Tumor-Associated Macrophages in Multiple Myeloma: Key Role in Disease Biology and Potential Therapeutic Implications
Abstract
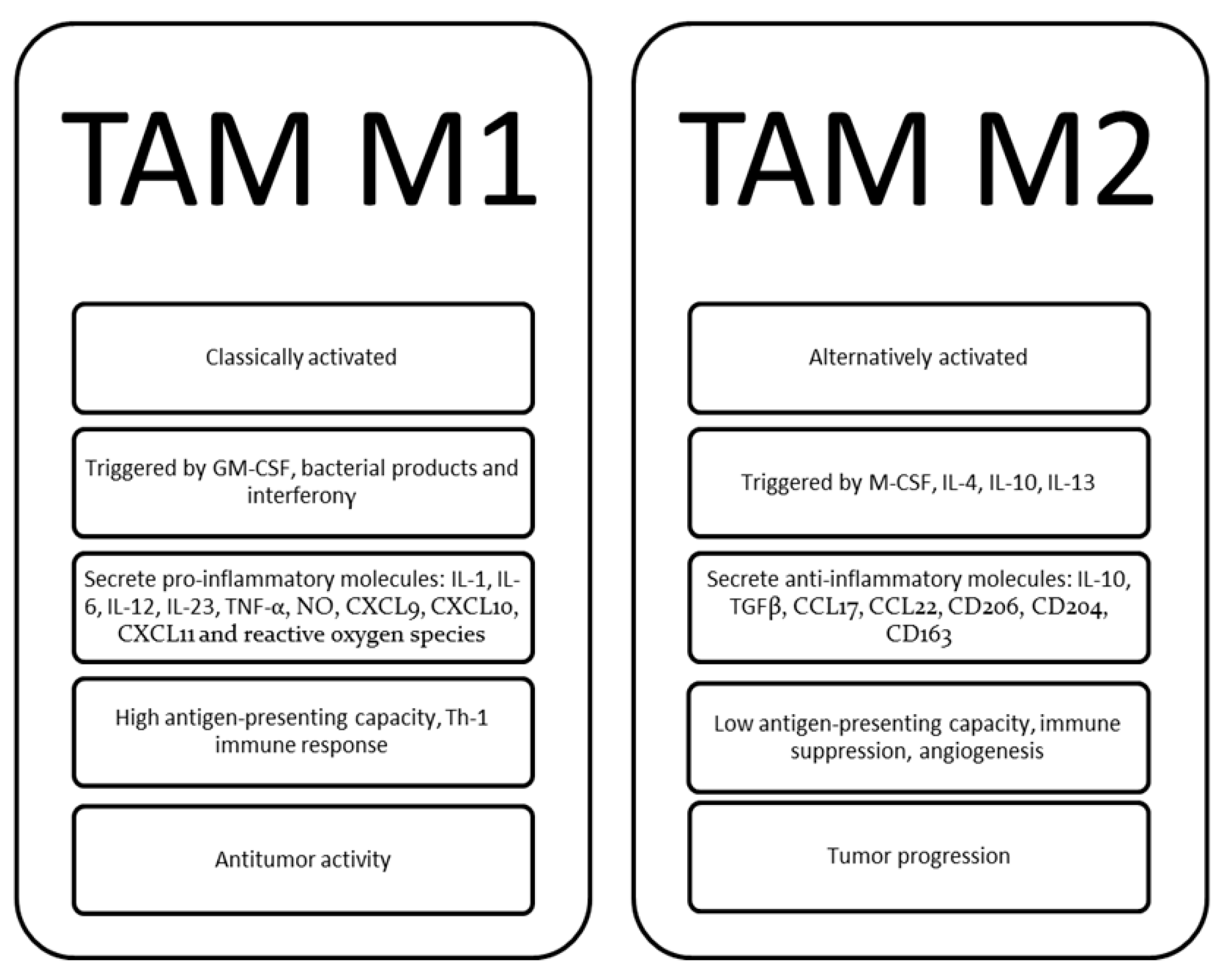
1. Introduction
2. Materials and Methods
3. TAM Role in the Pathophysiology and Progression of MM
3.1. TAM Accumulation
3.1.1. Mouse Model Studies
3.1.2. Human Studies
3.2. TAM and PC Migration, Homing and Proliferation
3.2.1. Mouse Model Studies
3.2.2. Human Studies
3.3. TAM and Angiogenesis
3.3.1. Mouse Model Studies
3.3.2. Human Studies
3.4. TAM and Immunosuppression
3.4.1. Mouse Model Studies
3.4.2. Human Studies
3.5. TAM and Drug Resistance
4. Clinical Studies of TAM in MM
5. TAM as Possible Therapeutic Target for MM
| Reference | Treatment | Mechanism of Action | Type of Study | Results |
|---|---|---|---|---|
| Sun et al. [26] | IL-10R blocking antibody | TAM reprogramming | In vitro, in vivo, ex vivo | Reduced MM cell proliferation. Overcame drug resistance to lenalidomide and dexamethasone |
| De Beule et al. [75] | JAK1/2 inhibitor AZD1480 | Overcome drug resistance | In vitro 5T33MM murine model | MM cells killing, reduced tumor burden, resensitize to bortezomib |
| Beider et al. [85] | Anti-CXCR4 antibody | Reduced TAM recruitment | Human MM cells | Disruption of MM cells-TME interaction, reduced MM cells proliferation |
| Opperman et al. [88] | Clodronate -liposome | TAM depletion | In vivo mouse model | Abrogates MM establishment, reduced tumor burden |
| Zhang et al. [102] | BMI1 inhibitor PTC596 | TAM depletion, antiangiogenic | In vivo mouse model | Reduced tumor burden, improved mice survival |
| Wang et al. [111] | Monoclonal antibody CS7 against CSF-1R | Reduced TAM recruitment and proliferation, TAM reprogramming | MM cells in vitro In vivo mouse models | Dose-dependent cell death, tumor-specific CD4+ T-cell response. Additive efficacy with bortezomib |
| Chen et al. [120] | BAFF inhibitor | Overcome drug resistance | Xenograft model | delayed tumor growth, resensitize to bortezomib |
| Chen et al. [122] | JAK1/2 inhibitor Ruxolitinib | TAM reprogramming | MM cells in vitro In vivo mouse models | Reduced tumor burden, resensitize to lenalidomide |
| Cucè et al. [130] | Trabectedin | Macrophage killing due to CCL2-CCR2 signaling axis inhibition, antiangiogenic | Human MM cells | Apoptosis trigger VEGF depletion NK cells upregulation |
| Vo et al. [133] | Lenalidomide | TAM depletion | In vivo mouse model | Reduced IL10 production, reduced tumor burden |
| Jensen et al. [136] | Agonistic anti-CD40 antibody | TAM reprogramming | In vivo mouse model | Reduced tumor burden, improved mice survival |
| Gutierrez-Gonzalez et al. [137] | GM-CSF and MIF blockade | TAM reprogramming | MM cells, patient samples, xenograft model | Increased cell death, reduced tumor burden in mice |
| Bonanno et al. [143] | IDO inhibitor | Reduced TAM-mediated immunosuppression | Patient cells | Reverted Tregs expansion, improved Th1 response |
| Rastgoo et al. [149] | Synthetic miR-155 | Inhibition of CD47-SIRPα do not eat me signal | MM cell lines and patient samples | MM cells phagocytosys by macrophages, apoptosis induction. Resensitize to bortezomib |
| Veitonmäki et al. [150] | BI-505, antibody against ICAM-1 | Targeting crosstalk TAM mm cells | MM cell lines, xenograft model | MM cell growth inhibition, reduced tumor burden |
5.1. TAM Recruitment
5.1.1. Mouse Model Studies
5.1.2. Human Studies
5.2. TAM Depletion
5.3. TAM Reprogramming
5.3.1. Mouse Model Studies
5.3.2. Human Studies
5.4. Restoration of T-Cell Response and Inhibition of CD47/SIRPα Don’t Eat Me Signal
5.5. Targeting the Cross-Talk between TAM and MM Cells to Overcome Drug Resistance
5.5.1. Mouse Model Studies
5.5.2. Human Studies
6. The Role of TAM in the Era of Novel Agents
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Minakata, D.; Fujiwara, S.I.; Yokoyama, D.; Noguchi, A.; Aoe, S.; Oyama, T.; Koyama, S.; Murahashi, R.; Nakashima, H.; Hyodo, K.; et al. Relapsed and refractory multiple myeloma: A systematic review and network meta-analysis of the efficacy of novel therapies. Br. J. Haematol. 2023, 200, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Kloock, C.; Comenzo, R. Relapsed/Refractory Multiple Myeloma: A Review of Available Therapies and Clinical Scenarios Encountered in Myeloma Relapse. Curr. Oncol. 2023, 30, 2322–2347. [Google Scholar] [CrossRef] [PubMed]
- Gozzetti, A.; Candi, V.; Papini, G.; Bocchia, M. Therapeutic advancements in multiple myeloma. Front. Oncol. 2014, 4, 241. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [CrossRef]
- Gozzetti, A.; Cerase, A.; Lotti, F.; Rossi, D.; Palumbo, A.; Petrucci, M.T.; Patriarca, F.; Nozzoli, C.; Cavo, M.; Offidani, M.; et al. Extramedullary intracranial localizations of multiple myeloma and treatment with novel agents: A retrospective survey of 50 patients. Cancer 2012, 118, 1574–1584. [Google Scholar] [CrossRef]
- Marcon, C.; Simeon, V.; Deias, P.; Facchin, G.; Corso, A.; Derudas, D.; Montefusco, V.; Offidani, M.; Petrucci, M.T.; Zambello, R.; et al. Experts’ consensus on the definition and management of high risk multiple myeloma. Front. Oncol. 2023, 12, 1096852. [Google Scholar] [CrossRef]
- Gozzetti, A.; Le Beau, M.M. Fluorescence in situ hybridization: Uses and limitations. Semin. Hematol. 2000, 37, 320–333. [Google Scholar] [CrossRef]
- Hagen, P.; Zhang, J.; Barton, K. High-risk disease in newly diagnosed multiple myeloma: Beyond the R-ISS and IMWG definitions. Blood Cancer J. 2022, 12, 83. [Google Scholar] [CrossRef]
- Gozzetti, A.; Cerase, A. Novel agents in CNS myeloma treatment. Cent. Nerv. Syst. Agents Med. Chem. 2014, 14, 23–27. [Google Scholar] [CrossRef]
- Kulig, P.; Milczarek, S.; Bakinowska, E.; Szalewska, L.; Baumert, B.; Machaliński, B. Lenalidomide in Multiple Myeloma: Review of Resistance Mechanisms, Current Treatment Strategies and Future Perspectives. Cancers 2023, 15, 963. [Google Scholar] [CrossRef]
- De Novellis, D.; Fontana, R.; Giudice, V.; Serio, B.; Selleri, C. Innovative Anti-CD38 and Anti-BCMA Targeted Therapies in Multiple Myeloma: Mechanisms of Action and Resistance. Int. J. Mol. Sci. 2022, 24, 645. [Google Scholar] [CrossRef]
- Gozzetti, A.; Ciofini, S.; Sicuranza, A.; Pacelli, P.; Raspadori, D.; Cencini, E.; Tocci, D.; Bocchia, M. Drug resistance and minimal residual disease in multiple myeloma. Cancer Drug Resist. 2022, 5, 171–183. [Google Scholar] [CrossRef]
- Leung-Hagesteijn, C.; Erdmann, N.; Cheung, G.; Keats, J.J.; Stewart, A.K.; Reece, D.E.; Chung, K.C.; Tiedemann, R.E. Xbp1s-negative tumor B cells and pre-plasmablasts mediate therapeutic proteasome inhibitor resistance in multiple myeloma. Cancer Cell. 2013, 24, 289–304. [Google Scholar] [CrossRef]
- Abraham, J.; Salama, N.N.; Azab, A.K. The role of P-glycoprotein in drug resistance in multiple myeloma. Leuk. Lymphoma 2015, 56, 26–33. [Google Scholar] [CrossRef]
- Besse, A.; Stolze, S.C.; Rasche, L.; Weinhold, N.; Morgan, G.J.; Kraus, M.; Bader, J.; Overkleeft, H.S.; Besse, L.; Driessen, C. Carfilzomib resistance due to ABCB1/MDR1 overexpression is overcome by nelfinavir and lopinavir in multiple myeloma. Leukemia 2018, 32, 391–401. [Google Scholar] [CrossRef]
- Gooding, S.; Ansari-Pour, N.; Towfic, F.; Ortiz Estévez, M.; Chamberlain, P.P.; Tsai, K.T.; Flynt, E.; Hirst, M.; Rozelle, D.; Dhiman, P.; et al. Multiple cereblon genetic changes are associated with acquired resistance to lenalidomide or pomalidomide in multiple myeloma. Blood 2021, 137, 232–237. [Google Scholar] [CrossRef]
- Chamberlain, P.P.; Lopez-Girona, A.; Miller, K.; Carmel, G.; Pagarigan, B.; Chie-Leon, B.; Rychak, E.; Corral, L.G.; Ren, Y.J.; Wang, M.; et al. Structure of the human Cereblon-DDB1-lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat. Struct. Mol. Biol. 2014, 21, 803–809. [Google Scholar] [CrossRef]
- Haertle, L.; Barrio, S.; Munawar, U.; Han, S.; Zhou, X.; Vogt, C.; Fernández, R.A.; Bittrich, M.; Ruiz-Heredia, Y.; Da Viá, M.; et al. Cereblon enhancer methylation and IMiD resistance in multiple myeloma. Blood 2021, 138, 1721–1726. [Google Scholar] [CrossRef]
- Kortüm, K.M.; Mai, E.K.; Hanafiah, N.H.; Shi, C.X.; Zhu, Y.X.; Bruins, L.; Barrio, S.; Jedlowski, P.; Merz, M.; Xu, J.; et al. Targeted sequencing of refractory myeloma reveals a high incidence of mutations in CRBN and Ras pathway genes. Blood 2016, 128, 1226–1233. [Google Scholar] [CrossRef]
- Gooding, S.; Ansari-Pour, N.; Kazeroun, M.; Karagoz, K.; Polonskaia, A.; Salazar, M.; Fitzsimons, E.; Sirinukunwattana, K.; Chavda, S.; Ortiz Estevez, M.; et al. Loss of COP9 signalosome genes at 2q37 is associated with IMiD resistance in multiple myeloma. Blood 2022, 140, 1816–1821. [Google Scholar] [CrossRef]
- Giannotta, C.; Autino, F.; Massaia, M. The immune suppressive tumor microenvironment in multiple myeloma: The contribution of myeloid-derived suppressor cells. Front Immunol. 2023, 13, 1102471. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.Y.; Chng, W.J.; Liu, H.; de Mel, S. Tumor-Associated Macrophages and Related Myelomonocytic Cells in the Tumor Microenvironment of Multiple Myeloma. Cancers 2022, 14, 5654. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, Y.; Hamidi, H.; Dos Santos, C.; Zhang, J.; Punnoose, E.; Li, W. Immune microenvironment characteristics in multiple myeloma progression from transcriptome profiling. Front. Oncol. 2022, 12, 948548. [Google Scholar] [CrossRef] [PubMed]
- Hervás-Salcedo, R.; Martín-Antonio, B. A Journey through the Inter-Cellular Interactions in the Bone Marrow in Multiple Myeloma: Implications for the Next Generation of Treatments. Cancers 2022, 14, 3796. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, N.; Perumal, D.; Rahman, A.; Kim-Schulze, S.; Yesil, J.; Auclair, D.; Adams, H., 3rd; Parekh, S.; Gnjatic, S.; Cho, H.J. High Dimensional Immune Profiling of Smoldering Multiple Myeloma Distinguishes Distinct Tumor Microenvironments. Clin Lymphoma Myeloma Leuk. 2022, 22, 853–862. [Google Scholar] [CrossRef]
- Sun, J.; Park, C.; Guenthner, N.; Gurley, S.; Zhang, L.; Lubben, B.; Adebayo, O.; Bash, H.; Chen, Y.; Maksimos, M.; et al. Tumor-associated macrophages in multiple myeloma: Advances in biology and therapy. J. Immunother. Cancer 2022, 10, e003975. [Google Scholar] [CrossRef]
- Opperman, K.S.; Vandyke, K.; Psaltis, P.J.; Noll, J.E.; Zannettino, A.C.W. Macrophages in multiple myeloma: Key roles and therapeutic strategies. Cancer Metastasis Rev. 2021, 40, 273–284. [Google Scholar] [CrossRef]
- Matula, Z.; Mikala, G.; Lukácsi, S.; Matkó, J.; Kovács, T.; Monostori, É.; Uher, F.; Vályi-Nagy, I. Stromal Cells Serve Drug Resistance for Multiple Myeloma via Mitochondrial Transfer: A Study on Primary Myeloma and Stromal Cells. Cancers 2021, 13, 3461. [Google Scholar] [CrossRef]
- Moschetta, M.; Mishima, Y.; Kawano, Y.; Manier, S.; Paiva, B.; Palomera, L.; Aljawai, Y.; Calcinotto, A.; Unitt, C.; Sahin, I.; et al. Targeting vasculogenesis to prevent progression in multiple myeloma. Leukemia 2016, 30, 1103–1115. [Google Scholar] [CrossRef]
- Ribatti, D.; Basile, A.; Ruggieri, S.; Vacca, A. Bone marrow vascular niche and the control of angiogenesis in multiple myeloma. Front. Biosci. (Landmark Ed) 2014, 19, 304–311. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Cencini, E.; Fabbri, A.; Sicuranza, A.; Gozzetti, A.; Bocchia, M. The Role of Tumor-Associated Macrophages in Hematologic Malignancies. Cancers 2021, 13, 3597. [Google Scholar] [CrossRef]
- DiPietro, L.A.; Wilgus, T.A.; Koh, T.J. Macrophages in Healing Wounds: Paradoxes and Paradigms. Int. J. Mol. Sci. 2021, 22, 950. [Google Scholar] [CrossRef]
- Blériot, C.; Chakarov, S.; Ginhoux, F. Determinants of Resident Tissue Macrophage Identity and Function. Immunity 2020, 52, 957–970. [Google Scholar] [CrossRef]
- Guerriero, J.L. Macrophages: The Road Less Traveled, Changing Anticancer Therapy. Trends Mol. Med. 2018, 24, 472–489. [Google Scholar] [CrossRef]
- Sica, A.; Schioppa, T.; Mantovani, A.; Allavena, P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: Potential targets of anti-cancer therapy. Eur. J. Cancer 2006, 42, 717–727. [Google Scholar] [CrossRef]
- Mantovani, A.; Schioppa, T.; Porta, C.; Allavena, P.; Sica, A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006, 25, 315–322. [Google Scholar] [CrossRef]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- Unver, N. Macrophage chemoattractants secreted by cancer cells: Sculptors of the tumor microenvironment and another crucial piece of the cancer secretome as a therapeutic target. Cytokine Growth Factor Rev. 2019, 50, 13–18. [Google Scholar] [CrossRef]
- Locati, M.; Mantovani, A.; Sica, A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv. Immunol. 2013, 120, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Song, J.X.; Wen, Y.; Li, R.W.; Dong, T.; Tang, Y.F.; Zhang, J.J.; Sa, Y.L. Phenotypic characterization of macrophages in the BMB sample of human acute leukemia. Ann. Hematol. 2020, 99, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Valencia, J.M.; Fernández-Sevilla, L.; Fraile-Ramos, A.; Sacedón, R.; Jiménez, E.; Vicente, A.; Varas, A. Acute Lymphoblastic Leukaemia Cells Impair Dendritic Cell and Macrophage Differentiation: Role of BMP4. Cells 2019, 8, 722. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.J.; Gu, Y.; Wang, C.Z.; Jin, Y.; Wen, X.M.; Ma, J.C.; Tang, L.J.; Mao, Z.W.; Qian, J.; Lin, J. The M2 macrophage marker CD206: A novel prognostic indicator for acute myeloid leukemia. Oncoimmunology 2019, 9, 1683347. [Google Scholar] [CrossRef]
- Hanna, B.S.; McClanahan, F.; Yazdanparast, H.; Zaborsky, N.; Kalter, V.; Rößner, P.M.; Benner, A.; Dürr, C.; Egle, A.; Gribben, J.G.; et al. Depletion of CLL-associated patrolling monocytes and macrophages controls disease development and repairs immune dysfunction in vivo. Leukemia 2016, 30, 570–579. [Google Scholar] [CrossRef]
- Audrito, V.; Serra, S.; Brusa, D.; Mazzola, F.; Arruga, F.; Vaisitti, T.; Coscia, M.; Maffei, R.; Rossi, D.; Wang, T.; et al. Extracellular nicotinamide phosphoribosyltransferase (NAMPT) promotes M2 macrophage polarization in chronic lymphocytic leukemia. Blood 2015, 125, 111–123. [Google Scholar] [CrossRef]
- Fiorcari, S.; Maffei, R.; Atene, C.G.; Potenza, L.; Luppi, M.; Marasca, R. Nurse-Like Cells and Chronic Lymphocytic Leukemia B Cells: A Mutualistic Crosstalk inside Tissue Microenvironments. Cells 2021, 10, 217. [Google Scholar] [CrossRef]
- García-Ortiz, A.; Rodríguez-García, Y.; Encinas, J.; Maroto-Martín, E.; Castellano, E.; Teixidó, J.; Martínez-López, J. The Role of Tumor Microenvironment in Multiple Myeloma Development and Progression. Cancers 2021, 13, 217. [Google Scholar] [CrossRef]
- Steidl, C.; Lee, T.; Shah, S.P.; Farinha, P.; Han, G.; Nayar, T.; Delaney, A.; Jones, S.J.; Iqbal, J.; Weisenburger, D.D.; et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N. Engl. J. Med. 2010, 362, 875–885. [Google Scholar] [CrossRef]
- Azambuja, D.; Natkunam, Y.; Biasoli, I.; Lossos, I.S.; Anderson, M.W.; Morais, J.C.; Spector, N. Lack of association of tumor-associated macrophages with clinical outcome in patients with classical Hodgkin’s lymphoma. Ann. Oncol. 2012, 23, 736–742. [Google Scholar] [CrossRef]
- Cencini, E.; Fabbri, A.; Rigacci, L.; Lazzi, S.; Gini, G.; Cox, M.C.; Mancuso, S.; Abruzzese, E.; Kovalchuk, S.; Goteri, G.; et al. Evaluation of the prognostic role of tumour-associated macrophages in newly diagnosed classical Hodgkin lymphoma and correlation with early FDG-PET assessment. Hematol. Oncol. 2017, 35, 69–78. [Google Scholar] [CrossRef]
- Gomez-Gelvez, J.C.; Salama, M.E.; Perkins, S.L.; Leavitt, M.; Inamdar, K.V. Prognostic Impact of Tumor Microenvironment in Diffuse Large B-Cell Lymphoma Uniformly Treated With R-CHOP Chemotherapy. Am. J. Clin. Pathol. 2016, 145, 514–523. [Google Scholar] [CrossRef]
- Wang, J.; Gao, K.; Lei, W.; Dong, L.; Xuan, Q.; Feng, M.; Wang, J.; Ye, X.; Jin, T.; Zhang, Z.; et al. Lymphocyte-to-monocyte ratio is associated with prognosis of diffuse large B-cell lymphoma: Correlation with CD163 positive M2 type tumor-associated macrophages, not PD-1 positive tumor-infiltrating lymphocytes. Oncotarget 2017, 8, 5414–5425. [Google Scholar] [CrossRef]
- Cencini, E.; Fabbri, A.; Schiattone, L.; Sicuranza, A.; Mecacci, B.; Granai, M.; Mancini, V.; Lazzi, S.; Bocchia, M.; Leoncini, L. Prognostic impact of tumor-associated macrophages, lymphocyte-to-monocyte and neutrophil-to-lymphocyte ratio in diffuse large B-cell lymphoma. Am. J. Blood Res. 2020, 10, 97–108. [Google Scholar] [CrossRef]
- Xu, X.; Li, Z.; Liu, J.; Zhu, F.; Wang, Z.; Wang, J.; Zhang, J.; Wang, H.; Zhai, Z. The prognostic value of tumour-associated macrophages in Non-Hodgkin’s lymphoma: A systematic review and meta-analysis. Scand. J. Immunol. 2020, 91, e12814. [Google Scholar] [CrossRef]
- Cencini, E.; Fabbri, A.; Bocchia, M. Prognostic role of M2 tumour-associated macrophages in lymphoproliferative disorders. J. Pathol. 2017, 242, 511–512. [Google Scholar] [CrossRef]
- Kridel, R.; Xerri, L.; Gelas-Dore, B.; Tan, K.; Feugier, P.; Vawda, A.; Canioni, D.; Farinha, P.; Boussetta, S.; Moccia, A.A.; et al. The Prognostic Impact of CD163-Positive Macrophages in Follicular Lymphoma: A Study from the BC Cancer Agency and the Lymphoma Study Association. Clin. Cancer Res. 2015, 21, 3428–3435. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Mills, C.D. Anatomy of a discovery: m1 and m2 macrophages. Front. Immunol. 2015, 6, 212. [Google Scholar] [CrossRef]
- Ricketts, T.D.; Prieto-Dominguez, N.; Gowda, P.S.; Ubil, E. Mechanisms of Macrophage Plasticity in the Tumor Environment: Manipulating Activation State to Improve Outcomes. Front. Immunol. 2021, 12, 642285. [Google Scholar] [CrossRef]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Qian, B.Z.; Soong, D.; Cassetta, L.; Noy, R.; Sugano, G.; Kato, Y.; Li, J.; Pollard, J.W. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 2015, 212, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, X.; Tsai, Y.; Yang, L.; Chuang, K.H.; Keng, P.C.; Lee, S.O.; Chen, Y. IL-6 Mediates Macrophage Infiltration after Irradiation via Up-regulation of CCL2/CCL5 in Non-small Cell Lung Cancer. Radiat. Res. 2017, 187, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Imamichi, T.; Weiss, J.M.; Sato, M.; Li, L.; Matsukawa, A.; Wang, J.M. Induction of Monocyte Chemoattractant Proteins in Macrophages via the Production of Granulocyte/Macrophage Colony-Stimulating Factor by Breast Cancer Cells. Front. Immunol. 2016, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.A.; Yang, B.; Tang, T.; Yang, Y.; Zhang, D.; Xiao, H.; Xu, J.; Wang, L.; Lin, L.; Jiang, J. Correlation of APE1 with VEGFA and CD163+ macrophage infiltration in bladder cancer and their prognostic significance. Oncol. Lett. 2020, 20, 2881–2887. [Google Scholar] [CrossRef]
- Tian, Y.; Matsui, S.; Touma, M.; Wu, Q.; Sugimoto, K. MicroRNA-342 inhibits tumor growth via targeting chemokine CXCL12 involved in macrophages recruitment/activation. Genes Cells 2018, 23, 1009–1022. [Google Scholar] [CrossRef]
- Gong, D.; Shi, W.; Yi, S.J.; Chen, H.; Groffen, J.; Heisterkamp, N. TGFbeta signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 2012, 13, 31. [Google Scholar] [CrossRef]
- Tan, I.L.; Arifa, R.D.N.; Rallapalli, H.; Kana, V.; Lao, Z.; Sanghrajka, R.M.; Sumru Bayin, N.; Tanne, A.; Wojcinski, A.; Korshunov, A.; et al. CSF1R inhibition depletes tumor-associated macrophages and attenuates tumor progression in a mouse sonic Hedgehog-Medulloblastoma model. Oncogene 2021, 40, 396–407. [Google Scholar] [CrossRef]
- Solinas, G.; Schiarea, S.; Liguori, M.; Fabbri, M.; Pesce, S.; Zammataro, L.; Pasqualini, F.; Nebuloni, M.; Chiabrando, C.; Mantovani, A.; et al. Tumor-conditioned macrophages secrete migration-stimulating factor: A new marker for M2-polarization, influencing tumor cell motility. J. Immunol. 2010, 185, 642–652. [Google Scholar] [CrossRef]
- Colegio, O.R.; Chu, N.Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef]
- Maller, O.; Drain, A.P.; Barrett, A.S.; Borgquist, S.; Ruffell, B.; Zakharevich, I.; Pham, T.T.; Gruosso, T.; Kuasne, H.; Lakins, J.N.; et al. Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat. Mater. 2021, 20, 548–559. [Google Scholar] [CrossRef]
- Oronsky, B.; Carter, C.; Reid, T.; Brinkhaus, F.; Knox, S.J. Just eat it: A review of CD47 and SIRP-α antagonism. Semin. Oncol. 2020, 47, 117–124. [Google Scholar] [CrossRef]
- Eladl, E.; Tremblay-LeMay, R.; Rastgoo, N.; Musani, R.; Chen, W.; Liu, A.; Chang, H. Role of CD47 in Hematological Malignancies. J. Hematol. Oncol. 2020, 13, 96. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, H.; Yu, J.; Tian, W.; Song, Y. Targeting CD47 for cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 180. [Google Scholar] [CrossRef]
- De Beule, N.; De Veirman, K.; Maes, K.; De Bruyne, E.; Menu, E.; Breckpot, K.; De Raeve, H.; Van Rampelbergh, R.; Van Ginderachter, J.A.; Schots, R.; et al. Tumour-associated macrophage-mediated survival of myeloma cells through STAT3 activation. J. Pathol. 2017, 241, 534–546. [Google Scholar] [CrossRef]
- Calcinotto, A.; Ponzoni, M.; Ria, R.; Grioni, M.; Cattaneo, E.; Villa, I.; Sabrina Bertilaccio, M.T.; Chesi, M.; Rubinacci, A.; Tonon, G.; et al. Modifications of the mouse bone marrow microenvironment favor angiogenesis and correlate with disease progression from asymptomatic to symptomatic multiple myeloma. Oncoimmunology 2015, 4, e1008850. [Google Scholar] [CrossRef]
- Beider, K.; Voevoda-Dimenshtein, V.; Zoabi, A.; Rosenberg, E.; Magen, H.; Ostrovsky, O.; Shimoni, A.; Weiss, L.; Abraham, M.; Peled, A.; et al. CXCL13 chemokine is a novel player in multiple myeloma osteolytic microenvironment, M2 macrophage polarization, and tumor progression. J. Hematol. Oncol. 2022, 15, 144. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Li, T.; Wang, Q.; Qian, J.; Lu, Y.; Zhang, M.; Bi, E.; Yang, M.; Reu, F.; et al. Chemokines CCL2, 3, 14 stimulate macrophage bone marrow homing, proliferation, and polarization in multiple myeloma. Oncotarget 2015, 6, 24218–24229. [Google Scholar] [CrossRef]
- Ribatti, D.; Moschetta, M.; Vacca, A. Microenvironment and multiple myeloma spread. Thromb. Res. 2014, 133, S102–S106. [Google Scholar] [CrossRef]
- Vacca, A.; Ribatti, D. Angiogenesis and vasculogenesis in multiple myeloma: Role of inflammatory cells. Recent Results Cancer Res. 2011, 183, 87–95. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Zhang, W.; Sun, R.; Liu, T.; Zheng, Y.; Wu, Y. Prognostic value of diametrically polarized tumor-associated macrophages in multiple myeloma. Oncotarget 2017, 8, 112685–112696. [Google Scholar] [CrossRef] [PubMed]
- Desantis, V.; Savino, F.D.; Scaringella, A.; Potenza, M.A.; Nacci, C.; Frassanito, M.A.; Vacca, A.; Montagnani, M. The Leading Role of the Immune Microenvironment in Multiple Myeloma: A New Target with a Great Prognostic and Clinical Value. J. Clin. Med. 2022, 11, 2513. [Google Scholar] [CrossRef] [PubMed]
- Scavelli, C.; Nico, B.; Cirulli, T.; Ria, R.; Di Pietro, G.; Mangieri, D.; Bacigalupo, A.; Mangialardi, G.; Coluccia, A.M.; Caravita, T.; et al. Vasculogenic mimicry by bone marrow macrophages in patients with multiple myeloma. Oncogene 2008, 27, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Panchabhai, S.; Kelemen, K.; Ahmann, G.; Sebastian, S.; Mantei, J.; Fonseca, R. Tumor-associated macrophages and extracellular matrix metalloproteinase inducer in prognosis of multiple myeloma. Leukemia 2016, 30, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Beider, K.; Bitner, H.; Leiba, M.; Gutwein, O.; Koren-Michowitz, M.; Ostrovsky, O.; Abraham, M.; Wald, H.; Galun, E.; Peled, A.; et al. Multiple myeloma cells recruit tumor-supportive macrophages through the CXCR4/CXCL12 axis and promote their polarization toward the M2 phenotype. Oncotarget 2014, 5, 11283–11296. [Google Scholar] [CrossRef] [PubMed]
- Canovas Nunes, S.; Manzoni, M.; Pizzi, M.; Mandato, E.; Carrino, M.; Quotti Tubi, L.; Zambello, R.; Adami, F.; Visentin, A.; Barilà, G.; et al. The small GTPase RhoU lays downstream of JAK/STAT signaling and mediates cell migration in multiple myeloma. Blood Cancer J. 2018, 8, 20. [Google Scholar] [CrossRef]
- Gao, R.; Miao, X.; Sun, C.; Su, S.; Zhu, Y.; Qian, D.; Ouyang, Z.; Duan, J. Frankincense and myrrh and their bioactive compounds ameliorate the multiple myeloma through regulation of metabolome profiling and JAK/STAT signaling pathway based on U266 cells. BMC Complement Med. Ther. 2020, 20, 96. [Google Scholar] [CrossRef]
- Opperman, K.S.; Vandyke, K.; Clark, K.C.; Coulter, E.A.; Hewett, D.R.; Mrozik, K.M.; Schwarz, N.; Evdokiou, A.; Croucher, P.I.; Psaltis, P.J.; et al. Clodronate-liposome mediated macrophage depletion abrogates multiple myeloma tumor establishment in vivo. Neoplasia 2019, 21, 777–787. [Google Scholar] [CrossRef]
- Ribatti, D.; Nico, B.; Vacca, A. Importance of the bone marrow microenvironment in inducing the angiogenic response in multiple myeloma. Oncogene 2006, 25, 4257–4266. [Google Scholar] [CrossRef]
- Aggarwal, R.; Ghobrial, I.M.; Roodman, G.D. Chemokines in multiple myeloma. Exp. Hematol. 2006, 34, 1289–1295. [Google Scholar] [CrossRef]
- Lentzsch, S.; Gries, M.; Janz, M.; Bargou, R.; Dorken, B.; Mapara, M.Y. Macrophage inflammatory protein 1-alpha (MIP-1 alpha) triggers migration and signaling cascades mediating survival and proliferation in multiple myeloma (MM) cells. Blood 2003, 101, 3568–3573. [Google Scholar] [CrossRef]
- Tai, Y.T.; Podar, K.; Catley, L.; Tseng, Y.H.; Akiyama, M.; Shringarpure, R.; Burger, R.; Hideshima, T.; Chauhan, D.; Mitsiades, N.; et al. Insulin-like growth factor-1 induces adhesion and migration in human multiple myeloma cells via activation of beta1-integrin and phosphatidylinositol3-kinase/AKT signaling. Cancer Res. 2003, 63, 5850–5858. [Google Scholar]
- Vande Broek, I.; Asosingh, K.; Vanderkerken, K.; Straetmans, N.; Van Camp, B.; Van Riet, I. Chemokine receptor CCR2 is expressed by human multiple myeloma cells and mediates migration to bone marrow stromal cell-produced monocyte chemotactic proteins MCP-1, -2 and -3. Br. J. Cancer 2003, 88, 855–862. [Google Scholar] [CrossRef]
- Kim, J.; Denu, R.A.; Dollar, B.A.; Escalante, L.E.; Kuether, J.P.; Callander, N.S.; Asimakopoulos, F.; Hematti, P. Macrophages and mesenchymal stromal cells support survival and proliferation of multiple myeloma cells. Br. J. Haematol. 2012, 158, 336–346. [Google Scholar] [CrossRef]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef]
- Matthes, T.; Manfroi, B.; Zeller, A.; Dunand-Sauthier, I.; Bogen, B.; Huard, B. Autocrine amplification of immature myeloid cells by IL-6 in multiple myeloma-infiltrated bone marrow. Leukemia 2015, 29, 1882–1890. [Google Scholar] [CrossRef]
- Kovacs, E. Interleukin-6 leads to interleukin-10 production in several human multiple myeloma cell lines. Does interleukin-10 enhance the proliferation of these cells? Leuk. Res. 2010, 34, 912–916. [Google Scholar] [CrossRef]
- Shi, Y.; Frost, P.; Hoang, B.; Benavides, A.; Gera, J.; Lichtenstein, A. IL-6-induced enhancement of c-Myc translation in multiple myeloma cells: Critical role of cytoplasmic localization of the rna-binding protein hnRNP A1. J. Biol. Chem. 2011, 286, 67–78. [Google Scholar] [CrossRef]
- Jasrotia, S.; Gupta, R.; Sharma, A.; Halder, A.; Kumar, L. Cytokine profile in multiple myeloma. Cytokine 2020, 136, 155271. [Google Scholar] [CrossRef]
- Mauer, J.; Chaurasia, B.; Goldau, J.; Vogt, M.C.; Ruud, J.; Nguyen, K.D.; Theurich, S.; Hausen, A.C.; Schmitz, J.; Brönneke, H.S.; et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat. Immunol. 2014, 15, 423–430. [Google Scholar] [CrossRef]
- Fuster, J.J.; Walsh, K. The good, the bad, and the ugly of interleukin-6 signaling. EMBO J. 2014, 33, 1425–1427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Huang, J.; Wang, F.; Ding, H.; Cui, Y.; Yang, Y.; Xu, J.; Luo, H.; Gao, Y.; Pan, L.; et al. BMI1 regulates multiple myeloma-associated macrophage’s pro-myeloma functions. Cell Death Dis. 2021, 12, 495. [Google Scholar] [CrossRef] [PubMed]
- Harney, A.S.; Arwert, E.N.; Entenberg, D.; Wang, Y.; Guo, P.; Qian, B.Z.; Oktay, M.H.; Pollard, J.W.; Jones, J.G.; Condeelis, J.S. Real-Time Imaging Reveals Local, Transient Vascular Permeability, and Tumor Cell Intravasation Stimulated by TIE2hi Macrophage-Derived VEGFA. Cancer Discov. 2015, 5, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Qiu, S.; Xiao, Q.; Wang, T.; Tian, X.; Chen, C.; Wang, X.; Han, J.; Zheng, H.; Shou, Y.; et al. Synergistic effects of multiple myeloma cells and tumor-associated macrophages on vascular endothelial cells in vitro. Med. Oncol. 2020, 37, 99. [Google Scholar] [CrossRef]
- Ria, R.; Melaccio, A.; Racanelli, V.; Vacca, A. Anti-VEGF Drugs in the Treatment of Multiple Myeloma Patients. J. Clin. Med. 2020, 9, 1765. [Google Scholar] [CrossRef]
- De Luisi, A.; Binetti, L.; Ria, R.; Ruggieri, S.; Berardi, S.; Catacchio, I.; Racanelli, V.; Pavone, V.; Rossini, B.; Vacca, A.; et al. Erythropoietin is involved in the angiogenic potential of bone marrow macrophages in multiple myeloma. Angiogenesis 2013, 16, 963–973. [Google Scholar] [CrossRef]
- Chen, H.; Campbell, R.A.; Chang, Y.; Li, M.; Wang, C.S.; Li, J.; Sanchez, E.; Share, M.; Steinberg, J.; Berenson, A.; et al. Pleiotrophin produced by multiple myeloma induces transdifferentiation of monocytes into vascular endothelial cells: A novel mechanism of tumor-induced vasculogenesis. Blood 2009, 113, 1992–2002. [Google Scholar] [CrossRef]
- Alexandrakis, M.G.; Goulidaki, N.; Pappa, C.A.; Boula, A.; Psarakis, F.; Neonakis, I.; Tsirakis, G. Interleukin-10 Induces Both Plasma Cell Proliferation and Angiogenesis in Multiple Myeloma. Pathol. Oncol. Res. 2015, 21, 929–934. [Google Scholar] [CrossRef]
- Tian, X.; Sun, M.; Wu, H.; Chen, C.; Li, H.; Qiu, S.; Wang, T.; Han, J.; Xiao, Q.; Chen, K. Exosome-derived miR-let-7c promotes angiogenesis in multiple myeloma by polarizing M2 macrophages in the bone marrow microenvironment. Leuk. Res. 2021, 105, 106566. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Cao, P.; Wang, H.; Liu, H.; Hua, L.; Xue, H.; Fu, R. Tumor-associated macrophages regulate the function of cytotoxic T lymphocyte through PD-1/PD-L1 pathway in multiple myeloma. Cancer Med. 2022, 11, 4838–4848. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, Y.; Li, R.; Jiang, Y.; Zheng, Y.; Qian, J.; Bi, E.; Zheng, C.; Hou, J.; Wang, S.; et al. Therapeutic effects of CSF1R-blocking antibodies in multiple myeloma. Leukemia 2018, 32, 176–183. [Google Scholar] [CrossRef]
- Zavidij, O.; Haradhvala, N.J.; Mouhieddine, T.H.; Sklavenitis-Pistofidis, R.; Cai, S.; Reidy, M.; Rahmat, M.; Flaifel, A.; Ferland, B.; Su, N.K.; et al. Single-cell RNA sequencing reveals compromised immune microenvironment in precursor stages of multiple myeloma. Nat. Cancer 2020, 1, 493–506. [Google Scholar] [CrossRef]
- Sun, J.; Muz, B.; Alhallak, K.; Markovic, M.; Gurley, S.; Wang, Z.; Guenthner, N.; Wasden, K.; Fiala, M.; King, J.; et al. Targeting CD47 as a Novel Immunotherapy for Multiple Myeloma. Cancers 2020, 12, 305. [Google Scholar] [CrossRef]
- Kim, D.; Wang, J.; Willingham, S.B.; Martin, R.; Wernig, G.; Weissman, I.L. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia 2012, 26, 2538–2545. [Google Scholar] [CrossRef]
- Yan, H.; Dong, M.; Liu, X.; Shen, Q.; He, D.; Huang, X.; Zhang, E.; Lin, X.; Chen, Q.; Guo, X.; et al. Multiple myeloma cell-derived IL-32γ increases the immunosuppressive function of macrophages by promoting indoleamine 2,3-dioxygenase (IDO) expression. Cancer Lett. 2019, 446, 38–48. [Google Scholar] [CrossRef]
- Palumbo, A.; Falco, P.; Falcone, A.; Benevolo, G.; Canepa, L.; Gay, F.; Larocca, A.; Magarotto, V.; Gozzetti, A.; Luraschi, A.; et al. Melphalan, prednisone, and lenalidomide for newly diagnosed myeloma: Kinetics of neutropenia and thrombocytopenia and time-to-event results. Clin. Lymphoma Myeloma 2009, 9, 145–150. [Google Scholar] [CrossRef]
- Zheng, Y.; Cai, Z.; Wang, S.; Zhang, X.; Qian, J.; Hong, S.; Li, H.; Wang, M.; Yang, J.; Yi, Q. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood 2009, 114, 3625–3628. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, J.; Qian, J.; Qiu, P.; Hanabuchi, S.; Lu, Y.; Wang, Z.; Liu, Z.; Li, H.; He, J.; et al. PSGL-1/selectin and ICAM-1/CD18 interactions are involved in macrophage-induced drug resistance in myeloma. Leukemia 2013, 27, 702–710. [Google Scholar] [CrossRef]
- Beyar-Katz, O.; Magidey, K.; Reiner-Benaim, A.; Barak, N.; Avivi, I.; Cohen, Y.; Timaner, M.; Avraham, S.; Hayun, M.; Lavi, N.; et al. Proinflammatory Macrophages Promote Multiple Myeloma Resistance to Bortezomib Therapy. Mol. Cancer Res. 2019, 17, 2331–2340. [Google Scholar] [CrossRef]
- Chen, J.; He, D.; Chen, Q.; Guo, X.; Yang, L.; Lin, X.; Li, Y.; Wu, W.; Yang, Y.; He, J.; et al. BAFF is involved in macrophage-induced bortezomib resistance in myeloma. Cell Death Dis. 2017, 8, e3161. [Google Scholar] [CrossRef]
- Xu, R.; Li, Y.; Yan, H.; Zhang, E.; Huang, X.; Chen, Q.; Chen, J.; Qu, J.; Liu, Y.; He, J.; et al. CCL2 promotes macrophages-associated chemoresistance via MCPIP1 dual catalytic activities in multiple myeloma. Cell Death Dis. 2019, 10, 781. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, M.; Sanchez, E.; Soof, C.M.; Bujarski, S.; Ng, N.; Cao, J.; Hekmati, T.; Zahab, B.; Nosrati, J.D.; et al. JAK1/2 pathway inhibition suppresses M2 polarization and overcomes resistance of myeloma to lenalidomide by reducing TRIB1, MUC1, CD44, CXCL12, and CXCR4 expression. Br. J. Haematol. 2020, 188, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Suyanı, E.; Sucak, G.T.; Akyürek, N.; Sahin, S.; Baysal, N.A.; Yağcı, M.; Haznedar, R. Tumor-associated macrophages as a prognostic parameter in multiple myeloma. Ann. Hematol. 2013, 92, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, W.M.; Xia, Z.J.; Liang, Y.; Lu, Y.; Lin, S.X.; Tang, H. High numbers of CD163+ tumor-associated macrophages correlate with poor prognosis in multiple myeloma patients receiving bortezomib-based regimens. J. Cancer 2019, 10, 3239–3245. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.N.; Abildgaard, N.; Maniecki, M.B.; Møller, H.J.; Andersen, N.F. Monocyte/macrophage-derived soluble CD163: A novel biomarker in multiple myeloma. Eur. J. Haematol. 2014, 93, 41–47. [Google Scholar] [CrossRef]
- Andersen, M.N.; Andersen, N.F.; Rødgaard-Hansen, S.; Hokland, M.; Abildgaard, N.; Møller, H.J. The novel biomarker of alternative macrophage activation, soluble mannose receptor (sMR/sCD206): Implications in multiple myeloma. Leuk. Res. 2015, 39, 971–975. [Google Scholar] [CrossRef]
- Storti, P.; Vescovini, R.; Costa, F.; Marchica, V.; Toscani, D.; Dalla Palma, B.; Craviotto, L.; Malavasi, F.; Giuliani, N. CD14+ CD16+ monocytes are involved in daratumumab-mediated myeloma cells killing and in anti-CD47 therapeutic strategy. Br. J. Haematol. 2020, 190, 430–436. [Google Scholar] [CrossRef]
- Rendtlew Danielsen, J.M.; Knudsen, L.M.; Dahl, I.M.; Lodahl, M.; Rasmussen, T. Dysregulation of CD47 and the ligands thrombospondin 1 and 2 in multiple myeloma. Br. J. Haematol. 2007, 138, 756–760. [Google Scholar] [CrossRef]
- Komohara, Y.; Noyori, O.; Saito, Y.; Takeya, H.; Baghdadi, M.; Kitagawa, F.; Hama, N.; Ishikawa, K.; Okuno, Y.; Nosaka, K.; et al. Potential anti-lymphoma effect of M-CSFR inhibitor in adult T-cell leukemia/lymphoma. J. Clin. Exp. Hematop. 2018, 58, 152–160. [Google Scholar] [CrossRef]
- Cucè, M.; Gallo Cantafio, M.E.; Siciliano, M.A.; Riillo, C.; Caracciolo, D.; Scionti, F.; Staropoli, N.; Zuccalà, V.; Maltese, L.; Di Vito, A.; et al. Trabectedin triggers direct and NK-mediated cytotoxicity in multiple myeloma. J. Hematol. Oncol. 2019, 12, 32. [Google Scholar] [CrossRef]
- Teixidó, J.; Martínez-Moreno, M.; Díaz-Martínez, M.; Sevilla-Movilla, S. The good and bad faces of the CXCR4 chemokine receptor. Int. J. Biochem. Cell. Biol. 2018, 95, 121–131. [Google Scholar] [CrossRef]
- Azab, A.K.; Runnels, J.M.; Pitsillides, C.; Moreau, A.S.; Azab, F.; Leleu, X.; Jia, X.; Wright, R.; Ospina, B.; Carlson, A.L.; et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood 2009, 113, 4341–4351. [Google Scholar] [CrossRef]
- Vo, M.C.; Jung, S.H.; Chu, T.H.; Lee, H.J.; Lakshmi, T.J.; Park, H.S.; Kim, H.J.; Rhee, J.H.; Lee, J.J. Lenalidomide and Programmed Death-1 Blockade Synergistically Enhances the Effects of Dendritic Cell Vaccination in a Model of Murine Myeloma. Front. Immunol. 2018, 9, 1370. [Google Scholar] [CrossRef]
- Nguyen-Pham, T.N.; Jung, S.H.; Vo, M.C.; Thanh-Tran, H.T.; Lee, Y.K.; Lee, H.J.; Choi, N.R.; Hoang, M.D.; Kim, H.J.; Lee, J.J. Lenalidomide Synergistically Enhances the Effect of Dendritic Cell Vaccination in a Model of Murine Multiple Myeloma. J. Immunother. 2015, 38, 330–339. [Google Scholar] [CrossRef]
- Chatziravdeli, V.; Katsaras, G.N.; Katsaras, D.; Doxani, C.; Stefanidis, I.; Zintzaras, E. A systematic review and meta-analysis of interventional studies of bisphosphonates and denosumab in multiple myeloma and future perspectives. J. Musculoskelet. Neuronal Interact. 2022, 22, 596–621. [Google Scholar]
- Jensen, J.L.; Rakhmilevich, A.; Heninger, E.; Broman, A.T.; Hope, C.; Phan, F.; Miyamoto, S.; Maroulakou, I.; Callander, N.; Hematti, P.; et al. Tumoricidal Effects of Macrophage-Activating Immunotherapy in a Murine Model of Relapsed/Refractory Multiple Myeloma. Cancer Immunol. Res. 2015, 3, 881–890. [Google Scholar] [CrossRef]
- Gutiérrez-González, A.; Martínez-Moreno, M.; Samaniego, R.; Arellano-Sánchez, N.; Salinas-Muñoz, L.; Relloso, M.; Valeri, A.; Martínez-López, J.; Corbí, Á.L.; Hidalgo, A.; et al. Evaluation of the potential therapeutic benefits of macrophage reprogramming in multiple myeloma. Blood 2016, 128, 2241–2252. [Google Scholar] [CrossRef]
- Hofmann, J.N.; Landgren, O.; Landy, R.; Kemp, T.J.; Santo, L.; McShane, C.M.; Shearer, J.J.; Lan, Q.; Rothman, N.; Pinto, L.A.; et al. A Prospective Study of Circulating Chemokines and Angiogenesis Markers and Risk of Multiple Myeloma and Its Precursor. JNCI Cancer Spectr. 2019, 4, pkz104. [Google Scholar] [CrossRef]
- Moreno, V.; Perets, R.; Peretz-Yablonski, T.; Fourneau, N.; Girgis, S.; Guo, Y.; Hellemans, P.; Verona, R.; Pendás, N.; Xia, Q.; et al. A phase 1 study of intravenous mitazalimab, a CD40 agonistic monoclonal antibody, in patients with advanced solid tumors. Investig. New Drugs. 2023, 41, 93–104. [Google Scholar] [CrossRef]
- Berenson, J.R.; Martinez, D.; Safaie, T.; Boccia, R.; Yang, H.; Moezi, M.; Lim, S.; Schwartz, G.; Eshaghian, S.; Swift, R.; et al. Ruxolitinib and methylprednisolone for treatment of patients with relapsed/refractory multiple myeloma. Br. J. Haematol. 2023, 200, 722–730. [Google Scholar] [CrossRef]
- Lauria, F.; Cencini, E.; Forconi, F. Alternative methods of cladribine administration. Leuk. Lymphoma 2011, 52, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lyu, H.; Pei, S.; Song, D.; Ni, J.; Liu, B. Cladribine in combination with entinostat synergistically elicits anti-proliferative/anti-survival effects on multiple myeloma cells. Cell Cycle 2018, 17, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, G.; Mariotti, A.; Procoli, A.; Folgiero, V.; Natale, D.; De Rosa, L.; Majolino, I.; Novarese, L.; Rocci, A.; Gambella, M.; et al. Indoleamine 2,3-dioxygenase 1 (IDO1) activity correlates with immune system abnormalities in multiple myeloma. J. Transl. Med. 2012, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Cencini, E.; Bocchia, M.; Fabbri, A. Nivolumab in relapsed/refractory Hodgkin lymphoma: Towards a new treatment strategy? Am. J. Blood. Res. 2021, 11, 261–265. [Google Scholar] [PubMed]
- Costa, F.; Vescovini, R.; Marchica, V.; Storti, P.; Notarfranchi, L.; Dalla Palma, B.; Toscani, D.; Burroughs-Garcia, J.; Catarozzo, M.T.; Sammarelli, G.; et al. PD-L1/PD-1 Pattern of Expression Within the Bone Marrow Immune Microenvironment in Smoldering Myeloma and Active Multiple Myeloma Patients. Front. Immunol. 2021, 11, 613007. [Google Scholar] [CrossRef]
- Cohen, Y.C.; Oriol, A.; Wu, K.L.; Lavi, N.; Vlummens, P.; Jackson, C.; Garvin, W.; Carson, R.; Crist, W.; Fu, J.; et al. Daratumumab With Cetrelimab, an Anti-PD-1 Monoclonal Antibody, in Relapsed/Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2021, 21, 46–54.e4. [Google Scholar] [CrossRef]
- Russ, A.; Hua, A.B.; Montfort, W.R.; Rahman, B.; Riaz, I.B.; Khalid, M.U.; Carew, J.S.; Nawrocki, S.T.; Persky, D.; Anwer, F. Blocking “don’t eat me” signal of CD47-SIRPα in hematological malignancies, an in-depth review. Blood Rev. 2018, 32, 480–489. [Google Scholar] [CrossRef]
- Advani, R.; Flinn, I.; Popplewell, L.; Forero, A.; Bartlett, N.L.; Ghosh, N.; Kline, J.; Roschewski, M.; LaCasce, A.; Collins, G.P.; et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 379, 1711–1721. [Google Scholar] [CrossRef]
- Rastgoo, N.; Wu, J.; Liu, A.; Pourabdollah, M.; Atenafu, E.G.; Reece, D.; Chen, W.; Chang, H. Targeting CD47/TNFAIP8 by miR-155 overcomes drug resistance and inhibits tumor growth through induction of phagocytosis and apoptosis in multiple myeloma. Haematologica 2020, 105, 2813–2823. [Google Scholar] [CrossRef]
- Veitonmäki, N.; Hansson, M.; Zhan, F.; Sundberg, A.; Löfstedt, T.; Ljungars, A.; Li, Z.C.; Martinsson-Niskanen, T.; Zeng, M.; Yang, Y.; et al. A human ICAM-1 antibody isolated by a function-first approach has potent macrophage-dependent antimyeloma activity in vivo. Cancer Cell 2013, 23, 502–515. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Tsakirakis, N.; Malandrakis, P.; Vitsos, P.; Metousis, A.; Orologas-Stavrou, N.; Ntanasis-Stathopoulos, I.; Kanellias, N.; Eleutherakis-Papaiakovou, E.; Pothos, P.; et al. Deep Phenotyping Reveals Distinct Immune Signatures Correlating with Prognostication, Treatment Responses, and MRD Status in Multiple Myeloma. Cancers 2020, 12, 3245. [Google Scholar] [CrossRef]
- Mougiakakos, D.; Bach, C.; Böttcher, M.; Beier, F.; Röhner, L.; Stoll, A.; Rehli, M.; Gebhard, C.; Lischer, C.; Eberhardt, M.; et al. The IKZF1–IRF4/IRF5 Axis Controls Polarization of Myeloma-Associated Macrophages. Cancer Immunol. Res. 2021, 9, 265–278. [Google Scholar] [CrossRef]
- Quach, H.; Ritchie, D.; Stewart, A.K.; Neeson, P.; Harrison, S.; Smyth, M.J.; Prince, H.M. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia 2009, 24, 22–32. [Google Scholar] [CrossRef]
- Kuwahara-Ota, S.; Shimura, Y.; Steinebach, C.; Isa, R.; Yamaguchi, J.; Nishiyama, D.; Fujibayashi, Y.; Takimoto-Shimomura, T.; Mizuno, Y.; Matsumura-Kimoto, Y.; et al. Lenalidomide and pomalidomide potently interfere with induction of myeloid-derived suppressor cells in multiple myeloma. Br. J. Haematol. 2020, 191, 784–795. [Google Scholar] [CrossRef]
- Paul, B.; Liedtke, M.; Khouri, J.; Rifkin, R.; Gandhi, M.D.; Kin, A.; Levy, M.Y.; Silbermann, R.; Cottini, F.; Sborov, D.W.; et al. A phase II multi-arm study of magrolimab combinations in patients with relapsed/refractory multiple myeloma. Future Oncol. 2023, in press. [Google Scholar] [CrossRef]
- van de Donk, N.W.; Usmani, S.Z. CD38 Antibodies in Multiple Myeloma: Mechanisms of Action and Modes of Resistance. Front. Immunol. 2018, 9, 2134. [Google Scholar] [CrossRef]
- Gozzetti, A.; Bacchiarri, F.; Sammartano, V.; Defina, M.; Sicuranza, A.; Mecacci, B.; Zappone, E.; Cencini, E.; Fabbri, A.; Raspadori, D.; et al. Long-Term Safety of Rapid Daratumumab Infusions in Multiple Myeloma Patients. Front. Oncol. 2020, 10, 570187. [Google Scholar] [CrossRef]
- Chong, L.L.; Soon, Y.Y.; Soekojo, C.Y.; Ooi, M.; Chng, W.J.; de Mel, S. Daratumumab-based induction therapy for multiple myeloma: A systematic review and meta-analysis. Crit. Rev. Oncol. 2020, 159, 103211. [Google Scholar] [CrossRef]
- Krejcik, J.; Casneuf, T.; Nijhof, I.S.; Verbist, B.; Bald, J.; Plesner, T.; Syed, K.; Liu, K.; Van De Donk, N.W.; Weiss, B.M.; et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016, 128, 384–394. [Google Scholar] [CrossRef]
- Richardson, P.G.; Facon, T.; Bensinger, W.I.; Leleu, X.; Campana, F.; Macé, S.; Chiron, M.; van de Velde, H.; Mikhael, J. Predictive biomarkers with isatuximab plus pomalidomide and dexamethasone in relapsed/refractory multiple myeloma. Blood Cancer J. 2021, 11, 55. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, L.; Acharya, C.; An, G.; Wen, K.; Qiu, L.; Munshi, N.C.; Tai, Y.-T.; Anderson, K.C. Targeting CD38 Suppresses Induction and Function of T Regulatory Cells to Mitigate Immunosuppression in Multiple Myeloma. Clin. Cancer Res. 2017, 23, 4290–4300. [Google Scholar] [CrossRef] [PubMed]
- Kakiuchi-Kiyota, S.; Ross, T.; Wallweber, H.A.; Kiefer, J.R.; Schutten, M.M.; Adedeji, A.O.; Cai, H.; Hendricks, R.; Cohen, S.; Myneni, S.; et al. A BCMA/CD16A bispecific innate cell engager for the treatment of multiple myeloma. Leukemia 2022, 36, 1006–1014. [Google Scholar] [CrossRef] [PubMed]

| Event | Mechanisms | Main Effects |
|---|---|---|
| TAM Accumulation within TME | Chemotactic factors and M2 TAM polarization driven by MM PC, CD206 up-regulation on TAM, JAK/STAT activation. | Recruitment of circulating monocytes, MM cells growth and survival, aggressive disease. |
| PC migration, homing, proliferation | M2 TAM production of chemotactic molecules and PC growth factors | Increased proliferation index of MM cells |
| Angiogenesis | Vascular mimicry, VEGF, FGF production by M2 TAM | Generate capillary-like vessels, disease progression |
| Immunosuppression | Inhibition of cytotoxic T cell response, overexpression of immune checkpoint proteins | Reduced cellular immune response against MM cells |
| Drug resistance | Activation of the Src, Erk1/2 kinase and c-myc pathway. BAFF production. NF-kB pathway and IL-6/JAK/STAT3 pathway. TAM M2 polarization. | Increased MM cells viability. Impaired drug-mediated apoptosis |
| Reference | Number of Patients | TAM Marker | Technique | Treatment | Survival Correlation |
|---|---|---|---|---|---|
| Chen et al. [81] | 240 | CD68 CD163 iNOS | IHC | MP = 12, VAD = 37, TD/MPT = 161, bortezomib or lenalidomide = 30 | Inferior PFS, OS |
| Panchabbai et al. [84] | 141 | CD163 | IHC Flow cytometry | NR | Inferior OS |
| Beyar-Katz et al. [119] | 34 | CD68 CCR2 | Flow cytometry | Bortezomib | Inferior OS |
| Suyani et al. [123] | 68 | CD68 CD163 | IHC | VAD = 37, MP = 10, thalidomide = 11, bortezomib = 5, lenalidomide = 1, no treatment = 4 | Inferior OS |
| Wang et al. [124] | 198 | CD163 | IHC | Proteasome inhibitors | Inferior PFS, OS |
| Andersen et al. [125] | 104 | CD163 | Soluble | High-dose therapy = 42 Chemotherapy = 62 | Inferior OS |
| Andersen et al. [126] | 104 | CD206 | Soluble | High-dose therapy = 42 Chemotherapy = 62 | Inferior OS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cencini, E.; Sicuranza, A.; Ciofini, S.; Fabbri, A.; Bocchia, M.; Gozzetti, A. Tumor-Associated Macrophages in Multiple Myeloma: Key Role in Disease Biology and Potential Therapeutic Implications. Curr. Oncol. 2023, 30, 6111-6133. https://doi.org/10.3390/curroncol30070455
Cencini E, Sicuranza A, Ciofini S, Fabbri A, Bocchia M, Gozzetti A. Tumor-Associated Macrophages in Multiple Myeloma: Key Role in Disease Biology and Potential Therapeutic Implications. Current Oncology. 2023; 30(7):6111-6133. https://doi.org/10.3390/curroncol30070455
Chicago/Turabian StyleCencini, Emanuele, Anna Sicuranza, Sara Ciofini, Alberto Fabbri, Monica Bocchia, and Alessandro Gozzetti. 2023. "Tumor-Associated Macrophages in Multiple Myeloma: Key Role in Disease Biology and Potential Therapeutic Implications" Current Oncology 30, no. 7: 6111-6133. https://doi.org/10.3390/curroncol30070455
APA StyleCencini, E., Sicuranza, A., Ciofini, S., Fabbri, A., Bocchia, M., & Gozzetti, A. (2023). Tumor-Associated Macrophages in Multiple Myeloma: Key Role in Disease Biology and Potential Therapeutic Implications. Current Oncology, 30(7), 6111-6133. https://doi.org/10.3390/curroncol30070455






