Capecitabine Plus Aromatase Inhibitor as First Line Therapy for Hormone Receptor Positive, HER2 Negative Metastatic Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Treatment and Evaluation
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics and Treatment
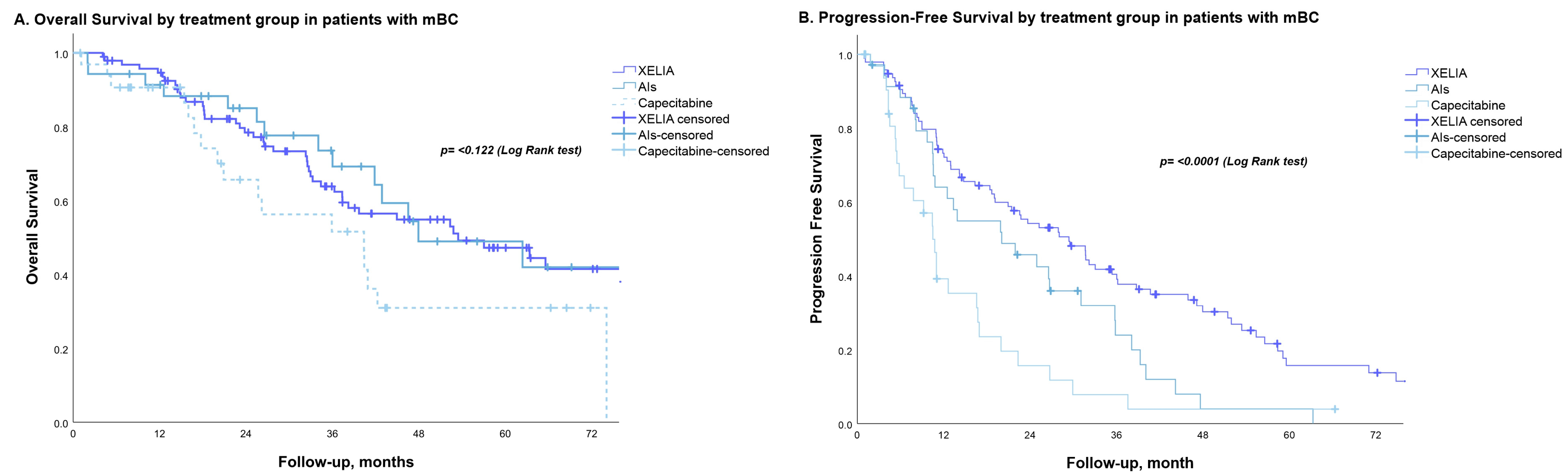
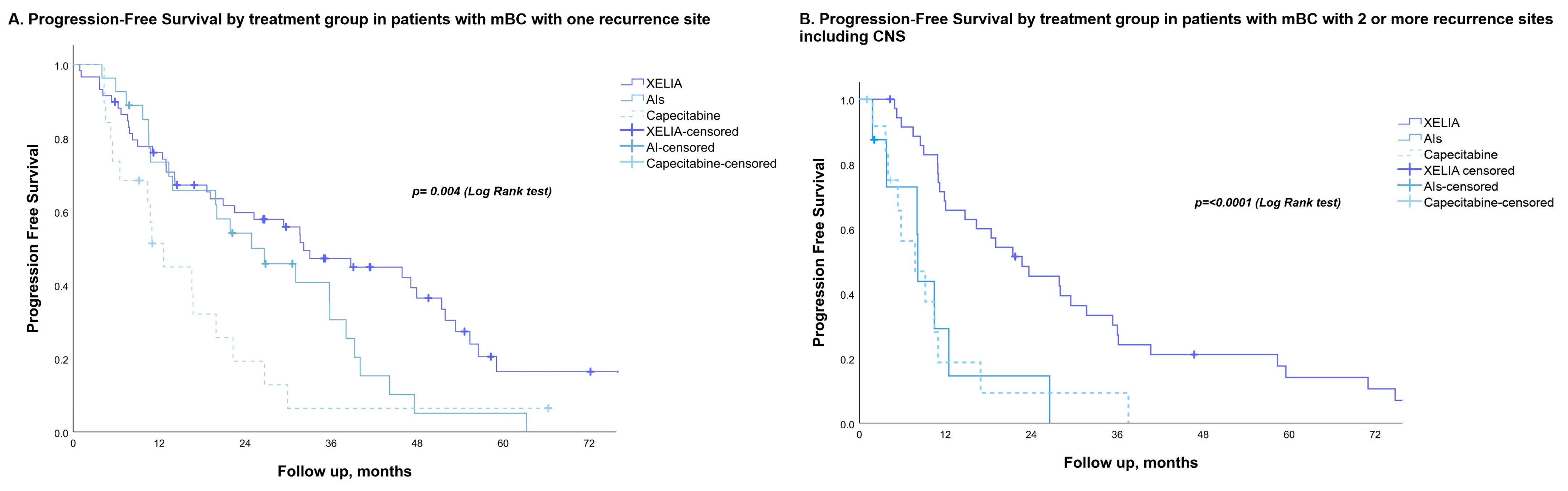
3.2. PFS, OS and Best Objective Response
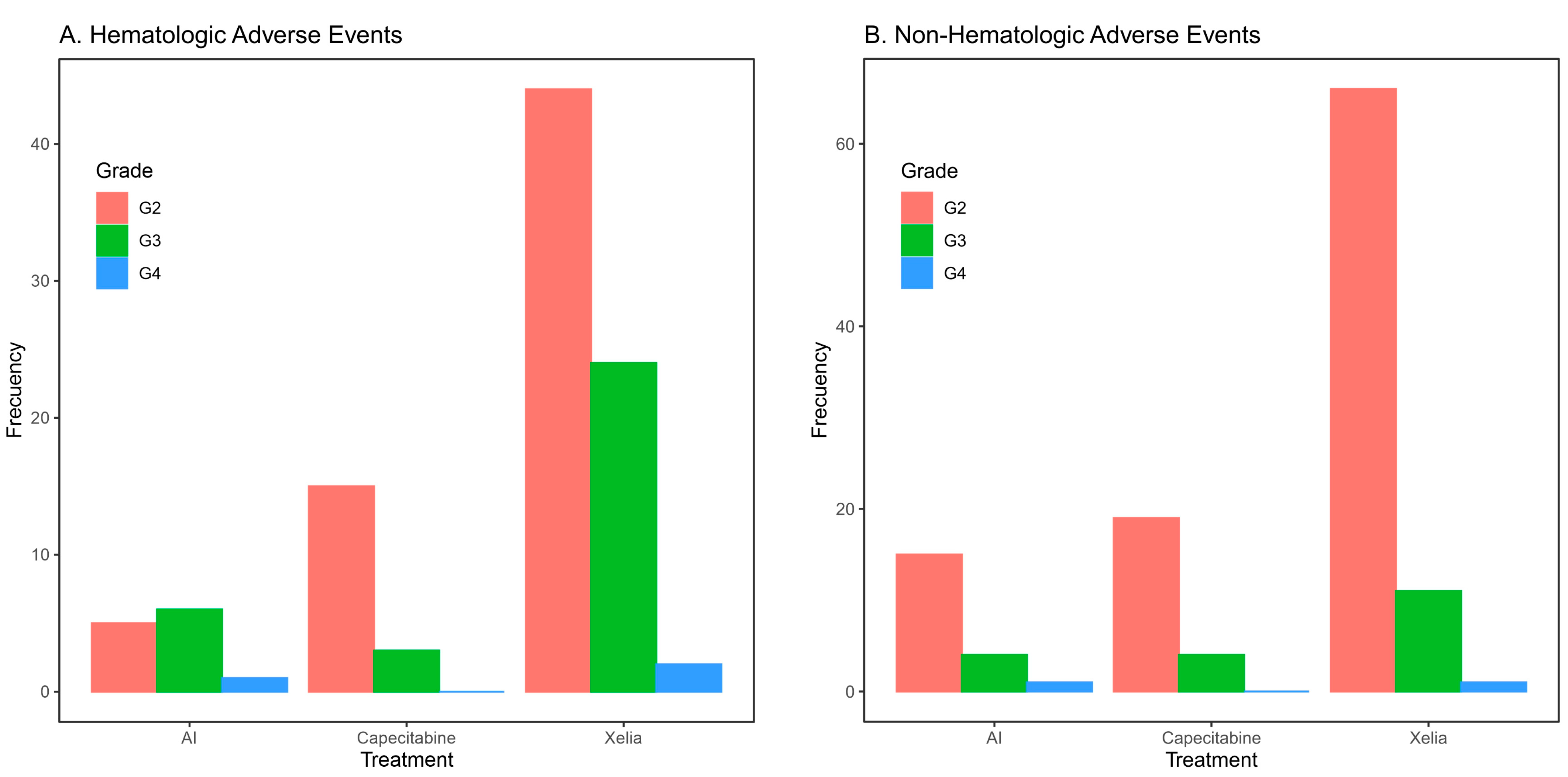
3.3. Efficacy and Safety Assessments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Leone, J.P.; Vallejo, C.T.; Hassett, M.J.; Leone, J.; Graham, N.; Tayob, N.; Freedman, R.A.; Tolaney, S.M.; Leone, B.A.; Winer, E.P.; et al. Factors associated with late risks of breast cancer-specific mortality in the SEER registry. Breast Cancer Res. Treat. 2021, 189, 203–212. [Google Scholar] [CrossRef]
- Lal, P.; Tan, L.K.; Chen, B. Correlation of HER-2 Status with Estrogen and Progesterone Receptors and Histologic Features in 3,655 Invasive Breast Carcinomas. Am. J. Clin. Pathol. 2005, 123, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Xiang, L.; Li, T.; Bai, Z. Cancer Hallmarks, Biomarkers and Breast Cancer Molecular Subtypes. J. Cancer 2016, 7, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Pietras, R.J. Biologic Basis of Sequential and Combination Therapies for Hormone-Responsive Breast Cancer. Oncol. 2006, 11, 704–717. [Google Scholar] [CrossRef]
- Mauri, D.; Pavlidis, N.; Polyzos, N.P.; Ioannidis, J.P.A. Survival with Aromatase Inhibitors and Inactivators Versus Standard Hormonal Therapy in Advanced Breast Cancer: Meta-analysis. Gynecol. Oncol. 2006, 98, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.; Martin, M.; Di Leo, A.; Im, S.-A.; Awada, A.; Forrester, T.; Frenzel, M.; Hardebeck, M.C.; Cox, J.; Barriga, S.; et al. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019, 5, 1–8. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.; Hu, P.; Falkson, G.; Tormey, D.; Abeloff, M.; for the Eastern Cooperative Oncology Group. Comparison of Chemotherapy with Chemohormonal Therapy as First-Line Therapy for Metastatic, Hormone-Sensitive Breast Cancer: An Eastern Cooperative Oncology Group Study. J. Clin. Oncol. 2000, 18, 262. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.; West, J.; Card, T.; Crooks, C.; Kirwan, C.C.; Grainge, M.J. When are breast cancer patients at highest risk of venous thromboembolism? A cohort study using English health care data. Blood 2016, 127, 849–857. [Google Scholar] [CrossRef]
- Moriya, Y.; Kataoka, M.; Yamaguchi, Y.; Sawada, N.; Yasuno, H.; Kondoh, K.; Evans, D.B.; Mori, K.; Hayashi, S.-I. Antitumor activity of chemoendocrine therapy in premenopausal and postmenopausal models with human breast cancer xenografts. Oncol. Rep. 2011, 27, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Rashad, N.; Abdelhamid, T.; Shouman, S.A.; Nassar, H.; Omran, M.A.; El Desouky, E.D.; Khaled, H. Capecitabine-Based Chemoendocrine Combination as First-Line Treatment for Metastatic Hormone-Positive Metastatic Breast Cancer: Phase 2 Study. Clin. Breast Cancer 2020, 20, 228–237. [Google Scholar] [CrossRef]
- Nukatsuka, M.; Saito, H.; Nakagawa, F.; Abe, M.; Uchida, J.; Shibata, J.; Matsuo, K.-I.; Noguchi, S.; Kiniwa, M. Oral fluoropyrimidine may augment the efficacy of aromatase inhibitor via the down-regulation of estrogen receptor in estrogen-responsive breast cancer xenografts. Breast Cancer Res. Treat. 2010, 128, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Kurebayashi, J.; Nukatsuka, M.; Nagase, H.; Nomura, T.; Hirono, M.; Yamamoto, Y.; Sugimoto, Y.; Oka, T.; Sonoo, H. Additive antitumor effect of concurrent treatment of 4-hydroxy tamoxifen with 5-fluorouracil but not with doxorubicin in estrogen receptor-positive breast cancer cells. Cancer Chemother. Pharmacol. 2006, 59, 515–525. [Google Scholar] [CrossRef]
- Montagna, E.; Cancello, G.; Dellapasqua, S.; Munzone, E.; Colleoni, M. Metronomic therapy and breast cancer: A systematic review. Cancer Treat. Rev. 2014, 40, 942–950. [Google Scholar] [CrossRef]
- Banys-Paluchowski, M.; Schütz, F.; Ruckhäberle, E.; Krawczyk, N.; Fehm, T. Metronomic Chemotherapy for Metastatic Breast Cancer—A Systematic Review of the Literature. Senol.-Z. Mammadiagnostik Und-Ther. 2016, 76, 525–534. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, F.; Liang, J.; Dai, X.; Wan, C.; Hong, X.; Zhang, K.; Liu, L. The efficacy and toxicity profile of metronomic chemotherapy for metastatic breast cancer: A meta-analysis. PLoS ONE 2017, 12, e0173693. [Google Scholar] [CrossRef] [PubMed]
- Krajnak, S.; Schnatz, C.; Almstedt, K.; Brenner, W.; Haertner, F.; Heimes, A.-S.; Lebrecht, A.; Makris, G.-M.; Schwab, R.; Hasenburg, A.; et al. Low-dose metronomic chemotherapy as an efficient treatment option in metastatic breast cancer—Results of an exploratory case–control study. Breast Cancer Res. Treat. 2020, 182, 389–399. [Google Scholar] [CrossRef]
- Velaei, K.; Samadi, N.; Barazvan, B.; Rad, J.S. Tumor microenvironment-mediated chemoresistance in breast cancer. Breast 2016, 30, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Deepak, K.; Vempati, R.; Nagaraju, G.P.; Dasari, V.R.; Nagini, S.; Rao, D.; Malla, R.R. Tumor microenvironment: Challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol. Res. 2020, 153, 104683. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Ma, F.; Wang, J.; Luo, Y.; Fan, Y.; Yuan, P.; Zhang, P.; Li, Q.; Li, Q.; et al. Safety and efficacy study of oral metronomic vinorelbine combined with trastuzumab (mNH) in HER2-positive metastatic breast cancer: A phase II trial. Breast Cancer Res. Treat. 2021, 188, 441–447. [Google Scholar] [CrossRef]
- Parra, K.; Valenzuela, P.; Lerma, N.; Gallegos, A.; Reza, L.; Rodriguez, G.; Emmenegger, U.; Di Desidero, T.; Bocci, G.; Felder, M.S.; et al. Impact of CTLA-4 blockade in conjunction with metronomic chemotherapy on preclinical breast cancer growth. Br. J. Cancer 2017, 116, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Kerbel, R.S.; Kamen, B.A. The anti-angiogenic basis of metronomic chemotherapy. Nat. Rev. Cancer 2004, 4, 423–436. [Google Scholar] [CrossRef]
- Natale, G.; Bocci, G. Does metronomic chemotherapy induce tumor angiogenic dormancy? A review of available preclinical and clinical data. Cancer Lett. 2018, 432, 28–37. [Google Scholar] [CrossRef]
- Cazzaniga, M.E.; Capici, S.; Cordani, N.; Cogliati, V.; Pepe, F.F.; Riva, F.; Cerrito, M.G. Metronomic Chemotherapy for Metastatic Breast Cancer Treatment: Clinical and Preclinical Data between Lights and Shadows. J. Clin. Med. 2022, 11, 4710. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Masuda, N.; Kamigaki, S.; Morimoto, T.; Saji, S.; Imoto, S.; Sasano, H.; Toi, M. Differential Involvement of Autophagy and Apoptosis in Response to Chemoendocrine and Endocrine Therapy in Breast Cancer: JBCRG-07TR. Int. J. Mol. Sci. 2019, 20, 984. [Google Scholar] [CrossRef] [PubMed]
- Hoon, S.-N.; Lau, P.K.H.; White, A.M.; Bulsara, M.K.; Banks, P.D.; Redfern, A.D. Capecitabine for hormone receptor-positive versus hormone receptor-negative breast cancer. Cochrane Database Syst. Rev. 2021, 2021, CD011220. [Google Scholar] [CrossRef]
- Shankar, A.; Roy, S.; Rath, G.K.; Julka, P.K.; Kamal, V.K.; Malik, A.; Patil, J.; Jeyaraj, P.A.; Mahajan, M.K. Aromatase Inhibition and Capecitabine Combination as 1st or 2nd Line Treatment for Metastatic Breast Cancer—A Retrospective Analysis. Asian Pac. J. Cancer Prev. 2015, 16, 6359–6364. [Google Scholar] [CrossRef]
- Shi, W.; Wang, X.; Bi, X.; Xia, W.; Huang, J.; Su, Y.; Huang, Z.; Yuan, Z. Combination of Aromatase Inhibitors with Metronomic Capecitabine: A New Chemoendocrine Treatment for Advanced Breast Cancer. J. Cancer Ther. 2019, 10, 146–156. [Google Scholar] [CrossRef]
- Bottini, A.; Generali, D.; Brizzi, M.P.; Fox, S.B.; Bersiga, A.; Bonardi, S.; Allevi, G.; Aguggini, S.; Bodini, G.; Milani, M.; et al. Randomized Phase II Trial of Letrozole and Letrozole Plus Low-Dose Metronomic Oral Cyclophosphamide As Primary Systemic Treatment in Elderly Breast Cancer Patients. J. Clin. Oncol. 2006, 24, 3623–3628. [Google Scholar] [CrossRef]
- Li, J.-W.; Zuo, W.-J.; Ivanova, D.; Jia, X.-Q.; Lei, L.; Liu, G.-Y. Metronomic capecitabine combined with aromatase inhibitors for new chemoendocrine treatment of advanced breast cancer: A phase II clinical trial. Breast Cancer Res. Treat. 2018, 173, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Caparica, R.; De Angelis, C.; Fêde, Â.; Werutsky, G.; de Azambuja, E. Metronomic chemotherapy combined with endocrine therapy: Are we challenging some dogmas? Expert Rev. Anticancer Ther. 2020, 20, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Abdelmaksoud, B.A.; Toam, M.M.; Fayed, A.A. Metronomic capecitabine with aromatase inhibitors for patients with metastatic hormone-receptor positive, HER2-negative breast cancer. Breast Cancer Manag. 2019, 8, BMT30. [Google Scholar] [CrossRef]
- Monteiro, M.; Nunes, N.; Crespo, J.; Abrahão, A.; Buscacio, G.; Lerner, L.; Sermoud, L.; Arakelian, R.; Piotto, G.; Lemos, C.; et al. Patient-centered Outcomes in Breast Cancer: Description of EQ-5D-5L and EORTC-QLQ-BR23 Measurements in Real-world Data and Their Association with Survival. Clin. Oncol. 2022, 34, 608–616. [Google Scholar] [CrossRef]
- Li, J.; Fu, F.; Yu, L.; Huang, M.; Lin, Y.; Mei, Q.; Lv, J.; Wang, C. Cyclin-dependent kinase 4 and 6 inhibitors in hormone receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer: A meta-analysis of randomized clinical trials. Breast Cancer Res. Treat. 2020, 180, 21–32. [Google Scholar] [CrossRef]
- Martín, M.; Loibl, S.; von Minckwitz, G.; Morales, S.; Martinez, N.; Guerrero, A.; Anton, A.; Aktas, B.; Schoenegg, W.; Muñoz, M.; et al. Phase III Trial Evaluating the Addition of Bevacizumab to Endocrine Therapy As First-Line Treatment for Advanced Breast Cancer: The Letrozole/Fulvestrant and Avastin (LEA) Study. J. Clin. Oncol. 2015, 33, 1045–1052. [Google Scholar] [CrossRef]
- Lerner, A.; Keshwani, K.; Okines, A.; Sanderson, B.; Board, R.; Flynn, M.; Sharkey, E.; Konstantis, A.; Roylance, R.; Hanna, D.; et al. A Multicentre Retrospective Study of Fulvestrant Use and Efficacy in Advanced/Metastatic Breast Cancer. Clin. Oncol. 2022, 34, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Dickler, M.N.; Barry, W.T.; Cirrincione, C.T.; Ellis, M.J.; Moynahan, M.E.; Innocenti, F.; Hurria, A.; Rugo, H.S.; Lake, D.E.; Hahn, O.; et al. Phase III Trial Evaluating Letrozole as First-Line Endocrine Therapy with or without Bevacizumab for the Treatment of Postmenopausal Women with Hormone Receptor–Positive Advanced-Stage Breast Cancer: CALGB 40503 (Alliance). J. Clin. Oncol. 2016, 34, 2602–2609. [Google Scholar] [CrossRef]
- Marschner, N.; Zacharias, S.; Lordick, F.; Hegewisch-Becker, S.; Martens, U.; Welt, A.; Hagen, V.; Gleiber, W.; Bohnet, S.; Kruggel, L.; et al. Association of Disease Progression with Health-Related Quality of Life Among Adults with Breast, Lung, Pancreatic, and Colorectal Cancer. JAMA Netw. Open 2020, 3, e200643. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, M.; Luo, X.; Li, H.; Shan, H.; Du, Q.; Zhai, Q. Pharmacoeconomic evaluations of CDK4/6 inhibitors plus endocrine therapy for advanced hormone receptor-positive (HR+) and human epidermal growth factor receptor-2 negative (HER2−) breast cancer: A systematic review. Ann. Transl. Med. 2022, 10, 233. [Google Scholar] [CrossRef]
- Salem, D.; Gado, N.M.; Abdelaziz, N.N.; Essa, A.E.; Abdelhafeez, Z.M.; Kamel, T.H. Phase II trial of metronomic chemotherapy as salvage therapy for patients with metastatic breast cancer. J. Egypt. Natl. Cancer Inst. 2008, 20, 134–140. [Google Scholar]
| Total (n = 163) | XELIA (n = 95) | HT (n = 35) | CTx (n = 33) | p-Value | |
|---|---|---|---|---|---|
| Age, years | |||||
| Median (range) | 56.00 (23–91) | 56.00 (34–87) | 56.00 (33–91) | 55.00 (23–85) 53.15 (±13.67) | 0.958 |
| Mean (standard deviation) | 56.77 (±12.48) | 57.46 (±11.40) | 58.29 (±13.79) | ||
| ECOG PS % (n/N) | |||||
| 0–1 | 83.4 (136/163) | 84.2 (80/95) | 82.9 (29/35) | 81.8 (27/33) | 0.945 |
| ≥2 | 16.6 (27/163) | 15.8 (15/95) | 17.1 (6/35) | 18.2 (6/33) | |
| Hormonal status % (n/N) | |||||
| Pre- or peri-menopausal | 22.7 (37/163) | 16.8 (16/95) | 28.6 (10/35) | 33.3 (11/33) | 0.097 |
| Post-menopausal | 77.3 (126/163) | 83.2 (79/95) | 71.4 (25/35) | 66.7 (22/33) | |
| Tumor status % (n/N) | |||||
| ≤T2 | 38.0 (62/163) | 41.1 (39/95) | 40.0 (14/35) | 27.3 (9/33) | 0.441 |
| T3 | 24.5 (40/163) | 22.1 (21/95) | 20.0 (7/35) | 36.4 (12/33) | |
| T4 | 37.4 (61/163) | 36.8 (35/95) | 40.0 (14/35) | 36.4 (12/33) | |
| Nodal status % (n/N) | |||||
| N1 | 36.2 (59/163) | 31.6 (30/95) | 45.3 (16/35) | 39.4 (13/33) | 0.823 |
| N2 | 31.3 (51/163) | 32.6 (31/95) | 25.7 (8/35) | 33.3 (11/33) | |
| N3 | 17.8 (29/163) | 18.9 (18/95) | 17.1 (6/35) | 15.2 (5/33) | |
| Disease state % (n/N) | |||||
| Recurrent disease | 80.4 (131/163) | 78.9 (75/95) | 74.3 (26/35) | 90.9 (30/33) | 0.195 |
| Metastatic disease | 19.6 (32/163) | 21.1 (20/95) | 25.7 (9/35) | 9.1 (3/33) | |
| Histological type % (n/N) | |||||
| IDC | 82.8 (135/163) | 84.2 (80/95) | 82.9 (29/35) | 78.8 (26/33) | 0.776 |
| ILC | 17.2 (28/163) | 15.8 (15/95) | 17.1 (6/35) | 21.2 (7/33) | |
| Ki-67% (n/N) | |||||
| <20 | 47.9 (46/96) | 42.9 (24/56) | 47.4 (9/19) | 61.9 (13/21) | 0.329 |
| ≥20 | 52.1 (50/96) | 57.1 (32/56) | 52.6 (10/19) | 38.1 (8/21) | |
| SBR % (n/N) | |||||
| Low | 9.1 (13/143) | 6.3 (5/80) | 18.8 (6/32) | 6.5 (2/31) | 0.098 |
| Medium/high | 90.9 (130/143) | 93.8 (75/80) | 81.3 (26/32) | 93.5 (29/31) | |
| ER % (n/N) | |||||
| Negative | 4.3 (7/163) | 5.3 (5/95) | 5.7 (2/35) | 0 (0/33) | 0.393 |
| Positive | 95.7 (156/163) | 94.7 (90/95) | 94.3 (33/35) | 100 (33/33) | |
| PgR % (n/N) | |||||
| Negative | 19.6 (32/163) | 21.1 (20/95) | 2.9 (1/35) | 33.3 (11/33) | 0.006 |
| Positive | 80.4 (131/163) | 78.9 (75/95) | 97.1 (34/35) | 66.7 (22/33) | |
| ER/PgR % (n/N) | |||||
| ER+, PgR+ | 76.1 (124/163) | 73.7 (70/95) | 91.4 (32/35) | 66.7 (22/33) | 0.021 |
| ER+, PgR− | 19.6 (32/163) | 21.1 (20/95) | 2.9 (1/35) | 33.3 (11/33) | |
| ER-, PgR+ | 4.3 (7/163) | 5.3 (5/95) | 5.7 (2/35) | 0 (0/33) | |
| Type of surgery % (n/N) | |||||
| BCS | 13.0 (17/131) | 17.3 (13/75) | 7.7 (2/26) | 6.7 (2/30) | 0.359 |
| MRM | 85.5 (112/131) | 81.3 (61/75) | 88.5 (23/26) | 93.3 (28/30) | |
| Previous neo/adjuvant chemotherapy % (n/N) | |||||
| Anthracycline-based | 2.3 (3/131) | 4.0 (3/75) | 0.0 (0/26) | 0.0 (0/30) | 0.209 |
| Anthracyclines/taxanes | 86.3 (113/131) | 84.0 (63/75) | 80.8 (21/26) | 96.7 (29/30) | |
| Others | 11.5 (15/131) | 12.0 (9/75) | 19.2 (5/26) | 3.3 (1/30) | |
| Adjuvant endocrine therapy % (n/N)+ | |||||
| Tamoxifen | 42.7 (56/131) | 38.7 (29/75) | 50.0 (13/26) | 46.7 (14/30) | 0.533 |
| Aromatase inhibitors | 57.3 (75/131) | 61.3 (46/75) | 50.0 (13/26) | 53.3 (16/30) | |
| Adjuvant radiotherapy % (n/N) | |||||
| Yes | 83.2 (109/131) | 81.3 (61/75) | 84.6 (22/26) | 86.7 (26/30) | 0.786 |
| No | 16.8 (22/131) | 18.7 (14/75) | 15.4 (4/26) | 13.3 (4/30) | |
| Disease-free interval % (n/N) | |||||
| ≤24 months | 30.5 (40/131) | 24.0 (18/75) | 34.6 (9/26) | 43.3 (13/30) | 0.133 |
| >24 months | 69.5 (91/131) | 76.0 (57/75) | 65.4 (17/26) | 56.7 (17/30) | |
| Number of metastases % (n/N) | |||||
| 1 place | 64.4 (105/163) | 62.1 (59/95) | 77.1 (27/35) | 57.6 (19/33) | 0.153 |
| 2 places | 22.1 (36/163) | 25.3 (24/95) | 14.3 (5/35) | 21.2 (7/33) | |
| ≥3 places | 7.4 (12/163) | 7.4 (7/95) | 8.6 (3/35) | 6.1 (2/33) | |
| CNS involvement * | 6.1 (10/163) | 5.3 (5/95) | 0.0 (0/35) | 15.2 (5/33) | |
| Disease site % (n/N) | |||||
| Visceral ** | 45.4 (74/163) | 47.4 (45/95) | 40.0 (14/35) | 45.5 (15/33) | 0.138 |
| Non-visceral | 9.8 (16/163) | 11.6 (11/95) | 8.6 (3/35) | 6.1 (2/33) | |
| Bone | 38.7 (63/163) | 35.8 (34/95) | 51.4 (18/35) | 33.3 (11/33) | |
| CNS *** | 6.1 (10/163) | 5.3 (5/95) | 0.0 (0/35) | 15.2 (5/33) | |
| Total (Events) | Median (95% CI) | p-Value | HR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| ECOG PS | 0.972 | ||||
| 0–1 | 136 (62) | 42.9 (31.1–54.7) | |||
| ≥2 | 27 (15) | 63.4 (33.9–92.8) | |||
| Hormonal status | 0.199 | ||||
| Pre- or peri-menopausal | 54 (19) | 46.5 (27.2–65.8) | |||
| Postmenopausal | 109 (58) | 52.3 (34.5–70.2) | |||
| Tumor status | 0.547 | ||||
| ≤T2 | 62 (25) | 62.4 (48.5–76.3) | |||
| T3 | 40 (21) | 40.9 (30.7–51.1) | |||
| T4 | 61 (31) | 41.8 (25.6–58.1) | |||
| Nodal status | 0.707 | ||||
| N0 | 24 (9) | 65.6 (29.7–101.4) | |||
| N1 | 59 (27) | 44.9 (36.7–53.2) | |||
| N2 | 51 (25) | 42.3 (33.4–51.1) | |||
| N3 | 29 (16) | 52.8 (25.7–79.9) | |||
| Disease state | 0.947 | ||||
| Recurrent disease | 131 (61) | 46.5 (33.1–60.0) | |||
| Metastatic disease | 32 (16) | 47.9 (16.2–79.7) | |||
| Histological subtype | 0.884 | ||||
| IDC | 135 (63) | 52.8 (35.5–70.1) | |||
| ILC | 28 (14) | 37.4 (26.4–48.3) | |||
| Ki67 | 0.507 | ||||
| <20 | 46 (17) | 62.39 (27.7–97.1) | |||
| ≥20 | 50 (24) | 46.5 (21.7–71.4) | |||
| Unknown | 67 (36) | 52.3 (32.2–72.5) | |||
| SBR | 0.085 | ||||
| Low | 13 (5) | 80.1 (31.7–128.6) | |||
| Intermediate and high | 130 (66) | 40.0 (34.3–47.5) | |||
| Unknown | 20 (6) | 63.4 (42.4–84.4) | |||
| ER | 0.451 | ||||
| Negative | 7 (4) | 18.1 (17.8–18.5) | |||
| Positive | 156 (73) | 47.9 (40.1–64.5) | |||
| PgR | 0.447 | ||||
| Negative | 32 (17) | 34.3 (11.5–57.3) | |||
| Positive | 131 (60) | 47.9 (28.1–67.8) | |||
| ER/PgR | 0.533 | ||||
| ER+, PgR+ | 124 (56) | 52.3 (32.6–72.1) | |||
| ER+, PgR− | 32 (17) | 34.4 (11.5–57.3) | |||
| ER−, PgR+ | 7 (4) | 18.1 (17.8–18.5) | |||
| Type of surgery | 0.387 | ||||
| BCS | 17 (5) | 98.9 (8.5–189.4) | |||
| MRM | 121 (61) | 44.9 (34.7–55.2) | |||
| None | 25 (11) | 36.3 (0–79.57) | |||
| Adjuvant endocrine Therapy | 0.748 | ||||
| Tamoxifen | 57 (26) | 52.3 (29.6–75.1) | |||
| Aromatase inhibitors | 106 (51) | 42.8 (26.9–58.8) | |||
| Disease-free interval % (n/N) | 0.993 | ||||
| Newly metastatic disease | 32 (16) | 47.9 (16.6–79.7) | |||
| ≤24 months | 40 (18) | 46.5 (24.3–68.7) | |||
| >24 months | 91 (43) | 44.9 (30.2–59.7) | |||
| Number of metastases % (n/N) | 0.010 | 1.129 (0.879–1.449) | 0.341 | ||
| 1 place | 105 (47) | 53.5 (34.9–72.1) | |||
| 2 places | 36 (22) | 34.4 (30.0–47.8) | |||
| ≥3 places | 12 (6) | 26.4 (9.5–43.3) | |||
| CNS involvement * | 10 (2) | X | |||
| Disease site % (n/N) | 0.068 | ||||
| Visceral | 84 (40) | 40.4 (21.4–59.4) | |||
| Non-visceral | 16 (3) | X | |||
| Bone | 63 (34) | 44.4 (37.3–52.6) | |||
| Treatment | 0.122 | 1.262 (0.947–1.683) | 0.112 | ||
| XELIA | 95 (45) | 53.5 (30.3–76.6) | |||
| Hormone therapy | 35 (15) | 47.9 (24.6–71.2) | |||
| Chemotherapy | 33 (17) | 40.4 (34.7–61.3) |
| Total (Events) | Median | p-Value | HR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| (95% CI) | |||||
| ECOG PS | |||||
| 0–1 | 136 (108) | 18.7 (12.3–25.1) | 0.215 | ||
| ≥2 | 27 (19) | 36.1 (16.9–55.4) | |||
| Hormonal status | |||||
| Pre- or peri-menopausal | 54 (44) | 16.3 (8.0–24.6) | 0.082 | ||
| Post-menopausal | 109 (83) | 22.5 (14.6–30.5) | |||
| Tumor status | |||||
| ≤T2 | 62 (44) | 28.0 (16.9–39.2) | 0.134 | ||
| T3 | 40 (34) | 18.7 (12.6–24.7) | |||
| T4 | 61 (49) | 19.0 (10.5–27.6) | |||
| Nodal status | |||||
| N0 | 24 (16) | 27.9 (13.0–42.7) | 0.184 | ||
| N1 | 59 (45) | 24.9 (18.5–31.3) | |||
| N2 | 51 (43) | 12.9 (7.2–18.7) | |||
| N3 | 29 (23) | 16.9 (5.9–27.9) | |||
| Disease state | |||||
| Recurrent disease | 131 (104) | 16.7 (10.2–23.2) | 0.039 | 0.805 (0.507–1.281) | 0.361 |
| Metastatic disease | 32 (23) | 31.1 (20.9–41.2) | |||
| Histological subtype | |||||
| IDC | 135 (104) | 20.9 (14.0–27.8) | 0.992 | ||
| ILC | 28 (23) | 20.0 (8.1–32.0) | |||
| Ki67 | |||||
| <20 | 46 (35) | 23.7 (10.1–37.2) | 0.428 | ||
| ≥20 | 50 (36) | 12.9 (4.7–20.2) | |||
| SBR | |||||
| Low | 13 (11) | 26.7 (15.3–38.0) | 0.731 | ||
| Intermediate and high | 130 (102) | 20.0 (14.9–25.1) | |||
| ER | |||||
| Negative | 7 (5) | 12.9 (0.1–25.8) | 0.952 | ||
| Positive | 156 (122) | 20.9 (14.9–27.2) | |||
| PgR | |||||
| Negative | 32 (21) | 19.1 (12.2–26.0) | 0.998 | ||
| Positive | 131 (106) | 20.9 (13.9–27.9) | |||
| ER/PgR | |||||
| ER+, PgR+ | 124 (101) | 20.9 (13.5–28.4) | 0.729 | ||
| ER+, PgR− | 32 (21) | 19.1 (12.2–26.0) | |||
| ER−, PgR+ | 7 (5) | 12.9 (0.1–25.8) | |||
| Type of surgery | |||||
| BCS | 17 (11) | 22.3 (0.0–60.60) | 0.731 | ||
| MRM | 112 (92) | 16.6 (10.9–22.2) | |||
| Adjuvant endocrine therapy | |||||
| Tamoxifen | 56 (48) | 19.9 (9.1–30.6) | 0.776 | ||
| Aromatase inhibitors | 75 (56) | 16.7 (10.7–22.7) | |||
| Disease-free interval % (n/N) | |||||
| Newly metastatic disease | 32 (23) | 31.0 (20.9–41.2) | 0.016 | 1.069 (0.690–1.657) | 0.765 |
| ≤24 months | 40 (30) | 10.5 (6.7–14.3) | |||
| >24 months | 91 (74) | 19.9 (13.8–26.1) | |||
| Number of metastases % (n/N) | |||||
| 1 place | 105 (78) | 25.2 (16.4–34.1) | 0.016 | 1.494 (1.051–2.125) | 0.025 |
| 2 places | 36 (32) | 16.3 (8.9–23.7) | |||
| ≥3 places | 12 (12) | 8.5 (4.6–12.4) | |||
| CNS involvement * | 10 (5) | 19.0 (0.0–60.9) | |||
| Disease site % (n/N) | |||||
| Visceral | 84 (69) | 13.9 (9.4–18.3) | 0.037 | 1.430 (1.070–1.912) | 0.016 |
| Non-visceral | 16 (10) | 11.0 (0–49.7) | |||
| Bone | 63 (48) | 29.4 (20.2–38.6) | |||
| Treatment | |||||
| XELIA | 95 (70) | 29.37 (20.91–37.84) | <0.001 | 1.669 (1.330–2.094) | <0.001 |
| Hormone therapy | 35(30) | 20.04 (7.29–32.79) | |||
| Best Objective Response | XELIA (n = 95) n (%) | HT (n = 35) n (%) | CTx (n = 33) n (%) | p-Value |
|---|---|---|---|---|
| Complete response | 10 (10.5) | 2 (5.7) | 0 (0.0) | 0.125 |
| Partial response | 18 (18.9) | 3 (8.6) | 3 (9.1) | 0.198 |
| Stable disease | 45 (47.4) | 18 (51.4) | 22 (66.7) | 0.160 |
| Progressive disease | 22 (23.2) | 12 (34.3) | 8 (24.2) | 0.426 |
| Overall response rate (PR + CR) | 28 (29.5) | 5 (14.3) | 3 (9.1) | 0.024 |
| Disease control rate (PR + CR + SD) | 73 (76.8) | 23 (65.7) | 25 (75.8) | 0.426 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado-Miranda, A.; Lara-Medina, F.U.; Muñoz-Montaño, W.R.; Zinser-Sierra, J.W.; Galeana, P.A.C.; Garza, C.V.; Sanchez Benitez, D.; Limón Rodríguez, J.A.; Arce Salinas, C.H.; Guijosa, A.; et al. Capecitabine Plus Aromatase Inhibitor as First Line Therapy for Hormone Receptor Positive, HER2 Negative Metastatic Breast Cancer. Curr. Oncol. 2023, 30, 6097-6110. https://doi.org/10.3390/curroncol30070454
Alvarado-Miranda A, Lara-Medina FU, Muñoz-Montaño WR, Zinser-Sierra JW, Galeana PAC, Garza CV, Sanchez Benitez D, Limón Rodríguez JA, Arce Salinas CH, Guijosa A, et al. Capecitabine Plus Aromatase Inhibitor as First Line Therapy for Hormone Receptor Positive, HER2 Negative Metastatic Breast Cancer. Current Oncology. 2023; 30(7):6097-6110. https://doi.org/10.3390/curroncol30070454
Chicago/Turabian StyleAlvarado-Miranda, Alberto, Fernando Ulises Lara-Medina, Wendy R. Muñoz-Montaño, Juan W. Zinser-Sierra, Paula Anel Cabrera Galeana, Cynthia Villarreal Garza, Daniel Sanchez Benitez, Jesús Alberto Limón Rodríguez, Claudia Haydee Arce Salinas, Alberto Guijosa, and et al. 2023. "Capecitabine Plus Aromatase Inhibitor as First Line Therapy for Hormone Receptor Positive, HER2 Negative Metastatic Breast Cancer" Current Oncology 30, no. 7: 6097-6110. https://doi.org/10.3390/curroncol30070454
APA StyleAlvarado-Miranda, A., Lara-Medina, F. U., Muñoz-Montaño, W. R., Zinser-Sierra, J. W., Galeana, P. A. C., Garza, C. V., Sanchez Benitez, D., Limón Rodríguez, J. A., Arce Salinas, C. H., Guijosa, A., & Arrieta, O. (2023). Capecitabine Plus Aromatase Inhibitor as First Line Therapy for Hormone Receptor Positive, HER2 Negative Metastatic Breast Cancer. Current Oncology, 30(7), 6097-6110. https://doi.org/10.3390/curroncol30070454





