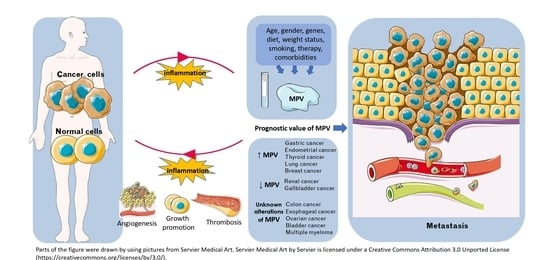
Relation of Mean Platelet Volume (MPV) with Cancer: A Systematic Review with a Focus on Disease Outcome on Twelve Types of Cancer
Abstract
1. Introduction
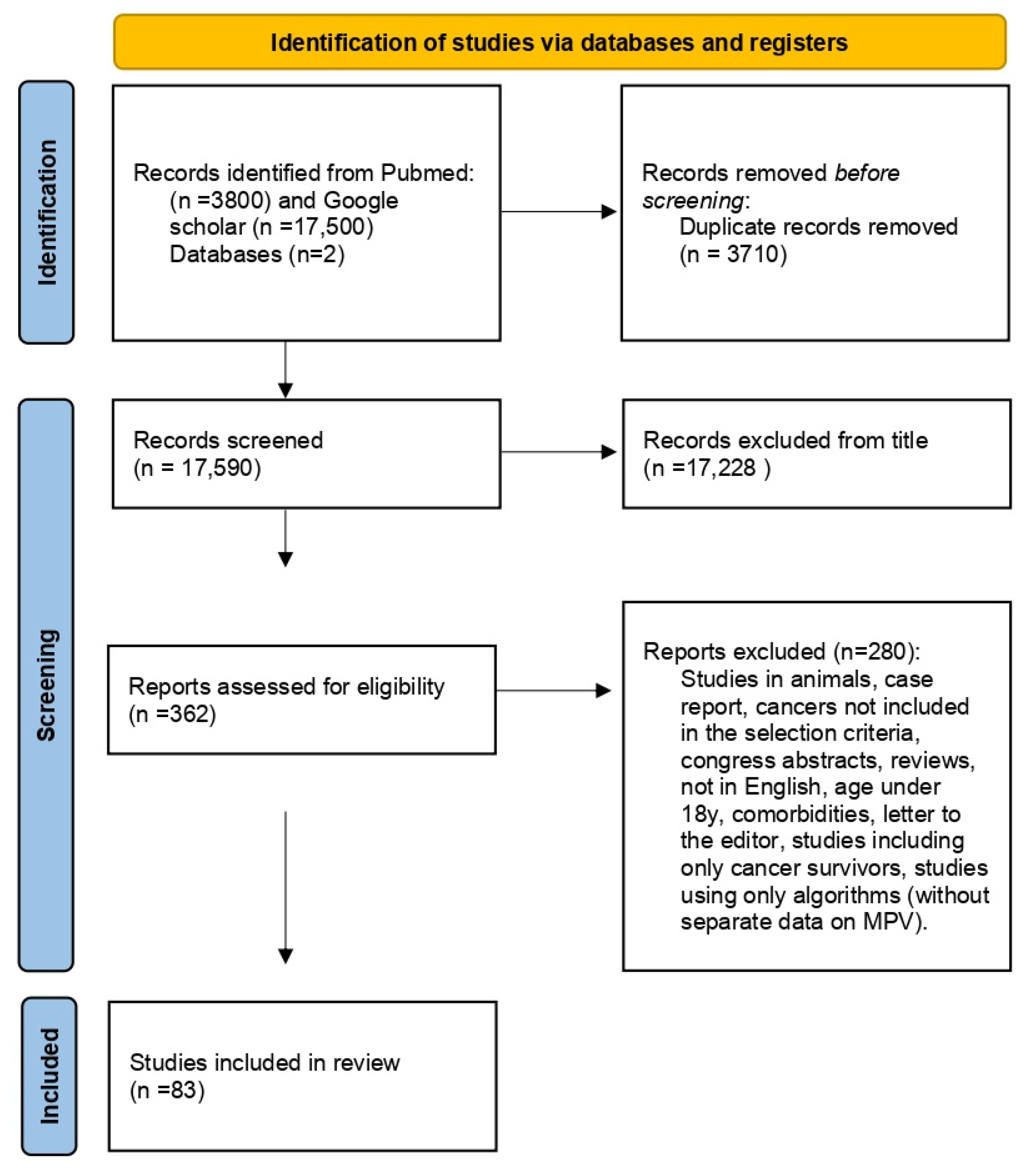
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Quality Assessment
2.4. Data Extraction
3. Results
3.1. Gastric Cancer
3.2. Colon Cancer
3.3. Esophageal Squamous Cell Carcinoma
3.4. Renal Cell Carcinoma
| Reference | Study Type | Methods/Number of Participants | Results | Reported Limitations |
|---|---|---|---|---|
| Gastric cancer | ||||
| Aksoy et al., 2019 Turkey [31] | Case control | 73 patients with gastric cancer 79 patients with intestinal metaplasia 70 healthy subjects |
|
|
| Matowicka-Karna et al., 2013 Poland [32] | Case control | 13 patients with early gastric cancer (group E) 18 patients with regionally advanced cancer (group A) 19 patients with metastatic cancer (group M) 40 healthy subjects |
|
|
| Pietrzyk et al., 2016 Poland [33] | Retrospective study- Case control | 61 patients with gastric cancer 61 healthy subjects |
|
|
| Kılınc¸alp et al., 2014 Turkey [34] | Retrospective study- Case control | 31 patients with gastric cancer 31 healthy subjects |
|
|
| Shen et al., 2016 China [3] | Retrospective study- Case control | 168 patients with resectable gastric cancer 30 healthy subjects (control group) |
|
|
| Lian et al., 2015 China [35] | Retrospective study | 128 inoperable gastric cancer patients: 53 patients with locally advanced gastric tumor 75 patients with relapsed or metastatic tumor |
|
|
| An et al., 2022 China [36] | Retrospective study | 401 patients who underwent gastric resection: 245 patients with stage I cancer 74 with stage II cancer 82 with stage III cancer |
|
|
| Manjunath et al., 2020 India [37] | Retrospective study | 149 patients with gastric cancer who had chemotherapy |
|
|
| Colon cancer | ||||
| Li et al., 2017 China [38] | Single-center retrospective study | 509 patients with colon cancer |
|
|
| Li et al., 2014 China [39] | Prospective study, case control | 256 participants: 128 patients with colon cancer 128 healthy participants (control group) |
|
|
| Tuncel T, et al., 2014 Turkey [40] | Retrospective study | 148 patients with colon cancer (53 metastatic, 95 nonmetastatic) |
|
|
| Liu, J. et al., 2020 China [41] | Retrospective study | 873 patients with stage II-III colorectal cancer |
|
|
| Sakin, A, et al., 2020 Turkey [42] | Retrospective study | 394 patients with Colorectal Cancer without metastasis |
|
|
| Wang, W. et al., 2021 China [46] | Retrospective study | 424 patients with colorectal cancer |
|
|
| Wang, P.et al., 2021 China [43] | Retrospective study | 75 patients with locally advanced rectal cancer treated with total mesorectal excision |
|
|
| Stojkovic, Lalosevic et al., 2019 Serbia [13] | Single-center prospective study | 300 newly diagnosed colon cancer patients 300 healthy volunteers (control group) |
|
|
| Chang, J., Lin, G., Ye, M. et al., 2019 China [19] | Single-center retrospective clinical study | 264 patients with metastatic colon cancer |
|
|
| Alsalman, A. et al., 2022 Qatar [44] | Cohort study | 97 colorectal cancer patients one week prior to surgery. |
|
|
| Huang L. et al., 2022 China [45] | Retrospective, case-control study | 251 patients with colon cancer 171 benign colon disease cases 187 healthy controls |
|
|
| Esophageal squamous cell carcinoma | ||||
| Feng et al., 2019 China [47] | Retrospective study | 277 resectable esophageal squamous cell carcinoma |
|
|
| Sun S-Y et al., 2018 China [20] | Retrospective single-center design | 457 patients with newly diagnosed locally advanced esophageal squamous cell carcinoma who have undergone radical esophagectomy 240 healthy subjects (control group) |
|
|
| Liu X. et al., 2022 China [48] | Retrospective study | 3210 patients with esophageal cancer that underwent esophagectomy |
|
|
| Zhou X., et al., 2021 China [49] | Retrospective study-case control | 314 early esophageal cancer patients 329 healthy individuals (control group) |
|
|
| Feng et al., 2019 China [47] | Retrospective study | 277 resectable esophageal squamous cell carcinoma |
|
|
| Surucu et al., 2015 Turkey [50] | Retrospective study | 52 patients with esophageal squamous cell cancer 52 with dyspepsia |
|
|
| Reference | Study Type | Methods/Number of Participants | Results | Reported Limitations |
|---|---|---|---|---|
| Renal cell carcinoma | ||||
| Yun. ZY et al., 2017 China [51] | Single-center retrospective study | 306 patients with renal cell carcinoma: 286 patients with locally confined disease 20 patients with locally advanced disease 290 patients with no metastasis 16 patients with metastasis |
|
|
| Yun, Z., Zhang, X., et al., 2017 China [18] | Cross-sectional study | 387 participants: 145 patients with renal cell carcinoma 110 patients with benign renal tumor 132 healthy control subjects (control group) |
|
|
| Prokopowicz et al., 2016 Poland [52] | Retrospective | 230 patients treated for renal cell carcinoma |
| |
3.5. Breast Cancer
3.6. Ovarian Cancer
3.7. Endometrial Cancer
3.8. Thyroid Cancer
3.9. Lung Cancer
| Reference | Study Type | Methods/Number of Participants | Results | Reported Limitations |
|---|---|---|---|---|
| Breast cancer | ||||
| Yao et al. 2014/China [53] | Observational | 608 women with breast cancer |
|
|
| Mantas et al. 2016/Greece [54] | Prospective | 53 patients with early breast cancer, who developed systemic metastases over a mean follow-up period of 65 months 37 patients that remained recurrence-free |
|
|
| Tanriverdi O. et al., 2016 Turkey [55] | Retrospective cohort study Case control | 121 women with breast cancer and bone metastases 71 women with breast cancer without metastases (control group) 39 healthy women (control group) |
|
|
| Mutlu H., et al., 2016 Turkey [56] | Retrospective study | 109 patients with locally advanced breast cancer |
|
|
| Gu M. et al., 2016 China [16] | Single-center retrospective study Case control | 340 women patients with newly diagnosed breast tumors: 170 women patients with invasive breast cancer 170 women with benign breast tumors (control group) |
|
|
| Sun H. et al., 2017 China [57] | Single-center retrospective study Case control | 110 patients with breast cancer 76 healthy females (control group) |
|
|
| Bozan MB., et al., 2022 Turkey [58] | Retrospective study | 83 women patients with breast cancer: 46 patients with nonmetastatic axilla 37 patients with metastatic axilla |
|
|
| Divsalar B., et al., 2021 Iran [59] | Retrospective study- Case control | −160 women patients with breast cancer −160 healthy controls (control group) |
|
|
| Ovarian cancer | ||||
| Qin, Yuan-Yuan et al., 2018 China [60] | Retrospective study | 326 patients with ovarian cancer 290 patients with benign ovarian cancer 162 healthy subjects (control group) |
|
|
| Kemal et al., 2014 Turkey [61] | Retrospective study | 113 ovarian cancer patients 90 healthy subjects (control group) |
|
|
| Ma et al., 2013 China [62] | Retrospective study | 182 patients with epithelial ovarian cancer 122 patients with benign ovarian tumor 150 healthy women |
|
|
| Kokcu et al., 2014 Turkey [63] | Retrospective study | 100 patients with epithelial ovarian cancer |
|
|
| Bakacak et al., 2016 Turkey [64] | Retrospective study | 185 benign cases 33 malignant cases following surgery for an initial diagnosis of adnexal mass and confirmed ovarian masses. |
|
|
| Yilmaz et al., 2017 Turkey [65] | Retrospective study | 33 patients with ovarian cancer 33 patients with benign tumors |
|
|
| Endometrial cancer | ||||
| Kurtoglu, Emel et al., 2015 Turkey [66] | Retrospective study | 114 patients surgically staged for endometrium adenocarcinoma (malign endometrium diseases) 105 patients who have undergone total abdominal or vaginal hysterectomy for benign uterine diseases |
|
|
| Zhang et al., 2020 China [67] | Retrospective | 144 patients with endometrial cancer (stage I: 32; II: 42; III: 48; and IV: 22) 104 patients with endometrial hyperplasia 80 healthy subjects |
|
|
| Temur et al., 2018 Turkey [68] | Retrospective | 763 patients with endometrial cancer |
|
|
| Karateket et al., 2015 Turkey [69] | Retrospective | 55 endometrial hyperplasia cases, 34 endometrial cancer cases 105 normal endometrial biopsy cases |
|
|
| Oge et al., 2013 Turkey [70] | Retrospective | 291 patients with endometrial cancer 250 women (control group) |
|
|
| Song et al., 2019 China [71] | Retrospective | 45 patients with endometrial cancer 143 malignant cases |
|
|
| Chen et al., 2020 China [72] | Retrospective | 1198 patients with endometrial cancer |
|
|
| Abide et al., 2018 Turkey [73] | Retrospective | 97 patients with endometrial carcinoma 135 patients with endometrial hyperplasia 184 healthy subjects |
|
|
| Reference | Study Type | Methods/Number of Participants | Results | Reported Limitations |
|---|---|---|---|---|
| Thyroid cancer | ||||
| Yu et al., 2017 China [74] | Cross-sectional | 280 patients with thyroid cancer 280 control subjects |
|
|
| Sit et al., 2019 Turkey [75] | Retrospective | 101 patients with malignant thyroid nodules 98 patients with benign thyroid nodules |
|
|
| Dincel et al., 2017 Turkey [76] | Retrospective | 65 papillary thyroid carcinoma patients 65 multi-nodular goiter patients 30 normal healthy subjects |
|
|
| Yildiz et al., 2019 Turkey [77] | Retrospective | 53 patients with papillary thyroid cancer 37 with nodular hyperplasia |
|
|
| Wen et al., 2018 China [96] | Retrospective | 558 patients newly diagnosed with papillary thyroid cancer |
|
|
| Kutluturk F. et al., 2019 Turkey [17] | Retrospective study | 58 patients with papillary thyroid carcinoma |
|
|
| Baldane S, Ipekci SH, Sozen M, Kebapcilar L. 2015 Turkey [14] | Retrospective study | 98 patients who underwent a total thyroidectomy: 66 patients with papillary thyroid cancer 32 patients with benign goiters 28 healthy subjects (control group) |
|
|
| Bayhan Ζ., et al., 2016 Turkey [78] | Retrospective study | 146 patients who underwent total thyroidectomy: 47 patients with malignant diseases of the thyroid 99 patients with benign diseases of the thyroid |
|
|
| Li, et al., 2022 Japan [80] | Retrospective study | 212 patients with papillary thyroid carcinoma |
|
|
| Li, C. et al., 2022 Japan [81] | Retrospective study | 68 patients diagnosed with medullary thyroid carcinoma who underwent surgery |
|
|
| Martin S. et al., 2021 Romania [79] | Retrospective study | 265 patients diagnosed with thyroid cancer 249 patients with histologically differentiated thyroid cancer 234 papillary thyroid carcinomas 15 follicular thyroid carcinomas 108 patients with benign thyroid pathology (control group) |
|
|
| Reference | Study Type | Methods/Number of Participants | Results | Reported Limitations |
|---|---|---|---|---|
| Lung cancer | ||||
| Cui et al., 2017 China [82] | Retrospective study | 270 patients with non-small-cell lung cancer |
|
|
| Hur et al., 2020 Korea [94] | Retrospective study | 116 patients with non-small-cell lung cancer |
|
|
| Sakin et al., 2019 Turkey [89] | Retrospective study | 90 patients with limited disease small-cell lung cancer |
|
|
| Shen et al., 2019 China [83] | Retrospective study | 138 patients with non-small-cell lung cancer who underwent etoposide-based first-line chemotherapy |
|
|
| Shi et al., 2018 China [84] | Retrospective study | 169 advanced and metastatic patients with non-small-cell lung cancer |
|
|
| Wang et al., 2019 China [83] | Retrospective study | 101 patients with resectable lung cancer |
|
|
| Watanabe et al., 2018 Japan [92] | Retrospective study | 82 advanced or recurrent patients with non-small-cell lung cancer with common EGFR mutation |
|
|
| Kumagai S., et al., 2015 Japan [93] | Retrospective study | 308 patients with non-small-cell lung cancer who underwent surgery |
|
|
| Omar M., et al., 2018 Turkey [90] | Retrospective study | 496 patients with non-small-cell lung cancer |
|
|
| Sakin A., Secmeler S., Arici S., et al., 2019 Turkey [12] | Retrospective study | 115 patients with locally advanced non-small-cell lung cancer who received chemotherapy |
|
|
| Kharel et al., 2022 [26] | Meta-analysis | 2421 patients with lung cancer |
|
|
| Ai L., et al., 2022 China [88] | Retrospective study | 703 lung adenocarcinoma patients: 270 malignant pleural effusion patients 433 tuberculous pleural effusion patients |
|
|
| Goksel S., et al., 2021 Turkey [91] | Retrospective, case-control study | 180 patients with lung cancer 180 healthy controls |
|
|
| Zhu X., et al., 2020 China [86] | Retrospective, case-control study | 209 patients with lung cancer 236 healthy subjects |
|
|
| Zu R., et al., 2020 China [87] | Prospective, case-control study | 245 participants 159 lung cancer patients 86 normal participants |
|
|
| Łochowsk M., et al., 2022 Poland [95] | Retrospective study | 532 patients with non-small-cell lung cancer staged IA–IIIA |
|
|
3.10. Bladder Cancer
3.11. Gallbladder Cancer
3.12. Multiple Myeloma
| Reference | Study Type | Methods/Number of Participants | Results | Reported Limitations |
|---|---|---|---|---|
| Bladder cancer | ||||
| Wang, Xin, et al., 2017 China [97] | Retrospective study | 218 patients with bladder cancer who have undergone radical cystectomy |
|
|
| Song et al., 2022 China [98] | Retrospective study | 271 patients after transurethral resection of bladder tumor |
|
|
| Liu et al., 2019 China [99] | Retrospective study | −210 subjects with bladder cancer −76 subjects with urothelial papilloma −132 healthy control subjects |
|
|
| Albayrak et al., 2016 Turkey [100] | prospective study | 86 patients with newly diagnosed non-muscle-invasive bladder cancer classified by the number of points assigned by the European Organization for Research and Treatment of Cancer risk tables. |
|
|
| Yildiz et al., 2021 Turkey [101] | prospective study | 94 consecutive patients newly diagnosed with non-muscle-invasive bladder cancer |
|
|
| Gallbladder Cancer | ||||
| Zhang, Xin et al., 2018 China [22] | Cross-sectional study | 213 participants: 104 patients with gallbladder cancer who had undergone surgical resection and had not received chemotherapy prior to surgery 109 healthy controls (control group) |
|
|
| Kucuk, S.; Mızrak, S. 2021 Turkey [102] | Retrospective, Cross-sectional study | 187 cholecystectomy specimens that were diagnosed as cholecystitis, dysplasia, and adenocarcinoma. |
|
|
| BV, P. et al., 2021 India [39] | Retrospective study | 73 patients with gallbladder cancer |
|
|
| Multiple myeloma | ||||
| Zhuang Q. et al., 2016 China [104] | Retrospective study | 62 patients with newly diagnosed multiple myeloma |
|
|
4. Discussion
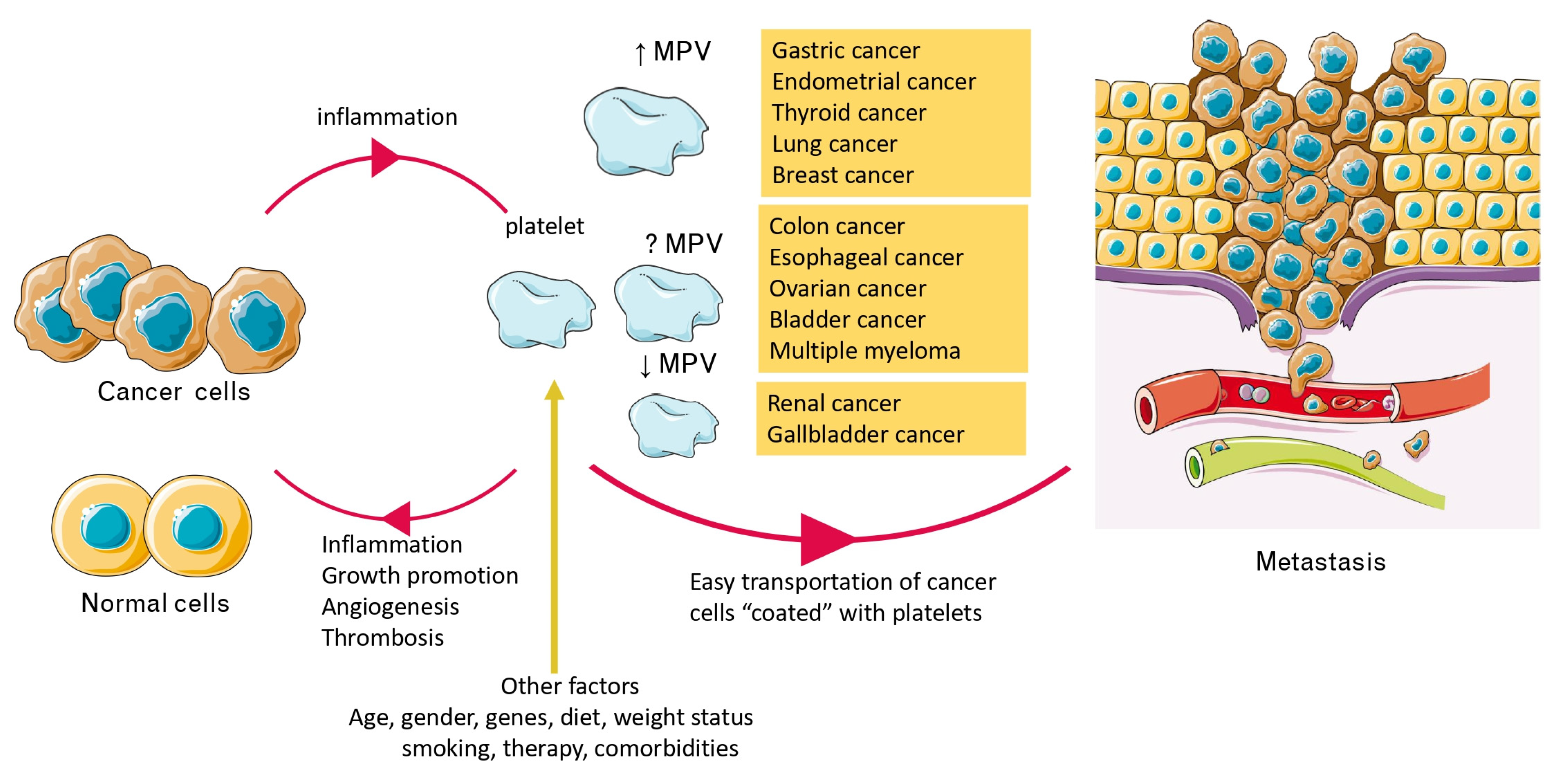
4.1. Alterations of MPV Values in Patients with Cancer and Relation to Survival
4.2. Cancer-Related Inflammation, Platelets, and MPV
4.3. MPV, Activated Platelets, Cancer Progression, and Metastasis
4.4. Other Factors Affecting MPV
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Atun, R.; Cavalli, F. The Global Fight against Cancer: Challenges and Opportunities. Lancet 2018, 391, 412–413. [Google Scholar] [CrossRef]
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P.; et al. Early Detection of Cancer. Science 2022, 375, eaay9040. [Google Scholar] [CrossRef]
- Shen, X.-M.; Xia, Y.-Y.; Lian, L.; Zhou, C.; Li, X.-L.; Han, S.-G.; Zheng, Y.; Gong, F.-R.; Tao, M.; Mao, Z.-Q.; et al. Mean Platelet Volume Provides Beneficial Diagnostic and Prognostic Information for Patients with Resectable Gastric Cancer. Oncol. Lett. 2016, 12, 2501–2506. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Guo, E.; Mao, X.; Miao, S. Emerging Roles of Platelets in Cancer Biology and Their Potential as Therapeutic Targets. Front. Oncol. 2022, 12, 939089. [Google Scholar] [CrossRef]
- Van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet Biology and Functions: New Concepts and Clinical Perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ehrenberg, A.; Toska, L.M.; Metz, L.M.; Klier, M.; Krueger, I.; Reusswig, F.; Elvers, M. Molecular Drivers of Platelet Activation: Unraveling Novel Targets for Anti-Thrombotic and Anti-Thrombo-Inflammatory Therapy. Int. J. Mol. Sci. 2020, 21, 7906. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Detopoulou, P.; Nomikos, T.; Pliakis, E.; Panagiotakos, D.B.; Antonopoulou, S. Mediterranean Wild Plants Reduce Postprandial Platelet Aggregation in Patients with Metabolic Syndrome. Metabolism 2012, 61, 325–334. [Google Scholar] [CrossRef]
- Detopoulou, P.; Nomikos, T.; Fragopoulou, E.; Antonopoulou, S.; Kotroyiannis, I.; Vassiliadou, C.; Panagiotakos, D.B.; Chrysohoou, C.; Pitsavos, C.; Stefanadis, C. Platelet Activating Factor (PAF) and Activity of Its Biosynthetic and Catabolic Enzymes in Blood and Leukocytes of Male Patients with Newly Diagnosed Heart Failure. Clin. Biochem. 2009, 42, 44–49. [Google Scholar] [CrossRef]
- Handtke, S.; Thiele, T. Large and Small Platelets—(When) Do They Differ? J. Thromb. Haemost. 2020, 18, 1256–1267. [Google Scholar] [CrossRef]
- Korniluk, A.; Koper-Lenkiewicz, O.M.; Kamińska, J.; Kemona, H.; Dymicka-Piekarska, V. Mean Platelet Volume (MPV): New Perspectives for an Old Marker in the Course and Prognosis of Inflammatory Conditions. Mediat. Inflamm. 2019, 2019, 9213074. [Google Scholar] [CrossRef]
- Kelesidis, T.; Papakonstantinou, V.; Detopoulou, P.; Fragopoulou, E.; Chini, M.; Lazanas, M.C.; Antonopoulou, S. The Role of Platelet-Activating Factor in Chronic Inflammation, Immune Activation, and Comorbidities Associated with HIV Infection. AIDS Rev. 2015, 17, 191–201. [Google Scholar]
- Sakin, A.; Secmeler, S.; Arici, S.; Geredeli, C.; Yasar, N.; Demir, C.; Aksaray, F.; Cihan, S. Prognostic Significance of Mean Platelet Volume on Local Advanced Non-Small Cell Lung Cancer Managed with Chemoradiotherapy. Sci. Rep. 2019, 9, 3959. [Google Scholar] [CrossRef]
- Stojkovic Lalosevic, M.; Pavlovic Markovic, A.; Stankovic, S.; Stojkovic, M.; Dimitrijevic, I.; Radoman Vujacic, I.; Lalic, D.; Milovanovic, T.; Dumic, I.; Krivokapic, Z. Combined Diagnostic Efficacy of Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Mean Platelet Volume (MPV) as Biomarkers of Systemic Inflammation in the Diagnosis of Colorectal Cancer. Dis. Markers 2019, 2019, 6036979. [Google Scholar] [CrossRef]
- Baldane, S.; Ipekci, S.H.; Sozen, M.; Kebapcilar, L. Mean Platelet Volume Could Be a Possible Biomarker for Papillary Thyroid Carcinomas. Asian Pac. J. Cancer Prev. 2015, 16, 2671–2674. [Google Scholar] [CrossRef][Green Version]
- Shen, W.-J.; Fu, S.; Li, N.; Li, L.-L.; Cao, Z.-G.; Li, C.; Liu, T.; Wang, R.-T. Decreased Mean Platelet Volume Is Associated with Cervical Cancer Development. Asian Pac. J. Cancer Prev. 2017, 18, 1769–1772. [Google Scholar] [CrossRef]
- Gu, M.; Zhai, Z.; Huang, L.; Zheng, W.; Zhou, Y.; Zhu, R.; Shen, F.; Yuan, C. Pre-Treatment Mean Platelet Volume Associates with Worse Clinicopathologic Features and Prognosis of Patients with Invasive Breast Cancer. Breast Cancer 2016, 23, 752–760. [Google Scholar] [CrossRef]
- Kutluturk, F.; Gul, S.S.; Sahin, S.; Tasliyurt, T. Comparison of Mean Platelet Volume, Platelet Count, Neutrophil/Lymphocyte Ratio and Platelet/Lymphocyte Ratio in the Euthyroid, Overt Hypothyroid and Subclinical Hyperthyroid Phases of Papillary Thyroid Carcinoma. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 859–865. [Google Scholar] [CrossRef]
- Yun, Z.-Y.; Zhang, X.; Liu, Z.-P.; Liu, T.; Wang, R.-T.; Chen, H. Association of Decreased Mean Platelet Volume with Renal Cell Carcinoma. Int. J. Clin. Oncol. 2017, 22, 1076–1080. [Google Scholar] [CrossRef]
- Chang, J.; Lin, G.; Ye, M.; Tong, D.; Zhao, J.; Zhu, D.; Yu, Q.; Zhang, W.; Li, W. Decreased Mean Platelet Volume Predicts Poor Prognosis in Metastatic Colorectal Cancer Patients Treated with First-Line Chemotherapy: Results from MCRC Biomarker Study. BMC Cancer 2019, 19, 15. [Google Scholar] [CrossRef]
- Sun, S.-Y.; Zhao, B.-Q.; Wang, J.; Mo, Z.-X.; Zhao, Y.-N.; Wang, Y.; He, J. The Clinical Implications of Mean Platelet Volume and Mean Platelet Volume/Platelet Count Ratio in Locally Advanced Esophageal Squamous Cell Carcinoma. Dis. Esophagus 2018, 31, dox125. [Google Scholar] [CrossRef]
- Yin, J.-B.; Wang, X.; Zhang, X.; Liu, L.; Wang, R.-T. Mean Platelet Volume Predicts Survival in Pancreatic Cancer Patients with Synchronous Liver Metastases. Sci. Rep. 2018, 8, 6014. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, Y.; Wang, X.; Liu, Z.; Liu, T.; Wang, R.-T. Mean Platelet Volume and Platelet Distribution Width Are Associated with Gallbladder Cancer. Asian Pac. J. Cancer Prev. 2018, 19, 351–355. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric Cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Pyo, J.-S.; Sohn, J.H.; Kang, G. Diagnostic and Prognostic Roles of the Mean Platelet Volume in Malignant Tumors: A Systematic Review and Meta-Analysis. Platelets 2016, 27, 722–728. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Tsujimoto, H.; Sugasawa, H.; Kouzu, K.; Itazaki, Y.; Sugihara, T.; Harada, M.; Ito, N.; Kishi, Y.; Ueno, H. Prognostic Value of Platelet-Related Measures for Overall Survival in Esophageal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2021, 164, 103427. [Google Scholar] [CrossRef]
- Kharel, S.; Shrestha, S.; Shakya, P.; Rawat, R.; Shilpakar, R. Prognostic Significance of Mean Platelet Volume in Patients with Lung Cancer: A Meta-Analysis. J. Int. Med. Res. 2022, 50, 3000605221084874. [Google Scholar] [CrossRef]
- Stang, A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 13 March 2023).
- Downes, M.J.; Brennan, M.L.; Williams, H.C.; Dean, R.S. Development of a Critical Appraisal Tool to Assess the Quality of Cross-Sectional Studies (AXIS). BMJ Open 2016, 6, e011458. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Aksoy, E.; Kantarci, S.; Torgutalp, M.; Akpinar, M.; Sapmaz, F.; Yalçın, G.; Uzman, M.; Şimşek, G.; Nazlıgül, Y. The Importance of Complete Blood Count Parameters in the Screening of Gastric Cancer. Gastroenterol. Rev. /Przegląd Gastroenterol. 2019, 14, 183–187. [Google Scholar] [CrossRef]
- Matowicka-Karna, J.; Kamocki, Z.; Polińska, B.; Osada, J.; Kemona, H. Platelets and Inflammatory Markers in Patients with Gastric Cancer. Clin. Dev. Immunol. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Pietrzyk, L.; Plewa, Z.; Denisow-Pietrzyk, M.; Zebrowski, R.; Torres, K. Diagnostic Power of Blood Parameters as Screening Markers in Gastric Cancer Patients. Asian Pac. J. Cancer Prev. 2016, 17, 4433–4437. [Google Scholar]
- Kılınçalp, S.; Ekiz, F.; Başar, Ö.; Ayte, M.R.; Çoban, Ş.; Yılmaz, B.; Altınbaş, A.; Başar, N.; Aktaş, B.; Tuna, Y.; et al. Mean Platelet Volume Could Be Possible Biomarker in Early Diagnosis and Monitoring of Gastric Cancer. Platelets 2014, 25, 592–594. [Google Scholar] [CrossRef]
- Lian, L.; Xia, Y.-Y.; Zhou, C.; Shen, X.-M.; Li, X.-L.; Han, S.-G.; Zheng, Y.; Gong, F.-R.; Tao, M.; Li, W. Mean Platelet Volume Predicts Chemotherapy Response and Prognosis in Patients with Unresectable Gastric Cancer. Oncol. Lett. 2015, 10, 3419–3424. [Google Scholar] [CrossRef][Green Version]
- An, S.; Eo, W.; Han, G.Y.; Park, S.; Lee, S. Preoperative Mean Platelet Volume Is a Prognostic Biomarker for Survival in Patients with Gastric Cancer: A Cohort Study. Medicine 2022, 101, e30504. [Google Scholar] [CrossRef]
- Manjunath, K.V.; Jonnada, P.; Anwar, A. Role of Mean Platelet Volume in the Prognosis of Locally Advanced Gastric Cancer: A Tertiary Cancer Center Experience. Cureus 2020, 12, e9109. [Google Scholar] [CrossRef]
- Li, N.; Yu, Z.; Zhang, X.; Liu, T.; Sun, Y.-X.; Wang, R.-T.; Yu, K.-J. Elevated Mean Platelet Volume Predicts Poor Prognosis in Colorectal Cancer. Sci. Rep. 2017, 7, 10261. [Google Scholar] [CrossRef]
- Li, J.-Y.; Li, Y.; Jiang, Z.; Wang, R.-T.; Wang, X.-S. Elevated Mean Platelet Volume Is Associated with Presence of Colon Cancer. Asian Pac. J. Cancer Prev. 2014, 15, 10501–10504. [Google Scholar] [CrossRef]
- Tuncel, T.; Ozgun, A.; Emirzeoglu, L.; Celik, S.; Bilgi, O.; Karagoz, B. Mean Platelet Volume as a Prognostic Marker in Metastatic Colorectal Cancer Patients Treated with Bevacizumab-Combined Chemotherapy. Asian Pac. J. Cancer Prev. 2014, 15, 6421–6423. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Yang, W.; Li, C.; Li, Z.; Zhang, C.; Chen, S.; Wu, G.; Xie, W.; Wei, C.; et al. Nomogram for Predicting Overall Survival in Stage II-III Colorectal Cancer. Cancer Med. 2020, 9, 2363–2371. [Google Scholar] [CrossRef]
- Sakin, A.; Sahin, S.; Sakin, A.; Karatas, F.; Sengul Samanci, N.; Yasar, N.; Arici, S.; Demir, C.; Geredeli, C.; Dikker, O.; et al. Mean Platelet Volume and Platelet Distribution Width Correlates with Prognosis of Early Colon Cancer. J. BUON 2020, 25, 227–239. [Google Scholar] [PubMed]
- Wang, P.; Wang, Z.; Liu, Y.; Xie, J.; Ren, Y. Prognostic Value of Platelet-Associated Biomarkers in Rectal Cancer Patients Received Neoadjuvant Chemoradiation: A Retrospective Study. Cancer Radiother. 2021, 25, 147–154. [Google Scholar] [CrossRef]
- Alsalman, A.; Al-Mterin, M.A.; Abu-Dayeh, A.; Alloush, F.; Murshed, K.; Elkord, E. Associations of Complete Blood Count Parameters with Disease-Free Survival in Right- and Left-Sided Colorectal Cancer Patients. J. Pers. Med. 2022, 12, 816. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Hu, Z.; Luo, R.; Li, H.; Yang, Z.; Qin, X.; Mo, Z. Predictive Values of the Selected Inflammatory Indexes in Colon Cancer. Cancer Control 2022, 29, 10732748221091332. [Google Scholar] [CrossRef]
- Wang, W.; Wang, G.; Fu, S.; Zhang, B.; Liu, Z.; Wang, R. Decreased Mean Platelet Volume Is Associated with Microsatellite Instability in Colorectal Cancer: A Propensity Score-Matched Analysis. Cancer Biomark. 2021, 31, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-F.; Sheng, C.; Zhao, Q.; Chen, P. Prognostic Value of Mean Platelet Volume/Platelet Count Ratio in Patients with Resectable Esophageal Squamous Cell Carcinoma: A Retrospective Study. PeerJ 2019, 7, e7246. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, K.; Tang, J.; Jiang, L.; Jiang, Y.; Wang, Q. Adjuvant Chemotherapy for Lymph Node Positive Esophageal Squamous Cell Cancer: The Prediction Role of Low Mean Platelet Volume. Front. Oncol. 2022, 12, 1067682. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, H.; Zhang, W.; Li, X.; Si, X.; Zhang, G. Predictive Value of Routine Blood Test in Patients with Early Esophageal Cancer: A Matched Case-Control Study. J. Cancer 2021, 12, 4739–4744. [Google Scholar] [CrossRef]
- Sürücü, E.; Demir, Y.; Şengöz, T. The Correlation between the Metabolic Tumor Volume and Hematological Parameters in Patients with Esophageal Cancer. Ann. Nucl. Med. 2015, 29, 906–910. [Google Scholar] [CrossRef]
- Yun, Z.-Y.; Zhang, X.; Liu, Y.-S.; Liu, T.; Liu, Z.-P.; Wang, R.-T.; Yu, K.-J. Lower Mean Platelet Volume Predicts Poor Prognosis in Renal Cell Carcinoma. Sci. Rep. 2017, 7, 6700. [Google Scholar] [CrossRef]
- Prokopowicz, G.; Życzkowski, M.; Nowakowski, K.; Bogacki, R.; Bryniarski, P.; Paradysz, A. Basic Parameters of Blood Count as Prognostic Factors for Renal Cell Carcinoma. BioMed Res. Int. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Liu, Y.; Jin, H.; Liu, X.; Fu, P.; Lv, K.; Wei, H.; Du, C.; Wang, S.; Wei, B. Prognostic Value of Preoperative Inflammatory Markers in Chinese Patients with Breast Cancer. OncoTargets Ther. 2014, 7, 1743. [Google Scholar] [CrossRef]
- Mantas, D.; Kostakis, I.D.; Machairas, N.; Markopoulos, C. White Blood Cell and Platelet Indices as Prognostic Markers in Patients with Invasive Ductal Breast Carcinoma. Oncol. Lett. 2016, 12, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Tanriverdi, O.; Menekse, S.; Teker, F.; Oktay, E.; Pilanc, K.; Günaldı, M.; Kocar, M.; Kacan, T.; Bahceci, A.; Avci, N.; et al. The Mean Platelet Volume May Predict the Development of Isolated Bone Metastases in Patients with Breast Cancer: A Retrospective Study of the Young Researchers Committee of the Turkish Oncology Group (TOG). J. BUON 2016, 21, 840–850. [Google Scholar] [PubMed]
- Mutlu, H.; Eryılmaz, M.K.; Musri, F.Y.; Gunduz, S.; Salim, D.K.; Coskun, H.S. Mean Platelet Volume as an Independent Predictive Marker for Pathologic Complete Response after Neoadjuvant Chemotherapy in Patients with Locally Advanced Breast Cancer. Asian Pac. J. Cancer Prev. 2016, 17, 2089–2092. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, H.; Yin, C.; Liu, Q.; Wang, F.; Yuan, C. Clinical Significance of Routine Blood Test-Associated Inflammatory Index in Breast Cancer Patients. Med. Sci. Monit. 2017, 23, 5090–5095. [Google Scholar] [CrossRef] [PubMed]
- Bozan, M.B.; Yazar, F.M.; Kale, I.T.; Topuz, S.; Bozan, A.A.; Boran, O.F. Immature Granulocyte Count and Delta Neutrophil Index as New Predictive Factors for Axillary Metastasis of Breast Cancer. J. Coll. Physicians Surg. Pak. 2022, 32, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Divsalar, B.; Heydari, P.; Habibollah, G.; Tamaddon, G. Hematological Parameters Changes in Patients with Breast Cancer. Clin. Lab. 2021, 67, 1832–1840. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wu, Y.; Xian, X.; Qin, J.; Lai, Z.; Liao, L.; Lin, F. Single and Combined Use of Red Cell Distribution Width, Mean Platelet Volume, and Cancer Antigen 125 for Differential Diagnosis of Ovarian Cancer and Benign Ovarian Tumors. J. Ovarian Res. 2018, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Kemal, Y.; Demirağ, G.; Ekiz, K.; Yücel, İ. Mean Platelet Volume Could Be a Useful Biomarker for Monitoring Epithelial Ovarian Cancer. J. Obstet. Gynaecol. 2014, 34, 515–518. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Y.; Sheng, H.; Tian, W.; Qi, Z.; Teng, F.; Xue, F. Prognostic Significance of Thrombocytosis, Platelet Parameters and Aggregation Rates in Epithelial Ovarian Cancer: Platelet Count, Aggregation Rate and EOC. J. Obstet. Gynaecol. Res. 2014, 40, 178–183. [Google Scholar] [CrossRef]
- Kokcu, A.; Kurtoglu, E.; Celik, H.; Tosun, M.; Malatyalıoglu, E.; Ozdemir, A.Z. May the Platelet to Lymphocyte Ratio Be a Prognostic Factor for Epithelial Ovarian Cancer? Asian Pac. J. Cancer Prev. 2014, 15, 9781–9784. [Google Scholar] [CrossRef] [PubMed]
- Bakacak, M.; Serin, S.; Ercan, O.; Kostu, B.; Bostanci, M.S.; Bakacak, Z.; Kiran, H.; Kiran, G. Utility of Preoperative Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios to Distinguish Malignant from Benign Ovarian Masses. J. Turk. Ger. Gynecol. Assoc. 2016, 17, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Coskun, E.I.; Sahin, N.; Ciplak, B.; Ekici, K. MPV, NLR, and Platelet Count: New Hematologic Markers in Diagnosis of Malignant Ovarian Tumor. Eur. J. Gynaecol. Oncol. 2017, 38, 346–349. [Google Scholar]
- Kurtoglu, E.; Kokcu, A.; Celik, H.; Sari, S.; Tosun, M. Platelet Indices May Be Useful in Discrimination of Benign and Malign Endometrial Lesions, and Early and Advanced Stage Endometrial Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 5397–5400. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.; Liang, K.; Ke, L.; Tang, S. Clinical Application of Red Cell Distribution Width, Mean Platelet Volume, and Cancer Antigen 125 Detection in Endometrial Cancer. J. Clin. Lab. Anal. 2020, 34, e23309. [Google Scholar] [CrossRef]
- Temur, I.; Kucukgoz Gulec, U.; Paydas, S.; Guzel, A.B.; Sucu, M.; Vardar, M.A. Prognostic Value of Pre-Operative Neutrophil/Lymphocyte Ratio, Monocyte Count, Mean Platelet Volume, and Platelet/Lymphocyte Ratio in Endometrial Cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 226, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Karateke, A.; Kaplanoglu, M.; Baloglu, A. Relations of Platelet Indices with Endometrial Hyperplasia and Endometrial Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 4905–4908. [Google Scholar] [CrossRef] [PubMed]
- Oge, T.; Yalcin, O.T.; Ozalp, S.S.; Isikci, T. Platelet Volume as a Parameter for Platelet Activation in Patients with Endometrial Cancer. J. Obstet. Gynaecol. 2013, 33, 301–304. [Google Scholar] [CrossRef]
- Song, J.; Lai, X.; Zhang, Y.; Zheng, X.; Su, J. Preoperative Platelet Morphology Parameters as Prognostic Predictors for Endometrial Malignant Carcinoma Stage and Progesterone Receptor. Medicine 2019, 98, e17818. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Q.; Zhang, Y.; Li, Q.; Ma, J.; Kong, F.; Ma, X. Nomograms Based on the Novel Platelet Index Score Predict Postoperative Prognosis in Endometrial Cancer. Gynecol. Oncol. 2020, 158, 689–697. [Google Scholar] [CrossRef]
- Yayla Abide, C.; Bostanci Ergen, E.; Cogendez, E.; Kilicci, C.; Uzun, F.; Ozkaya, E.; Karateke, A. Evaluation of Complete Blood Count Parameters to Predict Endometrial Cancer. J. Clin. Lab. Anal. 2018, 32, e22438. [Google Scholar] [CrossRef]
- Yu, Y.J.; Li, N.; Yun, Z.Y.; Niu, Y.; Xu, J.J.; Liu, Z.P.; Liu, T.; Wang, R.T.; Yu, K.J. Preoperative Mean Platelet Volume and Platelet Distribution Associated with Thyroid Cancer. Neoplasma 2017, 64, 594–598. [Google Scholar] [CrossRef]
- Sit, M. Mean Platelet Volume: An Overlooked Herald of Malignant Thyroid Nodules. Acta Clin. Croat. 2019, 58, 417. [Google Scholar] [CrossRef]
- Dincel, O.; Bayraktar, C. Evaluation of Platelet Indices as a Useful Marker in Papillary Thyroid Carcinoma. Bratisl. Lek. Listy 2017, 118, 153–155. [Google Scholar] [CrossRef]
- Yildiz, S.; Eker, E.; Ozturk, M.; Alay, M. A Comparison of Hemogram Parameters of Patients with Thyroid Papillary Cancer and Nodular Goiter. J. Pak. Med. Assoc. 2019, 69, 1642–1646. [Google Scholar] [CrossRef] [PubMed]
- Bayhan, Z.; Zeren, S.; Ozbay, I.; Kahraman, C.; Yaylak, F.; Tiryaki, C.; Ekici, M.F. Mean Platelet Volume as a Biomarker for Thyroid Carcinoma. Int. Surg. 2016, 101, 50–53. [Google Scholar] [CrossRef]
- Martin, S.; Mustata, T.; Enache, O.; Ion, O.; Chifulescu, A.; Sirbu, A.; Barbu, C.; Miron, A.; Giulea, C.; Andrei, F.; et al. Platelet Activation and Inflammation in Patients with Papillary Thyroid Cancer. Diagnostics 2021, 11, 1959. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, J.; Li, S.; Zhao, Y.; Liu, G.; Du, R.; Dionigi, G.; Liang, N.; Sun, H. Prognostic Significance of Inflammatory Markers LMR, PLR, MPV, FIB in Intermediate-and High-Risk Papillary Thyroid Carcinoma. Front. Endocrinol. 2022, 13, 984157. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, H.; Li, S.; Zhang, D.; Li, J.; Dionigi, G.; Liang, N.; Sun, H. Prognostic Impact of Inflammatory Markers PLR, LMR, PDW, MPV in Medullary Thyroid Carcinoma. Front. Endocrinol. 2022, 13, 861869. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Li, N.; Liu, X.; Yun, Z.; Niu, Y.; Zhang, Y.; Gao, B.; Liu, T.; Wang, R. Platelet Distribution Width Correlates with Prognosis of Non-Small Cell Lung Cancer. Sci. Rep. 2017, 7, 3456. [Google Scholar] [CrossRef]
- Shen, X.-B.; Wang, Y.; Shan, B.-J.; Lin, L.; Hao, L.; Liu, Y.; Wang, W.; Pan, Y.-Y. Prognostic Significance Of Platelet-To-Lymphocyte Ratio (PLR) And Mean Platelet Volume (MPV) During Etoposide-Based First-Line Treatment In Small Cell Lung Cancer Patients. Cancer Manag. Res. 2019, 11, 8965–8975. [Google Scholar] [CrossRef]
- Shi, L.; Li, Y.; Yu, T.; Wang, Z.; Zhou, C.; Xing, W.; Xu, G.; Tong, B.; Zheng, Y.; Zhou, J.; et al. Predictable Resistance and Overall Survival of Gemcitabine/Cisplatin by Platelet Activation Index in Non-Small Cell Lung Cancer. Med. Sci. Monit. 2018, 24, 8655–8668. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Wang, Y.-L.; Ge, X.-X.; Xu, M.-D.; Chen, K.; Wu, M.-Y.; Gong, F.-R.; Tao, M.; Wang, W.-J.; Shou, L.-M.; et al. Prognostic Values of Platelet-Associated Indicators in Resectable Lung Cancers. Technol. Cancer Res. Treat. 2019, 18, 153303381983726. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, Y.; Cui, Y. Absolute Neutrophil Count and Mean Platelet Volume in the Blood as Biomarkers to Detect Lung Cancer. Dis. Markers 2020, 2020, 1371964. [Google Scholar] [CrossRef]
- Zu, R.; Yu, S.; Yang, G.; Ge, Y.; Wang, D.; Zhang, L.; Song, X.; Deng, Y.; He, Q.; Zhang, K.; et al. Integration of Platelet Features in Blood and Platelet Rich Plasma for Detection of Lung Cancer. Clin. Chim. Acta. 2020, 509, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Li, J.; Ye, T.; Wang, W.; Li, Y. Use of Platelet Parameters in the Differential Diagnosis of Lung Adenocarcinoma-Associated Malignant Pleural Effusion and Tuberculous Pleural Effusion. Dis. Markers 2022, 2022, 5653033. [Google Scholar] [CrossRef]
- Sakin, A.; Yasar, N.; Arici, S.; Demir, C.; Geredeli, C.; Aksaray, F.; Isik, S.; Cihan, S. Effect of Pretreatment Platelet Parameters on Survival in Limited Disease Small Cell Lung Cancer. Asian Pac. J. Cancer Prev. 2019, 20, 1879–1885. [Google Scholar] [CrossRef][Green Version]
- Omar, M.; Tanriverdi, O.; Cokmert, S.; Oktay, E.; Yersal, O.; Pilancı, K.N.; Menekse, S.; Kocar, M.; Sen, C.A.; Ordu, C.; et al. Role of Increased Mean Platelet Volume (MPV) and Decreased MPV/Platelet Count Ratio as Poor Prognostic Factors in Lung Cancer. Clin. Respir. J. 2018, 12, 922–929. [Google Scholar] [CrossRef]
- Goksel, S.; Ozcelik, N.; Telatar, G.; Ardic, C. The Role of Hematological Inflammatory Biomarkers in the Diagnosis of Lung Cancer and in Predicting TNM Stage. Cancer Invest. 2021, 39, 514–520. [Google Scholar] [CrossRef]
- Watanabe, K.; Yasumoto, A.; Amano, Y.; Kage, H.; Goto, Y.; Yatomi, Y.; Takai, D.; Nagase, T. Mean Platelet Volume and Lymphocyte-to-Monocyte Ratio Are Associated with Shorter Progression-Free Survival in EGFR-Mutant Lung Adenocarcinoma Treated by EGFR Tyrosine Kinase Inhibitor. PLoS ONE 2018, 13, e0203625. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, S.; Tokuno, J.; Ueda, Y.; Marumo, S.; Shoji, T.; Nishimura, T.; Fukui, M.; Huang, C.-L. Prognostic Significance of Preoperative Mean Platelet Volume in Resected Non-Small-Cell Lung Cancer. Mol. Clin. Oncol. 2015, 3, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.Y.; Lee, H.Y.; Chang, H.J.; Choi, C.W.; Kim, D.H.; Eo, W.K. Preoperative Plateletcrit Is a Prognostic Biomarker for Survival in Patients with Non-Small Cell Lung Cancer. J. Cancer 2020, 11, 2800–2807. [Google Scholar] [CrossRef] [PubMed]
- Łochowski, M.; Rębowski, M.; Chałubińska-Fendler, J.; Zawadzka, I.; Łochowska, B.; Cieślik-Wolski, B.; Kozak, J. Prognostic Value of Selected Platelet Parameters of Patients Operated for Non-Small Cell Lung Cancer. J. Thorac. Dis. 2022, 14, 1374–1383. [Google Scholar] [CrossRef]
- Wen, W.; Wu, P.; Li, J.; Wang, H.; Sun, J.; Chen, H. Predictive Values of the Selected Inflammatory Index in Elderly Patients with Papillary Thyroid Cancer. J. Transl. Med. 2018, 16, 261. [Google Scholar] [CrossRef]
- Wang, X.; Cui, M.-M.; Xu, Y.; Liu, L.; Niu, Y.; Liu, T.; Liu, Z.-P.; Wang, R.-T.; Yu, K.-J. Decreased Mean Platelet Volume Predicts Poor Prognosis in Invasive Bladder Cancer. Oncotarget 2017, 8, 68115–68122. [Google Scholar] [CrossRef]
- Song, Y.; Tian, J.; Yang, L.; Zhang, Y.; Dong, Z.; Ding, H.; Wang, J.; Wang, Y.; Wang, H.; Wang, Z. Prognostic Value of Preoperative Platelet-Related Parameters and Plasma Fibrinogen in Patients with Non-Muscle Invasive Bladder Cancer after Transurethral Resection of Bladder Tumor. Future Oncol. 2022, 18, 2933–2942. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Y.; Cui, J.; Chen, S.; Shi, B. Evaluation of Platelet Distribution Width as a Diagnostic and Prognostic Biomarker in Bladder Neoplasm. Future Oncol. 2019, 15, 3797–3807. [Google Scholar] [CrossRef]
- Albayrak, S.; Zengin, K.; Tanik, S.; Atar, M.; Unal, S.H.; Imamoglu, M.A.; Gurdal, M. Can the Neutrophil-to-Lymphocyte Ratio Be Used to Predict Recurrence and Progression of Non-Muscle-Invasive Bladder Cancer? Kaohsiung J. Med. Sci. 2016, 32, 327–333. [Google Scholar] [CrossRef]
- Yıldız, H.A.; Değer, M.D.; Aslan, G. Prognostic Value of Preoperative Inflammation Markers in Non-muscle Invasive Bladder Cancer. Int. J. Clin. Pract. 2021, 75, e14118. [Google Scholar] [CrossRef]
- Kucuk, S.; Mızrak, S. Diagnostic Value of Inflammatory Factors in Patients with Gallbladder Cancer, Dysplasia, and Cholecystitis. Cancer Control 2021, 28, 10732748211033746. [Google Scholar] [CrossRef]
- Prakash, B.V.; Anwar, A.Z.; Harsha, R.; Arakeri, P.R.; Jonnada, P. Role of Mean Platelet Volume in the Prognosis of Gallbladder Carcinoma: A Tertiary Centre Experience. Cureus 2021, 13, e16389. [Google Scholar] [CrossRef]
- Zhuang, Q.; Xiang, L.; Xu, H.; Fang, F.; Xing, C.; Liang, B.; Yu, K.; Feng, J. The Independent Association of Mean Platelet Volume with Overall Survival in Multiple Myeloma. Oncotarget 2016, 7, 62640–62646. [Google Scholar] [CrossRef]
- Lukoseviciene, V.; Tikuisis, R.; Dulskas, A.; Miliauskas, P.; Ostapenko, V. Surgery for Triple-Negative Breast Cancer- Does the Type of Anaesthesia Have an Influence on Oxidative Stress, Inflammation, Molecular Regulators, and Outcomes of Disease? J. BUON 2018, 23, 290–295. [Google Scholar] [PubMed]
- Kushida, A.; Inada, T.; Shingu, K. Enhancement of Antitumor Immunity after Propofol Treatment in Mice. Immunopharmacol. Immunotoxicol. 2007, 29, 477–486. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69 (Suppl. 3), 4–10. [Google Scholar] [CrossRef] [PubMed]
- Salven, P.; Orpana, A.; Joensuu, H. Leukocytes and Platelets of Patients with Cancer Contain High Levels of Vascular Endothelial Growth Factor. Clin. Cancer Res. 1999, 5, 487–491. [Google Scholar]
- Fernandes, C.J.; Morinaga, L.T.K.; Alves, J.L.; Castro, M.A.; Calderaro, D.; Jardim, C.V.P.; Souza, R. Cancer-Associated Thrombosis: The When, How and Why. Eur. Respir. Rev. 2019, 28, 180119. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, M. Role of Platelets and Platelet Receptors in Cancer Metastasis. J. Hematol. Oncol. 2018, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Sabrkhany, S.; Kuijpers, M.J.E.; Oude Egbrink, M.G.A.; Griffioen, A.W. Platelets as Messengers of Early-Stage Cancer. Cancer Metastasis Rev. 2021, 40, 563–573. [Google Scholar] [CrossRef]
- Lavergne, M.; Janus-Bell, E.; Schaff, M.; Gachet, C.; Mangin, P. Platelet Integrins in Tumor Metastasis: Do They Represent a Therapeutic Target? Cancers 2017, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Dardik, R.; Kaufmann, Y.; Savion, N.; Rosenberg, N.; Shenkman, B.; Varon, D. Platelets Mediate Tumor Cell Adhesion to the Subendothelium under Flow Conditions: Involvement of Platelet GPIIb-IIIa and Tumor Cell ?V Integrins. Int. J. Cancer 1997, 70, 201–207. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kim, H.; Kim, H.-S.; Um, S.H.; Choi, J.-W.; Oh, B.-K. MMP-2 Detective Silicon Nanowire Biosensor Using Enzymatic Cleavage Reaction. J. Biomed. Nanotechnol. 2013, 9, 732–735. [Google Scholar] [CrossRef]
- Bendas, G.; Schlesinger, M. The GPIb-IX Complex on Platelets: Insight into Its Novel Physiological Functions Affecting Immune Surveillance, Hepatic Thrombopoietin Generation, Platelet Clearance and Its Relevance for Cancer Development and Metastasis. Exp. Hematol. Oncol. 2022, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Schoene, N.; Harris, W. Mean Platelet Volume as an Indicator of Platelet Activation: Methodological Issues. Platelets 2002, 13, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Beyan, C.; Kaptan, K.; Ifran, A. Platelet Count, Mean Platelet Volume, Platelet Distribution Width, and Plateletcrit Do Not Correlate with Optical Platelet Aggegation Responses in Healthy Volunteers. J. Thromb. Thrombolysis 2006, 22, 161–164. [Google Scholar] [CrossRef]
- Inagaki, N.; Kibata, K.; Tamaki, T.; Shimizu, T.; Nomura, S. Prognostic Impact of the Mean Platelet Volume/Platelet Count Ratio in Terms of Survival in Advanced Non-Small Cell Lung Cancer. Lung Cancer 2014, 83, 97–101. [Google Scholar] [CrossRef]
- Vázquez-Santiago, M.; Ziyatdinov, A.; Pujol-Moix, N.; Brunel, H.; Morera, A.; Soria, J.M.; Souto, J.C. Age and Gender Effects on 15 Platelet Phenotypes in a Spanish Population. Comput. Biol. Med. 2016, 69, 226–233. [Google Scholar] [CrossRef]
- Panova-Noeva, M.; Schulz, A.; Hermanns, M.I.; Grossmann, V.; Pefani, E.; Spronk, H.M.H.; Laubert-Reh, D.; Binder, H.; Beutel, M.; Pfeiffer, N.; et al. Sex-Specific Differences in Genetic and Nongenetic Determinants of Mean Platelet Volume: Results from the Gutenberg Health Study. Blood 2016, 127, 251–259. [Google Scholar] [CrossRef]
- Eicher, J.D.; Lettre, G.; Johnson, A.D. The Genetics of Platelet Count and Volume in Humans. Platelets 2018, 29, 125–130. [Google Scholar] [CrossRef]
- Toplak, H.; Wascher, T.C. Influence of Weight Reduction on Platelet Volume: Different Effects of a Hypocaloric Diet and a Very Low Calorie Diet. Eur. J. Clin. Investig. 1994, 24, 778–780. [Google Scholar] [CrossRef] [PubMed]
- Yazici, M.; Kaya, A.; Kaya, Y.; Albayrak, S.; Cinemre, H.; Ozhan, H. Lifestyle Modification Decreases the Mean Platelet Volume in Prehypertensive Patients. Platelets 2009, 20, 58–63. [Google Scholar] [CrossRef]
- Taskaynatan, H.; Alacacioglu, A.; Kucukzeybek, Y.; Varol, U.; Yildiz, Y.; Salman, T.; Oflazoglu, U.; Tarhan, M.O. Is Monitoring Mean Platelet Volume Necessary in Breast Cancer Patients? Open Med. 2018, 13, 450–455. [Google Scholar] [CrossRef]
- Demir, Y.; Üçler, R.; Sürücü, E.; Turan, M.; Balli, Z.; Şengöz, T. Temporary Changes in Neutrophil-to-Lymphocyte, Platelet-to-Lymphocyte Ratios, and Mean Platelet Volume Reflecting the Inflammatory Process after Radioiodine Therapy. Nucl. Med. Commun. 2016, 37, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Batista, T.R.; de Figueiredo, R.C.; Rios, D.R.A. Platelets Volume Indexes and Cardiovascular Risk Factors. Rev. Assoc. Med. Bras. 2018, 64, 554–559. [Google Scholar] [CrossRef]
- Pujani, M.; Chauhan, V.; Singh, K.; Rastogi, S.; Agarwal, C.; Gera, K. The Effect and Correlation of Smoking with Platelet Indices, Neutrophil Lymphocyte Ratio and Platelet Lymphocyte Ratio. Hematol. Transfus. Cell Ther. 2021, 43, 424–429. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Detopoulou, P.; Voulgaridou, G.; Papadopoulou, S. Cancer, Phase Angle and Sarcopenia: The Role of Diet in Connection with Lung Cancer Prognosis. Lung 2022, 200, 347–379. [Google Scholar] [CrossRef]
- Detopoulou, P.; Nomikos, T.; Fragopoulou, E.; Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C.; Antonopoulou, S. Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) Activity, Platelet-Activating Factor Acetylhydrolase (PAF-AH) in Leukocytes and Body Composition in Healthy Adults. Lipids Health Dis. 2009, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Detopoulou, P.; Fragopoulou, E.; Nomikos, T.; Yannakoulia, M.; Stamatakis, G.; Panagiotakos, D.B.; Antonopoulou, S. The Relation of Diet with PAF and Its Metabolic Enzymes in Healthy Volunteers. Eur. J. Nutr. 2015, 54, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Detopoulou, P.; Alepoudea, E.; Nomikos, T.; Kalogeropoulos, N.; Antonopoulou, S. Associations between Red Blood Cells Fatty Acids, Desaturases Indices and Metabolism of Platelet Activating Factor in Healthy Volunteers. Prostaglandins Leukot Essent. Fat Acids 2021, 164, 102234. [Google Scholar] [CrossRef]
- Detopoulou, P.; Tsiouda, T.; Pilikidou, M.; Palyvou, F.; Tsekitsidi, E.; Mantzorou, M.; Pezirkianidou, P.; Kyrka, K.; Methenitis, S.; Voulgaridou, G.; et al. Changes in Body Weight, Body Composition, Phase Angle, and Resting Metabolic Rate in Male Patients with Stage IV Non-Small-Cell Lung Cancer Undergoing Therapy. Medicina 2022, 58, 1779. [Google Scholar] [CrossRef] [PubMed]
- Detopoulou, P.; Panoutsopoulos, G.I.; Kalonarchi, G.; Alexatou, O.; Petropoulou, G.; Papamikos, V. Development of a Tool for Determining the Equivalence of Nutritional Supplements to Diabetic Food Exchanges. Nutrients 2022, 14, 3267. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Turner, A.; Sinclair, A.J. Relationship between Platelet Phospholipid FA and Mean Platelet Volume in Healthy Men. Lipids 2002, 37, 901–906. [Google Scholar] [CrossRef] [PubMed]
| P-Opulation |
| Patients with cancer |
| I-ntervention |
| Surgery, immunotherapy, radiotherapy, Chemotherapy |
| C-Comparison |
| Comparison of MPV in healthy and cancer patients |
| O-utcome |
| Relation of MPV to survival, severity of disease, metastasis Discriminatory and diagnostic capacity of MPV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Detopoulou, P.; Panoutsopoulos, G.I.; Mantoglou, M.; Michailidis, P.; Pantazi, I.; Papadopoulos, S.; Rojas Gil, A.P. Relation of Mean Platelet Volume (MPV) with Cancer: A Systematic Review with a Focus on Disease Outcome on Twelve Types of Cancer. Curr. Oncol. 2023, 30, 3391-3420. https://doi.org/10.3390/curroncol30030258
Detopoulou P, Panoutsopoulos GI, Mantoglou M, Michailidis P, Pantazi I, Papadopoulos S, Rojas Gil AP. Relation of Mean Platelet Volume (MPV) with Cancer: A Systematic Review with a Focus on Disease Outcome on Twelve Types of Cancer. Current Oncology. 2023; 30(3):3391-3420. https://doi.org/10.3390/curroncol30030258
Chicago/Turabian StyleDetopoulou, Paraskevi, George I. Panoutsopoulos, Marina Mantoglou, Periklis Michailidis, Ifigenia Pantazi, Spyros Papadopoulos, and Andrea Paola Rojas Gil. 2023. "Relation of Mean Platelet Volume (MPV) with Cancer: A Systematic Review with a Focus on Disease Outcome on Twelve Types of Cancer" Current Oncology 30, no. 3: 3391-3420. https://doi.org/10.3390/curroncol30030258
APA StyleDetopoulou, P., Panoutsopoulos, G. I., Mantoglou, M., Michailidis, P., Pantazi, I., Papadopoulos, S., & Rojas Gil, A. P. (2023). Relation of Mean Platelet Volume (MPV) with Cancer: A Systematic Review with a Focus on Disease Outcome on Twelve Types of Cancer. Current Oncology, 30(3), 3391-3420. https://doi.org/10.3390/curroncol30030258