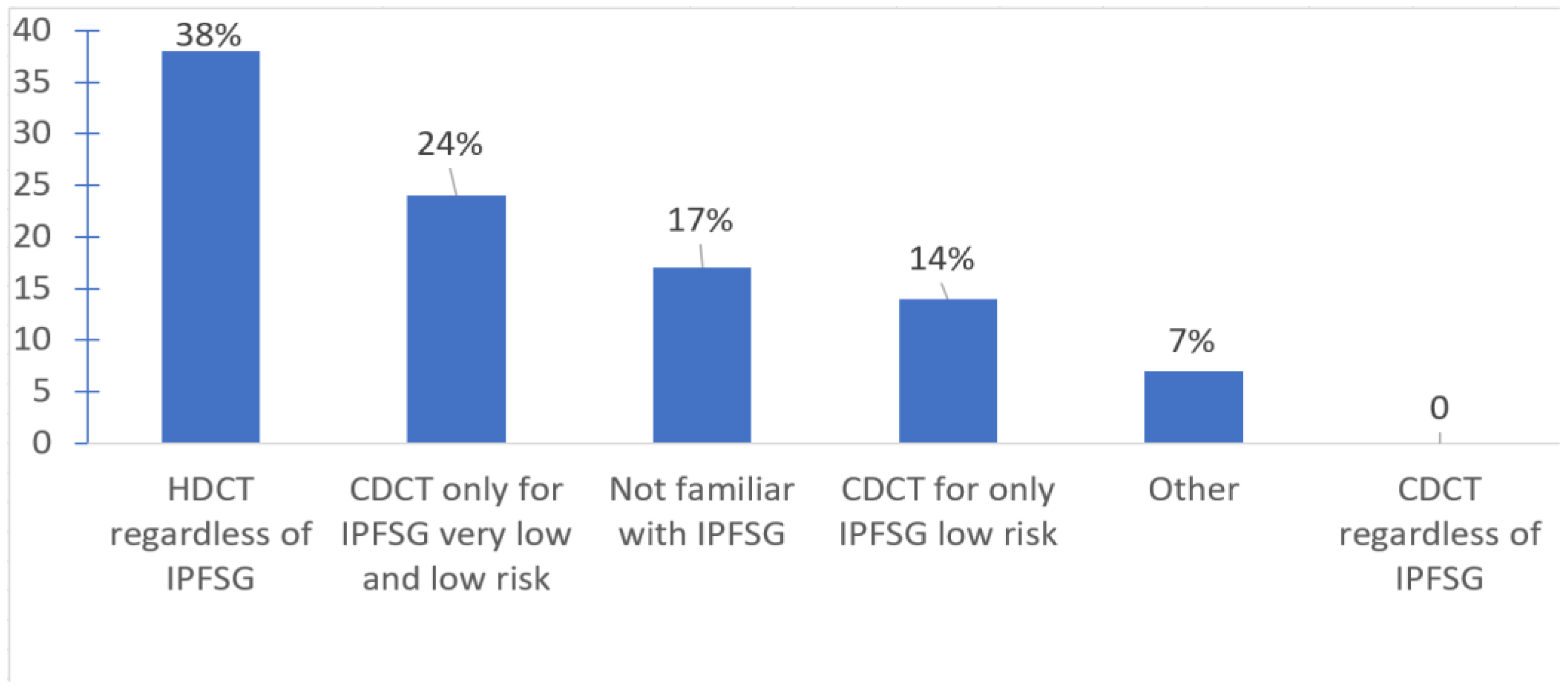
| 4. If salvage HDCT + ASCT is not available at your center, when do you typically refer patients with relapsed germ cell tumors for salvage HDCT + ASCT? | Upon the first relapse after first line of cisplatin | 6 | 8 | 75 |
| Upon further relapse after salvage CDCT | 1 | 12.5 | ||
| I do not usually refer a patient | 1 | 12.5 | ||
| 5. Is “bridging” CDCT given while waiting for HDCT + ASCT? | No. HDCT can be organized in 3 weeks | 5 | 20 | 25 |
| No. HDCT takes 3–6 weeks to organize, but no “bridging” CDCT is used | 2 | 10 | ||
| Yes | 13 | 65 | ||
| 6. Is disease response (biochemical and/or radiographic) to” bridging” CDCT required to proceed with salvage HDCT + ASCT at your center? | Always. Patients receive HDCT + ASCT only if evidence of disease response. | 1 | 13 | 7.7 |
| Never. Patients proceed to HDCT + ASCT regardless | 9 | 69.2 | ||
| Case by case discussion | 3 | 23.1 | ||
| 7. When do you initiate apheresis/collection after completion of “bridging” CDCT? | Within 4 weeks | 9 | 13 | 69.2 |
| Within 4–6 weeks | 1 | 6.9 | ||
| Within 6–8 weeks | 1 | 6.9 | ||
| I do not know | 2 | 15.3 | ||
| 8. Minimum number of collected CD34 cells required for salvage HDCT + ASCT to proceed | CD34+ cell count 2–3 × 106/kg | 6 | 18 | 33.3 |
| CD34+ cell count 3.1–4 × 106/kg | 1 | 5.6 | ||
| CD34+ cell count 4.1–6 × 106/kg | 0 | 0 | ||
| CD34+ cell count >6 × 106/kg | 1 | 5.6 | ||
| I do not know | 10 | 55.5 | ||
| 9. Initiation ASCT after peripheral stem cells collection | Within 2 weeks | 6 | 18 | 33.3 |
| Within 2–4 weeks | 6 | 33.3 | ||
| Within 4–6 weeks | 1 | 5.5 | ||
| I do not know | 5 | 27.7 | ||
| 10. Salvage HDCT + ASCT required planned admission to hospital | Yes | 15 | 18 | 83.3 |
| No | 3 | 16.7 | ||
| 11. Do the tumor markers and CT results post-first-cycle of HDCT + ASCT affect your decision to proceed with subsequent cycle of HDCT? | Yes. If disease progression, subsequent cycle of HDCT is abandoned. | 6 | 16 | 37.5 |
| No. Patient proceeds with subsequent cycle of HDCT regardless. | 6 | 37.5 | ||
| Case-by-case | 4 | 25 | ||
| 12. Surveillance investigations after completion of salvage HDCT + ASCT within the first year. | Tumor markers every 3 months | 16 | 24 | 50 |
| Imaging every 4 months | 16 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ezzi, E.M.; Zahralliyali, A.; Hansen, A.R.; Hamilton, R.J.; Crump, M.; Kuruvilla, J.; Wood, L.; Nappi, L.; Kollmannsberger, C.K.; North, S.A.; et al. The Use of Salvage Chemotherapy for Patients with Relapsed Testicular Germ Cell Tumor (GCT) in Canada: A National Survey. Curr. Oncol. 2023, 30, 6166-6176. https://doi.org/10.3390/curroncol30070458
Al-Ezzi EM, Zahralliyali A, Hansen AR, Hamilton RJ, Crump M, Kuruvilla J, Wood L, Nappi L, Kollmannsberger CK, North SA, et al. The Use of Salvage Chemotherapy for Patients with Relapsed Testicular Germ Cell Tumor (GCT) in Canada: A National Survey. Current Oncology. 2023; 30(7):6166-6176. https://doi.org/10.3390/curroncol30070458
Chicago/Turabian StyleAl-Ezzi, Esmail M., Amer Zahralliyali, Aaron R. Hansen, Robert J. Hamilton, Michael Crump, John Kuruvilla, Lori Wood, Lucia Nappi, Christian K. Kollmannsberger, Scott A. North, and et al. 2023. "The Use of Salvage Chemotherapy for Patients with Relapsed Testicular Germ Cell Tumor (GCT) in Canada: A National Survey" Current Oncology 30, no. 7: 6166-6176. https://doi.org/10.3390/curroncol30070458
APA StyleAl-Ezzi, E. M., Zahralliyali, A., Hansen, A. R., Hamilton, R. J., Crump, M., Kuruvilla, J., Wood, L., Nappi, L., Kollmannsberger, C. K., North, S. A., Winquist, E., Soulières, D., Hotte, S. J., & Jiang, D. M. (2023). The Use of Salvage Chemotherapy for Patients with Relapsed Testicular Germ Cell Tumor (GCT) in Canada: A National Survey. Current Oncology, 30(7), 6166-6176. https://doi.org/10.3390/curroncol30070458





