Developments in Checkpoint Inhibitor Therapy for the Management of Deficient Mismatch Repair (dMMR) Rectal Cancer
Abstract
1. Introduction
2. Epidemiology and Biology
3. Current Evidence for ICI in Non-Metastatic dMMR Colorectal Cancer
4. Current Management of dMMR Rectal Cancer
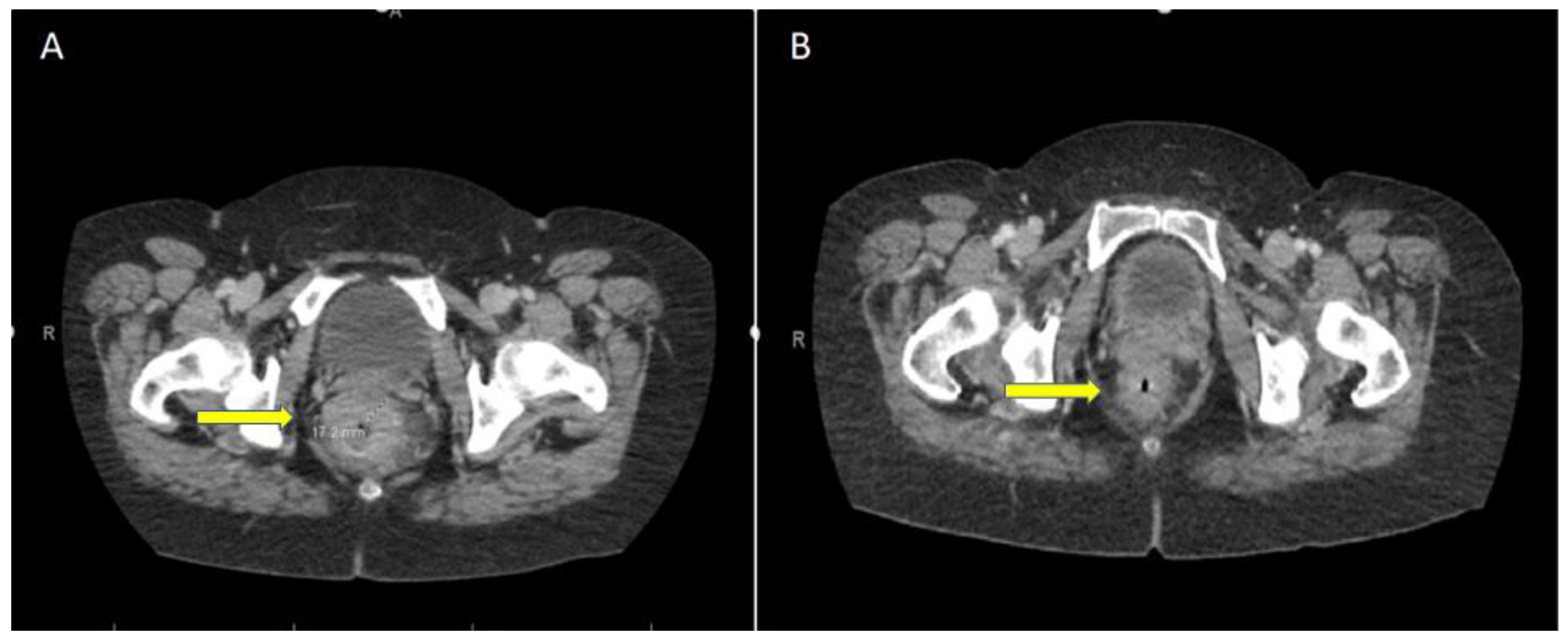
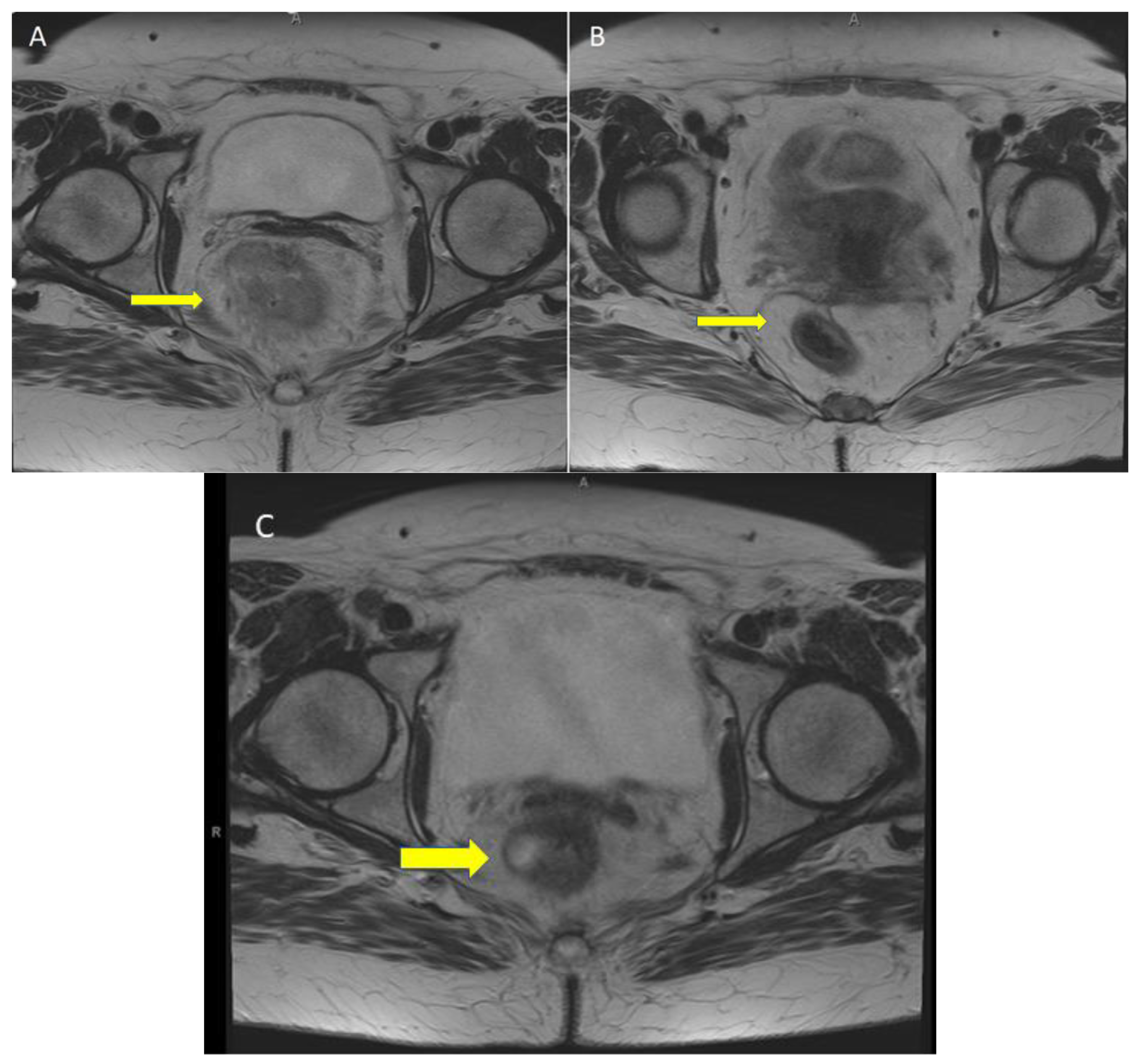
5. Case Report: ICI with Total Remission in dMMR laRC after a Single Cycle
6. ICI Toxicity Management
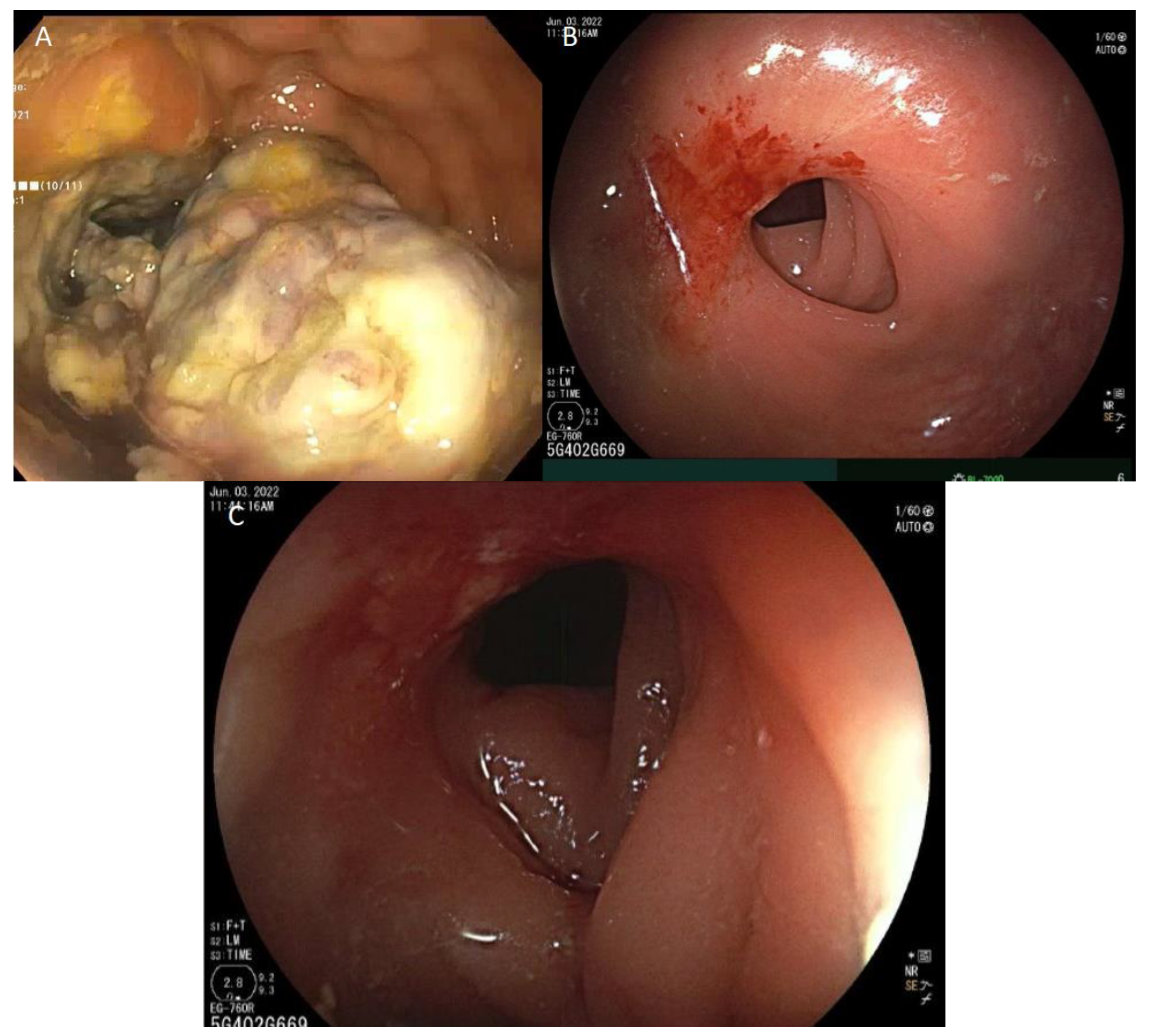
7. Response Assessment and Management of Immunotherapy-Related Rectal Stricture
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Vasen, H.F.; Blanco, I.; Aktan-Collan, K.; Gopie, J.P.; Alonso, A.; Aretz, S.; Bernstein, I.; Bertario, L.; Burn, J.; Capella, G.; et al. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): Recommendations by a group of European experts. Gut 2013, 62, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Fornaro, L.; Caparello, C.; Vivaldi, C.; Rotella, V.; Musettini, G.; Falcone, A.; Baldini, E.; Masi, G. Bevacizumab in the pre-operative treatment of locally advanced rectal cancer: A systematic review. World J. Gastroenterol. 2014, 20, 6081–6091. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Zhou, Y.; Cui, W.; Su, X.; Guo, Z.; Hidasa, I.; Li, Q.; Wang, Z.; Song, Y. The Addition of EGFR Inhibitors in Neoadjuvant Therapy for KRAS-Wild Type Locally Advanced Rectal Cancer Patients: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 706. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network NCCN Guidelines: Colon Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 10 September 2021).
- Dossa, F.; Chesney, T.R.; Acuna, S.A.; Baxter, N.N. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 501–513. [Google Scholar] [CrossRef]
- Papke, D.J., Jr.; Yurgelunm, M.B.; Noffsinger, A.E.; Turner, K.O.; Genta, R.M.; Redston, M. Prevalence of Mismatch-Repair Deficiency in Rectal Adenocarcinomas. N. Engl. J. Med. 2022, 387, 1714–1716. [Google Scholar] [CrossRef]
- Cercek, A.; Dos Santos Fernandes, G.; Roxburgh, C.S.; Ganesh, K.; Ng, S.; Sanchez-Vega, F.; Yaeger, R.; Segal, N.H.; Reidy-Lagunes, D.L.; Varghese, A.M.; et al. Mismatch Repair-Deficient Rectal Cancer and Resistance to Neoadjuvant Chemotherapy. Clin. Cancer Res. 2020, 26, 3271–3279. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef] [PubMed]
- Borelli, B.; Antoniotti, C.; Carullo, M.; Germani, M.M.; Conca, V.; Masi, G. Immune-Checkpoint Inhibitors (ICIs) in Metastatic Colorectal Cancer (mCRC) Patients beyond Microsatellite Instability. Cancers 2022, 14, 4974. [Google Scholar] [CrossRef]
- Boukouris, A.E.; Theochari, M.; Stefanou, D.; Papalambros, A.; Felekouras, E.; Gogas, H.; Ziogas, D.C. Latest evidence on immune checkpoint inhibitors in metastatic colorectal cancer: A 2022 update. Crit. Rev. Oncol. Hematol. 2022, 173, 103663. [Google Scholar] [CrossRef]
- Mas-Ponte, D.; McCullough, M.; Supek, F. Spectrum of DNA mismatch repair failures viewed through the lens of cancer genomics and implications for therapy. Clin. Sci. 2022, 136, 383–404. [Google Scholar] [CrossRef]
- Koopman, M.; Kortman, G.A.; Mekenkamp, L.; Ligtenberg, M.J.; Hoogerbrugge, N.; Antonini, N.F.; Punt, C.J.; van Krieken, J.H. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br. J. Cancer 2009, 100, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Baran, B.; Mert Ozupek, N.; Yerli Tetik, N.; Acar, E.; Bekcioglu, O.; Baskin, Y. Difference Between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterol. Res. 2018, 11, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Ashktorab, H.; Ahuja, S.; Kannan, L.; Llor, X.; Ellis, N.A.; Xicola, R.M.; Laiyemo, A.O.; Carethers, J.M.; Brim, H.; Nouraie, M. A meta-analysis of MSI frequency and race in colorectal cancer. Oncotarget 2016, 7, 34546–34557. [Google Scholar] [CrossRef] [PubMed]
- Barrow, E.; Robinson, L.; Alduaij, W.; Shenton, A.; Clancy, T.; Lalloo, F.; Hill, J.; Evans, D.G. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: A report of 121 families with proven mutations. Clin. Genet. 2009, 75, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Barnetson, R.A.; Tenesa, A.; Farrington, S.M.; Nicholl, I.D.; Cetnarskyj, R.; Porteous, M.E.; Campbell, H.; Dunlop, M.G. Identification and survival of carriers of mutations in DNA mismatch-repair genes in colon cancer. N. Engl. J. Med. 2006, 354, 2751–2763. [Google Scholar] [CrossRef] [PubMed]
- Peltomäki, P. Role of DNA mismatch repair defects in the pathogenesis of human cancer. J. Clin. Oncol. 2003, 21, 1174–1179. [Google Scholar] [CrossRef]
- Kim, J.K.; Chen, C.T.; Keshinro, A.; Khan, A.; Firat, C.; Vanderbilt, C.; Segal, N.; Stadler, Z.; Shia, J.; Balachandran, V.P.; et al. Intratumoral T-cell repertoires in DNA mismatch repair-proficient and -deficient colon tumors containing high or low numbers of tumor-infiltrating lymphocytes. Oncoimmunology 2022, 11, 2054757. [Google Scholar] [CrossRef]
- Karahan, B.; Argon, A.; Yıldırım, M.; Vardar, E. Relationship between MLH-1, MSH-2, PMS-2,MSH-6 expression and clinicopathological features in colorectal cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 4044–4053. [Google Scholar]
- Popat, S.; Houlston, R.S. A systematic review and meta-analysis of the relationship between chromosome 18q genotype, DCC status and colorectal cancer prognosis. Eur. J. Cancer 2005, 41, 2060–2070. [Google Scholar] [CrossRef]
- Gryfe, R.; Kim, H.; Hsieh, E.T.; Aronson, M.D.; Holowaty, E.J.; Bull, S.B.; Redston, M.; Gallinger, S. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N. Engl. J. Med. 2000, 342, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef]
- Vilar, E.; Gruber, S. Microsatellite instability in colorectal cancer—The stable evidence. Nat. Rev. Clin. Oncol. 2010, 7, 153–162. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, J.; Deng, Y.; Wang, H. Complete response in patients with locally advanced rectal cancer after neoadjuvant treatment with nivolumab. Oncoimmunology 2019, 8, e1663108. [Google Scholar] [CrossRef] [PubMed]
- Bando, H.; Tsukada, Y.; Inamori, K.; Togashi, Y.; Koyama, S.; Kotani, D.; Fukuoka, S.; Yuki, S.; Komatsu, Y.; Homma, S.; et al. Preoperative Chemoradiotherapy plus Nivolumab before Surgery in Patients with Microsatellite Stable and Microsatellite Instability-High Locally Advanced Rectal Cancer. Clin. Cancer Res. 2022, 28, 1136–1146. [Google Scholar] [CrossRef]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant Immunotherapy Leads to Pathological Responses in MMR-Proficient and MMR-Deficient Early-Stage Colon Cancers. Nat. Med. 2020, 26, 566–576. [Google Scholar] [CrossRef]
- Demisse, R.; Damle, N.; Kim, E.; Gong, J.; Fakih, M.; Eng, C.; Oesterich, L.; McKenny, M.; Ji, J.; Liu, J.; et al. Neoadjuvant Immunotherapy-Based Systemic Treatment in MMR-Deficient or MSI-High Rectal Cancer: Case Series. J. Natl. Compr. Cancer Netw. 2020, 18, 798–804. [Google Scholar] [CrossRef]
- Liu, D.X.; Li, D.D.; He, W.; Ke, C.F.; Jiang, W.; Tang, J.H.; Kong, L.H.; Li, Y.; Sui, Q.Q.; Xiao, B.Y.; et al. PD-1 blockade in neoadjuvant setting of DNA mismatch repair-deficient/microsatellite instability-high colorectal cancer. Oncoimmunology 2020, 9, 1711650. [Google Scholar] [CrossRef]
- Avallone, A.; De Stefano, A.; Pace, U.; Catteau, A.; Di Gennaro, E.; Tatangelo, F.; Boquet, I.; Cassata, A.; Costantini, S.; De Franciscis, S.; et al. 491p neoadjuvant nivolumabin early stage colorectal cancer. Ann. Oncol. 2020, 31, S449. [Google Scholar] [CrossRef]
- Lin, Z.; Cai, M.; Zhang, P.; Li, G.; Liu, T.; Li, X.; Cai, K.; Nie, X.; Tao, K.; Zhang, T. Short-Course Radiotherapy and Subsequent CAPOX Plus Camrelizumab Followed by Delayed Surgery for Locally Advanced Rectal Cancer:Short-Term Results of a Phase II Trial. J. Clin. Oncol. 2021, 39 (Suppl. 3), 63. [Google Scholar] [CrossRef]
- Salvatore, L.; Bensi, M.; Corallo, S.; Bergamo, F.; Tortora, G.; Pellegrini, I.; Rasola, C.; Borelli, B.; Tamburini, E.; Randon, G.; et al. Phase II Study of Preoperative (PREOP) Chemoradiotherapy (CTRT) Plus Avelumab (AVE) in Patients (PTS) With Locally Advanced Rectal Cancer(LARC): The AVANA Study. J. Clin. Oncol. 2021, 39 (Suppl. 15), 3511. [Google Scholar] [CrossRef]
- Hu, H.; Kang, L.; Zhang, J.; Wu, Z.; Wang, H.; Huang, M.; Lan, P.; Wu, X.; Wang, C.; Cao, W.; et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): A single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 2022, 7, 38–48. [Google Scholar] [CrossRef]
- Ludford, K.; Ho, W.J.; Thomas, J.V.; Raghav, K.P.S.; Murphy, M.B.; Fleming, N.D.; Lee, M.S.; Smaglo, B.G.; You, Y.N.; Tillman, M.M.; et al. Neoadjuvant Pembrolizumab in Localized Microsatellite Instability High/Deficient Mismatch Repair Solid Tumors. J. Clin. Oncol. 2023, 20, 138. [Google Scholar] [CrossRef] [PubMed]
- Azcue, P.; Encío, I.; Guerrero Setas, D.; Suarez Alecha, J.; Galbete, A.; Mercado, M.; Vera, R.; Gomez-Dorronsoro, M.L. PD-L1 as a Prognostic Factor in Early-Stage Colon Carcinoma within the Immunohistochemical Molecular Subtype Classification. Cancers 2021, 13, 1943. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, Y.; Wu, Z.; Zeng, F.; Song, B.; Zhang, Y.; Li, J.; Lui, S.; Wu, M. Tumor Mutational Burden Predicting the Efficacy of Immune Checkpoint Inhibitors in Colorectal Cancer: A Systematic Review and Meta-Analysis. Front Immunol. 2021, 12, 751407. [Google Scholar] [CrossRef]
- Westcott, P.M.K.; Sacks, N.J.; Schenkel, J.M.; Ely, Z.A.; Smith, O.; Hauck, H.; Jaeger, A.M.; Zhang, D.; Backlund, C.M.; Beytagh, M.C.; et al. Low neoantigen expression and poor T-cell priming underlie early immune escape in colorectal cancer. Nat. Cancer 2021, 2, 1071–1085. [Google Scholar] [CrossRef]
- Idos, G.E.; Kwok, J.; Bonthala, N.; Kysh, L.; Gruber, S.B.; Qu, C. The Prognostic Implications of Tumor Infiltrating Lymphocytes in Colorectal Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 3360. [Google Scholar] [CrossRef]
- Bruni, D.; Angell, H.K.; Galon, J. The Immune Contexture and Immunoscore in Cancer Prognosis and Therapeutic Efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Z.; Zhang, W.; Zhang, W.; Buzdin, A.; Mu, X.; Yan, Q.; Zhao, X.; Chang, H.H.; Duhon, M.; et al. FDA-Approved and Emerging Next Generation Predictive Biomarkers for Immune Checkpoint Inhibitors in Cancer Patients. Front. Oncol. 2021, 11, 683419. [Google Scholar] [CrossRef] [PubMed]
- de Rosa, N.; Rodriguez-Bigas, M.A.; Chang, G.J.; Veerapong, J.; Borras, E.; Krishnan, S.; Bednarski, B.; Messick, C.A.; Skibber, J.M.; Feig, B.W.; et al. DNA Mismatch Repair Deficiency in Rectal Cancer: Benchmarking Its Impact on Prognosis, Neoadjuvant Response Prediction, and Clinical Cancer Genetics. J. Clin. Oncol. 2016, 34, 3039–3046. [Google Scholar] [CrossRef]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.J.; Nanavaty, N.S.; Devanaboyina, M.; Stanbery, L.; Hamouda, D.; Edelman, G.; Dworkin, L.; Nemunaitis, J.J. The abscopal effect of radiation therapy. Future Oncol. 2021, 17, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Ebner, D.K.; Kamada, T.; Yamada, S. Abscopal effect in recurrent colorectal cancer treated with carbon-ion radiation therapy: 2 case reports. Adv. Radiat. Oncol. 2017, 2, 333–338. [Google Scholar] [CrossRef]
- George, T.; Yothers, G.; Rahma, O.; Hong, T.; Russell, M.; You, N.; Parker, W.; Jacobs, S.; Lucas, P.; Colangelo, L.; et al. Long-term results from NRG-GI002: A phase II clinical trial platform using total neoadjuvant therapy (TNT) in locally ad-vanced rectal cancer (LARC). J. Clin. Oncol. 2023, 41, 7. [Google Scholar] [CrossRef]
- Jain, V.; Remley, W.; Bunag, C.; Elfasi, A.; Chuquilin, M. Rituximab in Refractory Myositis and Acute Neuropathy Secondary to Checkpoint Inhibitor Therapy. Cureus 2022, 14, e25129. [Google Scholar] [CrossRef] [PubMed]
- Nassri, A.B.; Muenyi, V.; AlKhasawneh, A.; Ribeiro, B.S.; Scolapio, J.S.; Malespin, M.; de Melo, S.W., Jr. Ipilimumab and Nivolumab induced steroid-refractory colitis treated with infliximab: A case report. World J. Gastrointest. Pharmacol. Ther. 2019, 10, 29–34. [Google Scholar] [CrossRef]
- Stroud, C.R.; Hegde, A.; Cherry, C.; Naqash, A.R.; Sharma, N.; Addepalli, S.; Cherukuri, S.; Parent, T.; Hardin, J.; Walker, P. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J. Oncol. Pharm. Pract. 2019, 25, 551–557. [Google Scholar] [CrossRef]
- Burla, J.; Bluemel, S.; Biedermann, L.; Barysch, M.J.; Dummer, R.; Levesque, M.P.; Gubler, C.; Morell, B.; Rogler, G.; Scharl, M. Retrospective Analysis of Treatment and Complications of Immune Checkpoint Inhibitor-Associated Colitis: Histological Ulcerations as Potential Predictor for a Steroid-Refractory Disease Course. Inflamm. Intestig. Dis. 2020, 5, 109–116. [Google Scholar] [CrossRef]
- Somekawa, K.; Horita, N.; Kaneko, A.; Tagami, Y.; Fukuda, N.; Matsumoto, H.; Namkoong, H.; Fujiwara, Y.; Minegishi, K.; Fukumoto, T.; et al. Adverse events induced by nivolumab and ipilimumab combination regimens. Ther. Adv. Med. Oncol. 2022, 14, 17588359211058393. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, Y.; Liu, Z.; Lin, S.; Tan, J.; He, J.; Hu, F.; Wu, X.; Ghosh, S.; Chen, M.; et al. Intestinal strictures in Crohn’s disease: A 2021 update. Ther. Adv. Gastroenterol. 2022, 15, 17562848221104951. [Google Scholar] [CrossRef] [PubMed]
- Laurain, P.-A.; Guillo, L.; D’Amico, F.; Netter, P.; Danese, S.; Baumann, C.; Luc, A.; Clerc-Urmes, I.; Sofos, S.; Peyrin-Biroulet, L. Incidence of and risk factors for colorectal strictures in ulcerative colitis: A multicenter study. Clin. Gastroenterol. Hepatol. 2021, 19, 1899–1905.e1. [Google Scholar] [CrossRef] [PubMed]
- Kähler, K.C.; Eigentler, T.K.; Gesierich, A.; Heinzerling, L.; Loquai, C.; Meier, F.; Meiss, F.; Pföhler, C.; Schlaak, M.; Terheyden, P.; et al. Ipilimumab in Metastatic Melanoma Patients with Pre-Existing Autoimmune Disorders. Cancer Immunol. Cancer Immunol. Immunother. 2018, 67, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sbeih, H.; Faleck, D.M.; Ricciuti, B.; Mendelsohn, R.B.; Naqash, A.R.; Cohen, J.V.; Sellers, M.C.; Balaji, A.; Ben-Betzalel, G.; Hajir, I.; et al. Immune Checkpoint Inhibitor Therapy in Patients with Preexisting Inflammatory Bowel Disease. J. Clin. Oncol. 2020, 38, 576–583. [Google Scholar] [CrossRef]
- Tanaka, A.; Sadahiro, S.; Yasuda, M.; Shimizu, S.; Maeda, Y.; Suzuki, T.; Tokunaga, N.; Ogoshi, K. Endoscopic balloon dilation for obstructive colorectal cancer: A basic study on morphologic and pathologic features associated with perforation. Gastrointest. Endosc. 2010, 71, 799–805. [Google Scholar] [CrossRef]
| Author | Study | Year | dMMR/ pMMR | Location | Stage | Neoadjuvant Therapy Strategy | Efficacy | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Zhang [27] | Case Series N = 2 | 2019 | dMMR: 2 | Rectal | III | Both patients underwent nivolumab 3 mg/kg every 2 weeks for 6 cycles followed by total mesorectal excision. | 1. pCR 2. cCR | No Grade 3–4 adverse events (0%). |
| Bando [28] | VOLTAGE-A (Phase II) N = 44 | 2020 | dMMR: 5 pMMR: 39 | Rectal | II–III | Nivolumab 240 mg every 2 weeks ×5 cycles followed by radical surgery. | pCR dMMR 3/5 (60%) pMMR 11/37 (30%) | Grade 3 3/42 (4%) |
| Chalabi [29] | NICHE (Phase II) N = 41 | 2020 | dMMR: 21 pMMR: 20 | Colon | I–III | Ipilimumab (1 mg/kg) on day 1 followed by nivolumab (3 mg/kg) on day 1 + 15. Patients with pMMR tumors randomly assigned to receive celecoxib from day 1 until the day before surgery in addition to immunotherapy. Surgery was performed within 6 weeks following the last day of neoadjuvant therapy. | pCR dMMR 20/20 (100%) pMMR 4/15 (27%) MPR dMMR 19/20 (95%) pMMR 3/15 (20%) | Grade 3 5/40 (12%) |
| Demisse [30] | Case Series N = 3 | 2020 | dMMR: 3 | Rectal | II–III | 1. Pembrolizumab 200 mg every 3 weeks for 11 cycles. 2. Nivolumab 3 mg/kg and ipilimumab 1 mg/kg intravenously every 3 weeks for 7 cycles. 3. FOLFOX with concurrent pembrolizumab for 7 cycles followed by low anterior resection. | pCR 3/3 (100%) | 1 patient discontinued treatment due to grade II fatigue. No grade 3–4 adverse events were reported. |
| Liu * [31] | Case Series N = 8 | 2020 | dMMR: 8 | Colorectal | II–IV II–III: 4 IV: 4 | 1. Pembrolizumab 240 mg for 2 cycles with neoadjuvant XELOX (oxaliplatin and capecitabine) followed by subtotal colectomy. 2. Pembrolizumab 200 mg + ipilimumab 50 mg for 4 cycles. 3. Nivolumab 140 mg for 12 cycles and anterior resection. 4. Pembrolizumab 200 mg for 4 cycles followed by right hemicolectomy with lymph node dissection. | 1. pCR 2. No response 3. pCR 4. pR | Grade 3 1/8 (13%) |
| Avallone [32] | NICOLE (Phase II) N = 22 | 2021 | dMMR: 3 pMMR: 19 | Colon | I–III | Nivolumab 240 mg on day 1 + 15. Surgery after 5 weeks. | MPR dMMR 0/3 (0%) pMMR 3/19 (16%) | Grade 3 1/22 (5%) |
| Lin [33] | NCT04231552 (Phase II) N = 29 | 2021 | dMMR: 1 pMMR: 26 | Rectal | II–III | 5 × 5 Gy short course radiation therapy followed by 21 days of CAPOX (oxaliplatin 130 mg/m2 intravenously, day 1; capecitabine 1000 mg/m2 orally twice daily, days 1–14) plus camrelizumab (200 mg intravenously, day 1), followed by radical surgery after 1 week. | pCR dMMR 1/1 (100%) pMMR 12/26 (46%) | Grade 3 8/30 (26.7%) |
| Salvatore [34] | AVANA (Phase II) N = 101 | 2021 | dMMR: 1 pMMR: 38 Other: 57 | Rectal | II–III | CTRT (capecitabine 825 mg/sqm/bid 5 days/week + 50.4 Gy in 28 fractions over 5.5 weeks) pluse 6 cycles of avelumab 10 mg/kg every 2 weeks followed by total mesorectal excision at 8–10 weeks after the end of CTRT. | pCR Total 22/96 (23%) MPR Total 59/96 (62%) | Grade 3 8/96 (8%) Grade 4 4/96 (4%) Avelumab interrupted in 9/101 (9%) due to treatment toxicity. |
| Cercek [9] | NCT04165772 (Phase II) N = 12 | 2022 | dMMR: 12 | Rectal | II–III | Dostarlimab (500 mg) administered every 3 weeks for 6 months followed by standard radiation therapy (5040 cGy in 28 fractions) with concurrent administration of capecitabine. Patients with clinical complete response after induction of anti-PD1 or chemoradiotherapy underwent non-operative follow-up. | cCR dMMR 12/12 (100%) | No Grade 3–4 adverse events (0%). |
| Hu [35] | PICC (Phase II) N = 53 | 2022 | dMMR: 34 | Colorectal | II–III | 34 participants randomly assigned to either toripalimab (n = 17) or toripalimab + celecoxib (n = 17) for 6 months followed by colectomy. Toripalimab was administered every 2 weeks for 6 months. Celecoxib group received additional 200 mg oral celecoxib twice daily for 6 months. | Toripalimab pCR 11/17 (65%) Toripalimab + celecoxib pCR 15/17 (88%) | Grade 3 1/34 (3%) |
| Ludford [36] | NCT04082572 (Phase II) N = 35 | 2023 | dMMR: 27 | Colorectal | II/II | Pembrolizumab 200 mg once every 3 weeks for 6 months followed by surgical resection with an option to continue therapy for 1 year followed by observation. | pCR 11/14 (79%) | Grade 3 2/35 (6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, A.; Pedraza, R.; Kennecke, H. Developments in Checkpoint Inhibitor Therapy for the Management of Deficient Mismatch Repair (dMMR) Rectal Cancer. Curr. Oncol. 2023, 30, 3672-3683. https://doi.org/10.3390/curroncol30040279
Su A, Pedraza R, Kennecke H. Developments in Checkpoint Inhibitor Therapy for the Management of Deficient Mismatch Repair (dMMR) Rectal Cancer. Current Oncology. 2023; 30(4):3672-3683. https://doi.org/10.3390/curroncol30040279
Chicago/Turabian StyleSu, Alan, Rodrigo Pedraza, and Hagen Kennecke. 2023. "Developments in Checkpoint Inhibitor Therapy for the Management of Deficient Mismatch Repair (dMMR) Rectal Cancer" Current Oncology 30, no. 4: 3672-3683. https://doi.org/10.3390/curroncol30040279
APA StyleSu, A., Pedraza, R., & Kennecke, H. (2023). Developments in Checkpoint Inhibitor Therapy for the Management of Deficient Mismatch Repair (dMMR) Rectal Cancer. Current Oncology, 30(4), 3672-3683. https://doi.org/10.3390/curroncol30040279





