Robotic Lobectomy without Complete Fissure for Non-Small Cell Lung Cancer: Technical Aspects and Perioperative Outcomes of the Tunnel Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
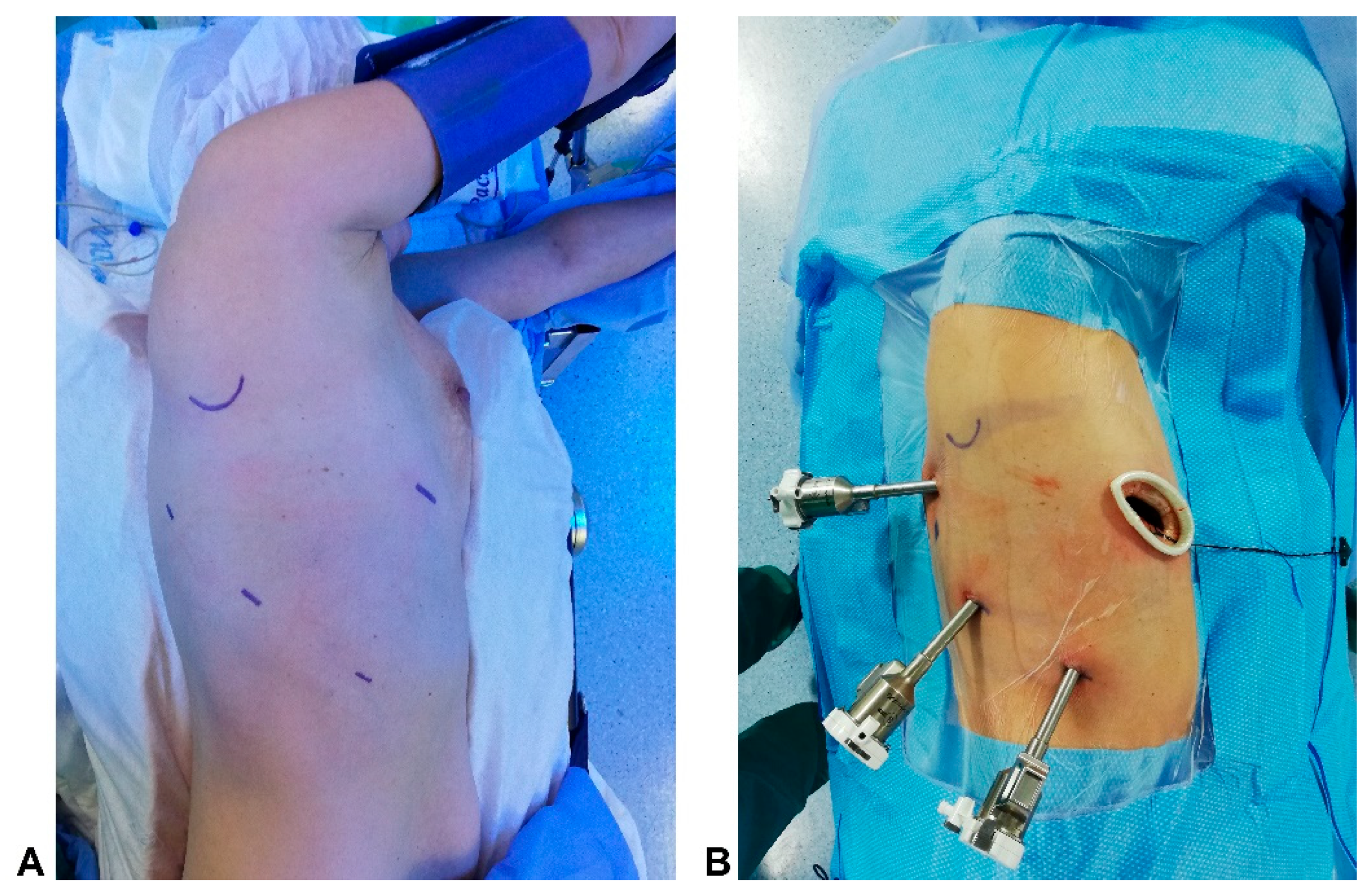
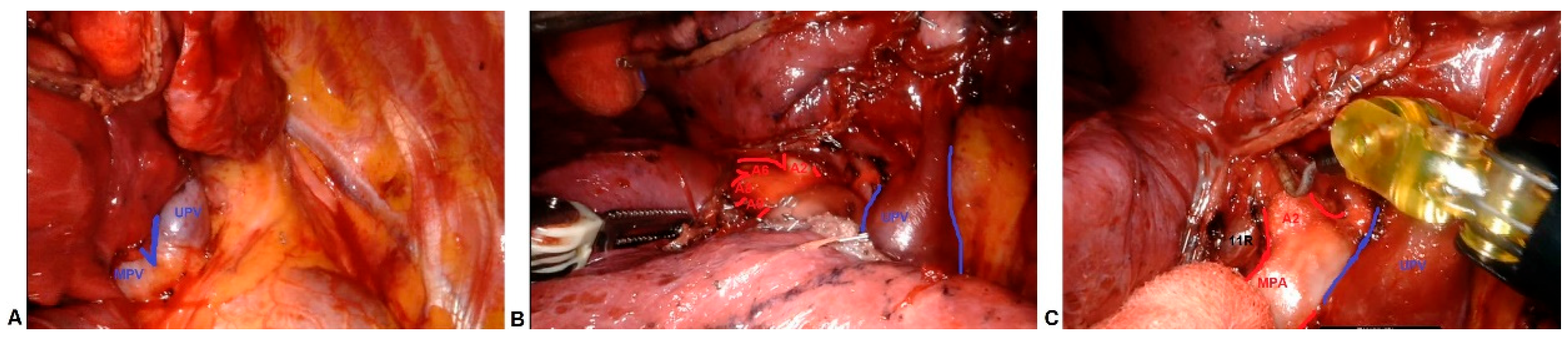
2.2. Technique
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spaggiari, L.; Sedda, G.; Maisonneuve, P.; Tessitore, A.; Casiraghi, M.; Petrella, F.; Galetta, D. A Brief Report on Survival after Robotic Lobectomy for Early-Stage Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2019, 14, 2176–2180. [Google Scholar] [CrossRef] [PubMed]
- Tajè, R.; Gallina, F.T.; Forcella, D.; Alessandrini, G.; Papale, M.; Sardellitti, F.; Pierconti, F.; Coccia, C.; Ambrogi, V.; Facciolo, F.; et al. Multimodal evaluation of locoregional anaesthesia efficacy on postoperative pain after robotic pulmonary lobectomy for NSCLC: A pilot study. J. Robot. Surg. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Gallina, F.T.; Tajè, R.; Forcella, D.; Gennari, V.; Visca, P.; Pierconti, F.; Coccia, C.; Cappuzzo, F.; Sperduti, I.; Facciolo, F.; et al. Perioperative outcomes of robotic lobectomy for early-stage non-small cell lung cancer in elderly patients. Front. Oncol. 2022, 12, 1055418. [Google Scholar] [CrossRef] [PubMed]
- Gallina, F.T.; Tajè, R.; Forcella, D.; Corzani, F.; Cerasoli, V.; Visca, P.; Coccia, C.; Pierconti, F.; Sperduti, I.; Cecere, F.L.; et al. Oncological Outcomes of Robotic Lobectomy and Radical Lymphadenectomy for Early-Stage Non-Small Cell Lung Cancer. J. Clin. Med. 2022, 11, 2173. [Google Scholar] [CrossRef] [PubMed]
- Bille, A.; Woo, K.M.; Ahmad, U.; Rizk, N.P.; Jones, D.R. Incidence of occult pN2 disease following resection and mediastinal lymph node dissection in clinical stage I lung cancer patients. Eur. J. Cardiothorac. Surg. Off. J. Eur. Assoc. Cardiothorac. Surg. 2017, 51, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Decaluwe, H.; Sokolow, Y.; Deryck, F.; Stanzi, A.; Depypere, L.; Moons, J.; Van Raemdonck, D.; De Leyn, P. Thoracoscopic tunnel technique for anatomical lung resections: A ‘fissure first, hilum last’ approach with staplers in the fissureless patient. Interact. Thorac. Surg. 2015, 21, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Decaluwé, H. Video-assisted thoracic surgery tunnel technique: An alternative fissureless approach for anatomical lung resections. Video-Assist. Thorac. Surg. 2017, 2, 45. [Google Scholar] [CrossRef]
- Stamenovic, D.; Bostanci, K.; Messerschmidt, A.; Jahn, T.; Schneider, T. Fissureless fissure-last video-assisted thoracoscopic lobectomy for all lung lobes: A better alternative to decrease the incidence of prolonged air leak? Eur. J. Cardiothorac. Surg. 2016, 50, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Zhu, Z.H.; Yan, T.D.; Wang, Q.; Jiang, G.; Liu, L.; Liu, D.; Wang, Z.; Shao, W.; Black, D.; et al. Video-assisted thoracic surgery versus open thoracotomy for non-small-cell lung cancer: A propensity score analysis based on a multi-institutional registry. Eur. J. Cardiothorac. Surg. 2013, 44, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Voltolini, L.; Bongiolatti, S.; Gonfiotti, A. Fissureless fissure-last video assisted thoracoscopic lobectomy: Always? Never? Sometimes. J. Thorac. Dis. 2018, 10 (Suppl. 26), S3135–S3137. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, J.; Mantovani, S.; Dolciami, M.; Bassi, M.; Venuta, F.; Anile, M. Fissureless Technique Might Prevent the Middle Lobe Impairment After Right Upper Lobectomy. Surg. Innov. 2021, 28, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.G.; Chansky, K.; Van Schil, P.; Nicholson, A.G.; Boubia, S.; Brambilla, E.; Donington, J.; Galateau-Sallé, F.; Hoffmann, H.; Infante, M.; et al. The IASLC Lung Cancer Staging Project: Analysis of Resection Margin Status and Proposals for Residual Tumor Descriptors for Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2020, 15, 344–359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, Q.; Huang, Y.; Ouyang, L.; Luo, F. Updated Evaluation of Robotic- and Video-Assisted Thoracoscopic Lobectomy or Segmentectomy for Lung Cancer: A Systematic Review and Meta-Analysis. Front Oncol. 2022, 12, 853530. [Google Scholar] [CrossRef] [PubMed]
- Licht, P.B.; Jorgensen, O.D.; Ladegaard, L. Is Video-assisted Thoracoscopic Lobectomy a Superior Approach to Open Lobectomy in the Treatment of Lung Cancer? Ann. Thorac. Surg. 2020, 109, 1633–1639. [Google Scholar]
- Gossot, D.; Boddaert, G.; Mariolo, A.V.; Seguin-Givelet, A. Sublobar resection for early-stage lung cancer: The issue of nodal upstaging. Eur. J. Cardiothorac. Surg. 2022, 62, ezac481. [Google Scholar] [CrossRef] [PubMed]
- Chiappetta, M.; Leuzzi, G.; Sperduti, I.; Bria, E.; Mucilli, F.; Lococo, F.; Spaggiari, L.; Ratto, G.B.; Filosso, P.L.; Facciolo, F. Lymph-node ratio predicts survival among the different stages of non-small-cell lung cancer: A multicentre analysis. Eur. J. Cardiothorac. Surg. 2019, 55, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Gallina, F.T.; Melis, E.; Forcella, D.; Mercadante, E.; Marinelli, D.; Ceddia, S.; Cappuzzo, F.; Vari, S.; Cecere, F.L.; Caterino, M.; et al. Nodal Upstaging Evaluation After Robotic-Assisted Lobectomy for Early-Stage Non-small Cell Lung Cancer Compared to Video-Assisted Thoracic Surgery and Thoracotomy: A Retrospective Single Center Analysis. Front. Surg. 2021, 8, 666158. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, G.; Abbas, A.E.S.; Muriana, P.; Lembo, R.; Bottoni, E.; Perroni, G.; Testori, A.; Dieci, E.; Bakhos, C.T.; Car, S.; et al. Perioperative Outcome of Robotic Approach Versus Manual Videothoracoscopic Major Resection in Patients Affected by Early Lung Cancer: Results of a Randomized Multicentric Study (ROMAN Study). Front. Oncol. 2021, 11, 726408. [Google Scholar] [CrossRef] [PubMed]
- Zirafa, C.; Aprile, V.; Ricciardi, S.; Romano, G.; Davini, F.; Cavaliere, I.; Alì, G.; Fontanini, G.; Melfi, F. Nodal upstaging evaluation in NSCLC patients treated by robotic lobectomy. Surg. Endosc. 2019, 33, 153–158. [Google Scholar] [CrossRef] [PubMed]
| Variables | RATS | VATS | p-Value |
|---|---|---|---|
| Age (years) | 0.3 | ||
| Median (range) | 63.50 (47–79) | 67.30 (49–78) | |
| Smoking history | 0.5 | ||
| Yes (%) | 22 (73.3) | 24 (80) | |
| No (%) | 8 (26.7) | 6 (20) | |
| Comorbidities | 0.2 | ||
| COPD (%) | 14 | 12 | |
| Cardiac diseases (%) | 8 | 5 | |
| Diabetes mellitus (%) | 3 | 2 | |
| Other cancers (%) | 1 | 0 | |
| Other disease (%) | 5 | 7 | |
| No (%) | 19 | 9 | |
| Duration of Surgery (min.) | 0.1 | ||
| Median (range) | 147 (109–174) | 131 (113–189) | |
| Daily drainage (mL) | 0.09 | ||
| Median (range) | 182 (142–239) | 171 (134–234) | |
| Median hospital stay (days) | 0.6 | ||
| Nr (range) | 5 (4–8) | 5 (4–7) | |
| Postoperative morbidity rate | 0.5 | ||
| Nr (%) | 5 (16.7) | 6 (20) | |
| Postoperative complication | |||
| Air leaks (%) | 4 (8.8) | 3 (6.7) | |
| Pneumonia (%) | - | 1 (2.2) | |
| Arrythmia (%) | 3 (6.6) | 2 (4.4) | |
| Others (%) | - | 2 (4.4) | |
| Histology | 0.6 | ||
| Adenocarcinoma (%) | 40 (88.8) | 39 (86.6) | |
| Squamous (%) | 6 (13.3) | 5 (11.1) | |
| pT | 0.5 | ||
| T1 (%) | 27 (60.0) | 26 (57.7) | |
| T2 (%) | 15 (33.3) | 14 (31.1) | |
| T3 (%) | 3 (6.6) | 5 (11.1) | |
| pN | 0.2 | ||
| N0 | 36 (80.0) | 39 (86.7) | |
| N1 | 8 (17.8) | 6 (13.3) | |
| N2 | 1 (3.3) | 0 (0) | |
| Hilar Lymph nodes resected | 0.04 | ||
| Median (range) | 7 (4–15) | 4 (2–10) | |
| Station 10 | 3 (2–4) | 3 (2–4) | 0.2 |
| Station 11 | 5 (2–6) | 3 (1–4) | 0.07 |
| Station 12 | 4 (1–6) | 1 (1–2) | 0.03 |
| Hilar Upstaging rate | |||
| Nr (%) | 6 (13.3) | 2 (4.4) | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallina, F.T.; Forcella, D.; Melis, E.; Facciolo, F. Robotic Lobectomy without Complete Fissure for Non-Small Cell Lung Cancer: Technical Aspects and Perioperative Outcomes of the Tunnel Technique. Curr. Oncol. 2023, 30, 5898-5905. https://doi.org/10.3390/curroncol30060441
Gallina FT, Forcella D, Melis E, Facciolo F. Robotic Lobectomy without Complete Fissure for Non-Small Cell Lung Cancer: Technical Aspects and Perioperative Outcomes of the Tunnel Technique. Current Oncology. 2023; 30(6):5898-5905. https://doi.org/10.3390/curroncol30060441
Chicago/Turabian StyleGallina, Filippo Tommaso, Daniele Forcella, Enrico Melis, and Francesco Facciolo. 2023. "Robotic Lobectomy without Complete Fissure for Non-Small Cell Lung Cancer: Technical Aspects and Perioperative Outcomes of the Tunnel Technique" Current Oncology 30, no. 6: 5898-5905. https://doi.org/10.3390/curroncol30060441
APA StyleGallina, F. T., Forcella, D., Melis, E., & Facciolo, F. (2023). Robotic Lobectomy without Complete Fissure for Non-Small Cell Lung Cancer: Technical Aspects and Perioperative Outcomes of the Tunnel Technique. Current Oncology, 30(6), 5898-5905. https://doi.org/10.3390/curroncol30060441






