Abstract
(1) Background: The health-related quality of life (HRQOL) of colorectal cancer (CRC) survivors >10 years post-diagnosis is understudied. We aimed to compare the HRQOL of CRC survivors 14–24 years post-diagnosis to that of age- and sex-matched non-cancer controls, stratified by demographic and clinical factors. (2) Methods: We used data from 506 long-term CRC survivors and 1489 controls recruited from German population-based multi-regional studies. HRQOL was assessed with the European Organization for Research and Treatment of Cancer Quality of Life Core-30 (EORTC QLQ-C30) questionnaire. We estimated differences in the HRQOL of CRC survivors and controls with multiple regression, adjusted for age at survey, sex, and education, where appropriate. (3) Results: CRC survivors reported poorer social functioning but better health status/QOL than controls. CRC survivors, in general, had higher levels of symptom burden, and in particular diarrhea and constipation, regardless of demographic or clinical factors. In stratified analyses, HRQOL differed by age, sex, cancer type, and having a permanent stoma. (4) Conclusions: Although CRC survivors may have a comparable health status/QOL to controls 14–24 years after diagnosis, they still live with persistent bowel dysfunction that can negatively impact aspects of functioning. Healthcare providers should provide timely and adapted follow-up care to ameliorate potential long-term suffering.
1. Introduction
Earlier detection and better treatment have improved the relative 5-year survival after colorectal cancer (CRC) [1,2,3,4], even among survivors diagnosed at an older age [5]. Among stage I–III CRC survivors, minimal excess mortality for colon cancer may be achieved within six years post-diagnosis, and nine years post-diagnosis for rectal cancer [6].
Besides survival, health-related quality of life (HRQOL) is now considered an important outcome of cancer treatments [7]. For most CRC survivors, treatment side effects such as pain or fatigue are acute and impairments do recede back to pre-treatment levels, with concurrent improvements in HRQOL [8]. Therefore, the majority of CRC survivors can expect to live a large proportion of their remaining life in relatively good health [9]. Nevertheless, a significant proportion of survivors continue to struggle with the sequelae of CRC; for example, treatment-related symptoms that can negatively influence aspects of HRQOL years after diagnosis [10,11].
In our previous study on long-term CRC survivors 5–16 years post-diagnosis, we noted that survivors reported a global health/quality of life (QOL) comparable to that of age-matched non-cancer controls [12]. Nevertheless, specific problems with constipation and diarrhea persist, and younger (<65 years) CRC survivors reported lower levels of role and social functioning, and higher levels of financial problems.
There is a paucity of research on the HRQOL of CRC survivors extending >10 years post-diagnosis [13,14,15,16]. We recently reported that the overall global health/QOL of survivors of colorectal, breast, and prostate cancers 14–24 years after diagnosis was comparable to that of non-cancer controls [17]. The current study builds on that publication with a focus on CRC survivors and more in-depth analyses of the associations of factors such as age at survey and cancer type (colon and rectal) on HRQOL of CRC survivors in comparison to a matched non-cancer population.
Our main study objectives were to compare the HRQOL of CRC survivors 14–24 years post-diagnosis to that of non-cancer controls. Furthermore, we were interested in whether HRQOL was associated with demographic (age at survey, sex, education) and clinical (cancer type, treatment, disease status) factors.
2. Materials and Methods
2.1. CAESAR Study
The population-based CAncEr Survivorship—A multi-Regional (CAESAR) study aimed to describe the long-term HRQOL of colorectal, breast, and prostate cancer survivors. The German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) conducted the study in collaboration with six epidemiologic cancer registries in Germany (Bremen, Hamburg, North Rhine-Westphalia, Rhineland-Palatinate, Schleswig-Holstein, and Saarland). This study used data from five cancer registries as no CRC survivors were recruited from Schleswig-Holstein for logistical reasons. Cancer survivors diagnosed between January 1994 and June 2004, as registered in the participating cancer registries, and aged between 20 and 75 years at diagnosis, were eligible. Details of the initial study have been described elsewhere [18]. Initial recruitment was in 2008–2010, 5–16 years post-diagnosis. Between 2018 and 2019, a follow-up assessment was conducted among survivors (14–24 years post-diagnosis) who had given consent at initial recruitment to be re-contacted and who were still alive [17]. Of the 2704 (62.9%) participants who returned a full-length questionnaire at follow-up assessment, 506 (19%) were CRC survivors. Further details about the response have been previously reported, namely that CRC survivors were less likely to participate at follow-up in contrast to breast or prostate cancer survivors [17].
2.2. LinDe Study
An individual level HRQOL from a representative sample of the German population was accessed from the Lebensqualität in DEutschland (‘Quality of life in Germany’, LinDE) study [18]. Eligible participants aged 18 and above, stratified by age and sex, were randomly selected from the general German population via regional municipal offices. Data collection was conducted between 2013 and 2014. Potential participants received detailed study information and a questionnaire by mail. Non-respondents received two follow-up reminder mails and a telephone contact (or one mailed reminder or home visit, if necessary). Further details of sample selection are reported elsewhere [18]. For the current study, we selected as controls those participants who completed a full questionnaire, were cancer-free, and of comparable age to the CRC sample at CAESAR follow-up.
2.3. HRQOL Assessment
HRQOL and financial difficulty were assessed with the European Organization for Research and Treatment of Cancer Quality of Life Core-30 (EORTC QLQ-C30) questionnaire [19]. This 30-item questionnaire consists of five functional scales (physical, role, cognitive, emotional, social), a global health/QOL scale, and nine items/scales on symptoms and financial impact. Answers are ranged from 1 (not at all) to 4 (very much), and from 1 (‘very poor’) to 7 (‘excellent’) for items in the global health/QOL scale. All raw scores were linearly transformed to scales of 0–100 using standard procedures [20]. Higher functioning and global health/QOL scores indicate better function or health status; higher scores on symptoms and financial difficulty indicate greater symptom burden and financial problems.
2.4. Demographics and Clinical Data
The CAESAR questionnaire contained questions concerning sociodemographic factors and clinical history. Self-reported data include primary treatment received, disease recurrence since index cancer (recurrence, metastasis, new primary cancer), and comorbid conditions. Participating cancer registries provided clinical data such as date of diagnosis and the tumor stage (from the initial 2008–2010 data collection). Other self-reported data collected in the initial data survey include monthly household income, education, type of employment (e.g., employee, self-employed), and work situation (e.g., full/part-time). The classification of cancer site was according to the International Classification of Diseases-10 codes.
Relevant data from surveys in 2008–2010 and 2018–2019 were combined for this analysis.
2.5. Statistical Analyses
We compared sociodemographic differences between CRC survivors and population controls with Cochran–Mantel–Haenszel tests. Although the age distribution of the population controls reflected a stratified sampling scheme, controls were significantly younger than CRC survivors. Therefore, we used direct standardization for further comparisons of sample characteristics, using the age and sex distribution of CRC survivors as standard.
We used multiple linear regression to derive least square mean HRQOL scores of CRC survivors and controls. In models stratified by disease status, we categorized CRC survivors accordingly: stage I–III at diagnosis and with no reports of recurrence/metastasis/new cancer at follow-up (‘disease-free’); stage IV at diagnosis or self-report of subsequent recurrence/metastasis/new cancer after CRC diagnosis (’active disease’). Linear regression models were adjusted for age (in 5-year bands: 45–49, 50–54…90–94), sex, and education at the time of the survey, where appropriate. Covariates included for adjustment in the models were selected a priori, based on previous associations with HRQOL reported in the literature [21,22]. Although employment, relationship status, and comorbidities differed between survivors and controls, we did not include these as covariates in the models because they may reflect the situation at the time of survey. Potential differences in those variables between CRC survivors could be a consequence of the cancer.
Missing data were imputed using the Markov chain Monte Carlo method with 25 repetitions to reduce possible bias due to missing values (generally <10%). All analyses were conducted with SAS (version 9.4 for Windows, SAS Institute Inc., Cary, NC, USA). Statistical significance is determined at p < 0.05 (two-sided). The p-values were not adjusted for multiple testing, referring to the individual tests rather than a global test for differences.
3. Results
3.1. Non-Response Analysis
Of the 1216 CRC participants in the initial CAESAR survey, 292 (24%) had died before follow-up. Of the survivors eligible for the follow-up survey (n = 924), 506 (55%) returned a completed questionnaire (Figure 1).
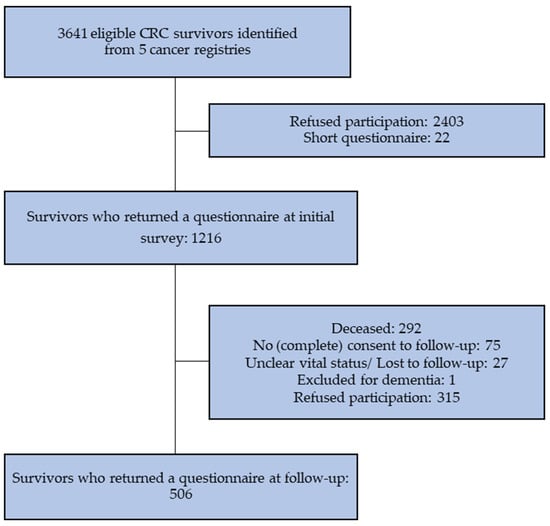
Figure 1.
Flowchart of inclusion of CRC survivors.
Respondents to the follow-up survey were younger at initial survey, more likely to be male, and to be treated surgically (Table 1). Non-respondents were more likely to be diagnosed with colon cancer and less likely to have received chemotherapy or radiotherapy. Those who had died before follow-up were older at time of diagnosis, were more often male, had stage III or IV CRC, and had a permanent stoma.

Table 1.
Characteristics of sample according to response to follow-up survey.
3.2. Characteristics of CRC Survivors and Controls
CRC survivors were older and more likely to be male, when compared with the controls (Table 2). Even after age and sex standardization, there remained significant differences in the characteristics between the two groups. CRC survivors were less likely to be employed, more likely to be in a partnered relationship, and generally less likely to have cardiovascular, gastro-intestinal, or neuro-skeletal comorbidities.

Table 2.
Characteristics of CRC survivors and population controls.
3.3. Characteristics of CRC Survivors by Cancer Type
There were no significant differences in characteristics between colon and rectal cancer survivors except for treatment. Rectal cancer survivors were more likely to have received chemotherapy and radiotherapy, and to have a permanent stoma (Table 3).

Table 3.
Characteristics of cancer survivors by cancer type.
3.4. HRQOL of CRC Survivors and Controls
In general, CRC survivors reported levels of functioning comparable to controls, except for social functioning that was statistically significantly lower than controls (Figure 2). CRC survivors reported a better health status/QOL than controls. With respect to symptom burden, CRC survivors reported less pain but more problems with dyspnea, constipation, diarrhea, and nausea/vomiting than controls.
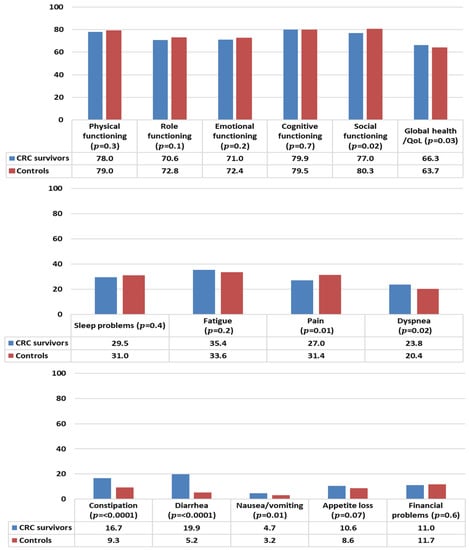
Figure 2.
Mean EORTC QLQ-C30 scores of colorectal cancer (CRC) survivors and controls. Models are adjusted for age at survey, sex, and education. All results are based on 25 imputations of missing values.
3.5. HRQOL of CRC Survivors and Controls, Stratified by Demographic Factors
3.5.1. By Age at Survey
CRC survivors who were <75 years old tended to report lower functioning scores than age-matched controls, although significant differences were noted only in emotional functioning, cognitive functioning, and social functioning (Figure 3). Survivors who were 75–84 years old reported better cognitive functioning and global health/QOL than controls in the same age group. In terms of symptom burden, CRC survivors in the 65–74 years age group reported higher levels of fatigue, dyspnea, and appetite loss than age-matched controls. Survivors in the age groups < 65 and 65–74 years were more likely to report higher levels of nausea/vomiting than controls in similar age groups. Regardless of age, CRC survivors reported higher levels of constipation and diarrhea.
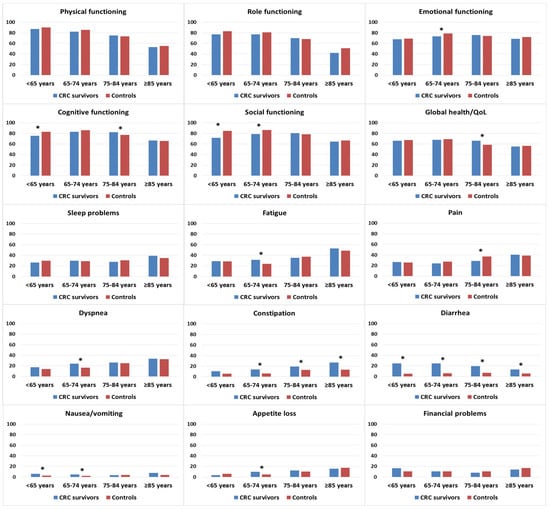
Figure 3.
Mean EORTC QLQ-C30 scores of colorectal cancer (CRC) survivors and controls, stratified by age at survey. Models are adjusted for age at survey, sex, and education. Asterisks (*) indicate significantly differences (p < 0.05) between the subgroups. All results are based on 25 imputations of missing values.
3.5.2. By Education
There were no differences in functioning when stratified by years of education, with the exception of cognitive and social functioning. In these two subscales, CRC survivors with 10–11 years of education reported lower scores than controls with equivalent education (Figure 4). Among those with ≤9 years of education, CRC survivors reported higher levels of global health/QOL than controls. For symptom burden, CRC survivors reported significantly more problems with constipation and diarrhea than controls, irrespective of education level. Increased problems with nausea/vomiting in CRC survivors compared to controls were observed in the group with 10–11 years of education.
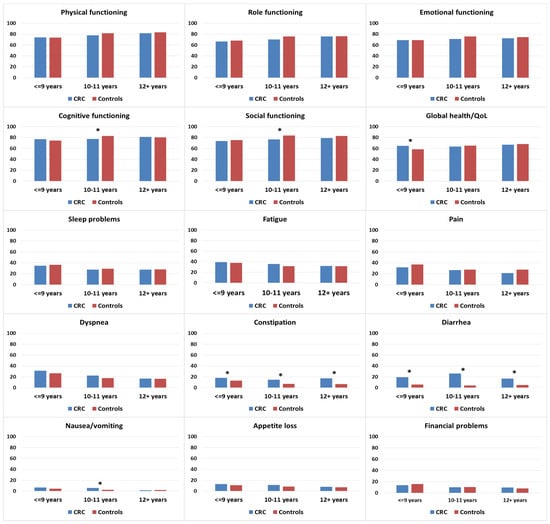
Figure 4.
Mean EORTC QLQ-C30 scores of colorectal cancer (CRC survivors) and controls, stratified by education. Models are adjusted for age at survey and sex. Asterisks (*) indicate significantly significant differences (p < 0.05) between the subgroups. All results are based on 25 imputations of missing values.
3.6. HRQOL of Survivors by Cancer Type and Controls, Stratified by Sex
The functioning and global health/QOL scores of female colon and rectal cancer survivors were comparable with those of female controls (Figure 5). Female rectal cancer survivors were more likely to be fatigued and have appetite loss when compared with female controls. Female colon cancer survivors experienced more problems with nausea/vomiting when compared with female controls. Female colon and rectal cancer survivors reported significantly more problems with constipation than female controls. For diarrhea, female rectal cancer survivors reported significantly higher levels than female colon cancer survivors and controls.
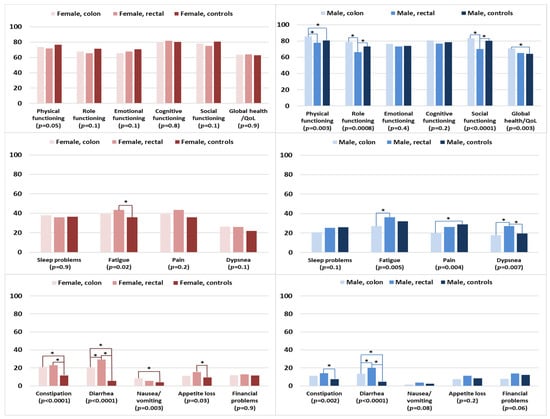
Figure 5.
Mean EORTC QLQ-C30 scores of survivors (colon and rectal cancer) and controls, stratified by sex. Models are adjusted for age at survey and education. The p-values shown indicate the global comparison between cancer survivors and controls, separated by sex. The line spans and asterisks (*) indicate the subgroups that showed significant differences in pairwise comparison. For example, the line span for physical functioning in male colon survivors is across three columns, indicating that male colon survivors reported significantly higher physical functioning when compared with male controls. All results are based on 25 imputations of missing values.
Among the male participants, rectal cancer survivors reported the lowest levels of functioning, namely in the domains of physical functioning (versus colon cancer survivors), and role and social functioning in comparison with colon cancer survivors and controls (Figure 5). In contrast, male colon cancer survivors reported better physical functioning and global health/QOL than male controls. Male rectal cancer survivors tended to report higher levels of symptom burden than male colon survivors or controls, notably for fatigue, dyspnea, constipation, and diarrhea. Male colon cancer survivors reported lower levels of pain but higher levels of diarrhea when compared with male controls.
3.7. HRQOL of Survivors Stratified by Clinical Factors, and Controls
3.7.1. By Stoma Status
CRC survivors with a permanent stoma reported significantly lower levels of physical, role, and social functioning when compared with stoma-free CRC survivors or controls (Figure 6). Stoma-free survivors reported better global health/QOL than controls. CRC survivors with a stoma were more likely to report higher levels of fatigue and dyspnea when compared with colon cancer survivors and controls. Stoma-free CRC survivors reported less pain than controls but higher levels of constipation, in comparison with CRC survivors with permanent stoma or controls. CRC survivors, regardless of stoma status, reported more problems with diarrhea than controls.
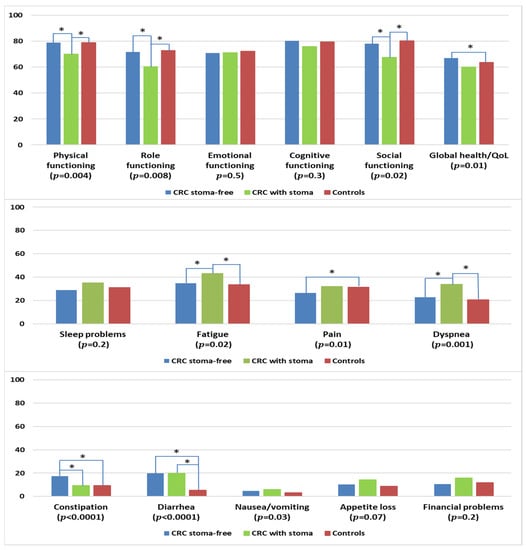
Figure 6.
Mean EORTC QLQ-C30 scores of colorectal cancer (CRC) survivors with and without permanent stoma, and controls. Models are adjusted for age at survey, sex, and education. The p-values shown indicate the global comparison between cancer survivors with and without stoma and controls. The line spans and asterisks (*) indicate the subgroups that showed significant differences in pairwise comparison. For example, the line span for global health/QOL is across three columns, indicating that stoma-free CRC survivors reported significantly higher global health/QOL when compared with controls. All results are based on 25 imputations of missing values.
3.7.2. By Disease Status
CRC survivors with active disease reported lower levels of emotional functioning when compared with disease-free survivors or controls (Figure 7). Survivors who remained disease-free reported higher levels of global health/QOL and lower levels of pain than controls. CRC survivors, regardless of disease status, had more problems with constipation and diarrhea than controls. Survivors with active disease reported more problems with nausea/vomiting than disease-free survivors or controls.
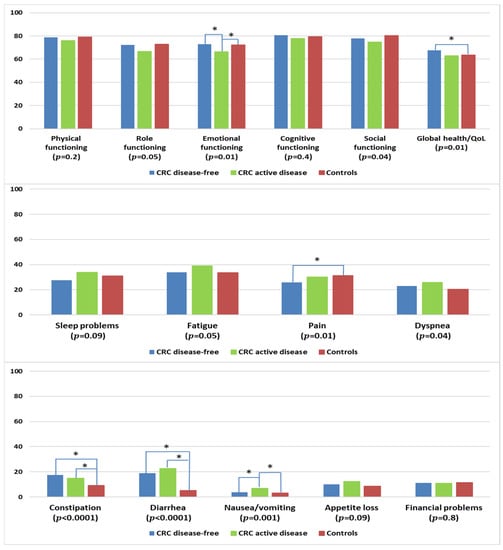
Figure 7.
Mean EORTC QLQ-C30 scores of colorectal cancer (CRC) survivors by disease status, and controls. Models are adjusted for age at survey, sex, and education. Disease-free: stage I–III at diagnosis and remaining disease-free at follow-up according to self-report; active disease: stage IV at diagnosis or reported subsequent recurrence, metastasis, or primary cancer. The p-values shown indicate the global comparison between cancer survivors with and without active disease and controls. The line spans and asterisks (*) indicate the subgroups that showed significant differences in pairwise comparison. For example, the line span for global health/QOL is across three columns, indicating that disease-free CRC survivors reported significantly higher global health/QOL when compared with controls. All results are based on 25 imputations of missing values.
4. Discussion
With CRC survivors living longer, it is important to have a better understanding of the quality of this prolonged survival. We found that CRC survivors 14–24 years after diagnosis, in general, had comparable levels of functioning and global health/QOL to age- and sex-matched population controls. Nevertheless, we see persistent deficits in aspects of functioning and a higher symptom burden in subgroup analyses.
We observed a consistent pattern of lower levels of social functioning among CRC survivors, most prominent among survivors who were <75 years old, with 10–11 years of education, male rectal cancer survivors, and survivors with a permanent stoma. In addition, constipation and diarrhea remain as problems of significance among survivors in comparison with controls, irrespective of age, sex, education, cancer type, and disease status. Treatments for CRC have been associated with chronic bowel dysfunction [11,23], of which the impact on HRQOL is significant [24]. It is intuitive that persistent bowel problems could hinder daily activities and restrict social participation [25]. CRC survivors described feelings of embarrassment and a loss of control resulting from bowel dysfunction, and struggles to self-manage these problems, e.g., toilet mapping, extra planning, or diet changes [26,27].
Male rectal cancer survivors and survivors with permanent stoma reported similar patterns of deficits, namely poorer levels of physical, role, and social functioning, and higher levels of fatigue, dyspnea, and diarrhea than comparison groups. These results are consistent with concerns commonly shared by individuals living with a stoma [28]. Problems associated with a stoma are well documented [29,30], and can pose a threat to body image and self-confidence [25]. A stoma can restrict physical, role (including work) and social functioning [25], and reduce HRQOL [31].
Survivors younger than 75 years at survey were more likely to have deficits in emotional, cognitive, and social functioning. Keeping in mind that our study involved survivors who were diagnosed up to 24 years previously, this would suggest that poorer emotional and social functioning scores observed closer to the time since diagnosis can persist in survivors who were diagnosed at a younger age [32]. Furthermore, in the study by Dunn et al., survivors who were diagnosed at younger age not only had consistently poorer emotional and social functioning up to 5 years post-diagnosis, but reported consistently lower life satisfaction [32]. With a trend in increasing incidence of CRC among persons younger than 50 years of age [33], these results indicate that greater attention to the adaption process of life after cancer for younger CRC survivors is paramount. A better identification of vulnerable survivors and the timely addressing, e.g., closer to time since diagnosis, of potential physical, psychosocial, and financial needs of young-onset CRC survivors may reduce the long-term burden of CRC.
4.1. Clinical Implications
Despite the improved prognosis after CRC diagnosis, a significant proportion of survivors are living with long-term symptoms and functioning impairments. Existing clinical guidelines on CRC follow-up care tend to focus on the detection of early recurrences, the timing of follow-ups, and appropriate diagnostic tools, but few guidelines provide adequate recommendations for the management of long-term symptoms [34]. For example, the German guideline provides recommendations that CRC survivors should be encouraged to improve their health and QOL. However, the guideline does not give specifics on how or from whom this encouragement should be provided, but states that survivors benefit if they self-manage their symptoms and side effects [35]. Bowel dysfunction remains a significant problem for CRC survivors. Although there are treatments for bowel problems including recommendations of diet adjustments [36,37,38], survivors may not be aware of such help. There is a discrepancy between healthcare professionals reporting the provision of dietary advice and the proportion of survivors remembering that such advice was provided [39]. Studies have shown that survivors respond better to clinician-provided recommendations for lifestyle change [40], and are positive about the multidisciplinary approach to symptom management adopted by survivorship care clinics [41]. Changes in diagnostics and treatments for CRC in recent years (e.g., the advent of minimally invasive surgery) [42,43], and increasing awareness of unmet needs, such as the provision of psycho-oncological support could have improved the HRQOL of survivors with a more recent diagnosis [44,45]. However, a significant proportion of long-term survivors may not have benefitted from these recent improvements in survivorship care [46]. Reasons could include an unawareness of the potential persisting or late effects of treatment and help thereof in survivors and clinicians, was ‘lost’ to follow-up when transitioning from acute to long-term survivorship or fragmented delivery of health care. Collectively, these results suggest that clinical guidelines need to be updated by incorporating emerging evidence on supportive care interventions, to include more comprehensive recommendations for the management of the long-term symptoms of CRC survivors [34]. Recently, the European Society for Medical Oncology (ESMO) published an Expert Consensus Statement on Cancer Survivorship, highlighting the need for the provision of care plans for survivors to monitor and manage the physical effects of cancer [47]. Recommendations include the provision of early (during active treatment phase) educational and self-management support to anticipate possible late or persistent survivorship care needs. Furthermore, at-risk survivors could benefit from a coordinated multidisciplinary approach to symptom management, from earlier detection to established referral pathways to relevant specialists [47,48].
4.2. Strengths and Limitations
Our population-based study focused on the long-term HRQOL of CRC survivors 14–24 years after diagnosis, a group that is currently understudied. Another strength is the adequate sample size that allowed for subgroup analyses. However, our study has several limitations to consider. Although our sample size is adequate, nevertheless with a response rate of 55%, our results may not be generalizable to CRC survivors of a similar vintage. There were significant differences in characteristics between respondents and non-respondents. This potential participation bias may influence the reported mean HRQOL estimates. Participants provided clinical data in the context of the initial study round in 2008–2010. As some participants were diagnosed up to 16 years before the initial survey, the possibility of under-reporting or problem with recall (e.g., wrong time frame, misclassification of endoscopic or laparoscopic surgery) has to be discussed. It is unlikely that almost 20% of all participants did not undergo surgery for primary treatment. In addition, the participants who were classified as stoma-free could have had a temporary stoma although we do not know the duration and when the stoma was removed. We reported on the cross-sectional associations of clinical and demographic factors with HRQOL, and therefore the results should not be interpreted as causal associations.
5. Conclusions
In conclusion, while CRC survivors 14–24 years after diagnosis may have a comparable health status/QOL to age- and sex-matched controls, they still live with persistent bowel dysfunction that can negatively impact aspects of functioning. Health care providers should regularly screen for potential unmet needs and provide timely follow-up care that is adapted to the needs of CRC survivors, to ameliorate potential long-term suffering.
Author Contributions
Conceptualization, M.S.Y.T. and V.A.; Data curation, M.S.Y.T., D.D., L.W., L.K.-G., L.J., H.B. (Heike Bertram), A.E., B.H., A.N., A.W. and S.R.Z.; Formal analysis, M.S.Y.T.; Funding acquisition, H.B. (Hermann Brenner) and V.A.; Project administration, D.D. and L.J.; Writing—original draft, M.S.Y.T. Writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the German Cancer Aid (No. 108262, 110231, and 70112089). The funding source was neither involved in the collection, interpretation, and analysis of the data, nor in the decision for the writing and submission of this report for publication.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the ethics committee of the Medical Faculty of the University of Heidelberg (CAESAR: S438/2008, LinDe: S499/2012), and by the responsible local ethics committees of the participating cancer registries (CAESAR).
Informed Consent Statement
All participants provided written informed consent.
Data Availability Statement
Data is available from the authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- André, T.; Meyerhardt, J.; Iveson, T.; Sobrero, A.; Yoshino, T.; Souglakos, I.; Grothey, A.; Niedzwiecki, D.; Saunders, M.; Labianca, R.; et al. Effect of duration of adjuvant chemotherapy for patients with stage III colon cancer (IDEA collaboration): Final results from a prospective, pooled analysis of six randomised, phase 3 trials. Lancet Oncol. 2020, 21, 1620–1629. [Google Scholar] [CrossRef]
- Cardoso, R.; Guo, F.; Heisser, T.; Hackl, M.; Ihle, P.; De Schutter, H.; Van Damme, N.; Valerianova, Z.; Atanasov, T.; Májek, O.; et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: An international population-based study. Lancet Oncol. 2021, 22, 1002–1013. [Google Scholar] [CrossRef]
- van Gijn, W.; Marijnen, C.A.; Nagtegaal, I.D.; Kranenbarg, E.M.; Putter, H.; Wiggers, T.; Rutten, H.J.; Påhlman, L.; Glimelius, B.; van de Velde, C.J. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011, 12, 575–582. [Google Scholar] [CrossRef]
- Jansen, L.; Castro, F.A.; Gondos, A.; Krilaviciute, A.; Barnes, B.; Eberle, A.; Emrich, K.; Hentschel, S.; Holleczek, B.; Katalinic, A.; et al. Recent cancer survival in Germany: An analysis of common and less common cancers. Int. J. Cancer 2014, 136, 2649–2658. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger-Raab, A.; Werner, J.; Friess, H.; Hölzel, D.; Engel, J. Age and Outcome in Gastrointestinal Cancers: A Population-Based Evaluation of Oesophageal, Gastric and Colorectal Cancer. Visc. Med. 2017, 33, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, S.M.; Dickman, P.W.; De Wilt, J.H.; Verhoeven, R. Conditional Survival and Cure of Patients with Colon or Rectal Cancer: A Population-Based Study. J. Natl. Compr. Cancer Netw. 2020, 18, 1230–1237. [Google Scholar] [CrossRef]
- United States Department of Health and Human Services Food and Drug Administration. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims; United States Department of Health and Human Services Food and Drug Administration: Silver Spring, MD, USA, 2009.
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Soerjomataram, I.; Thong, M.S.Y.; Ezzati, M.; Lamont, E.B.; Nusselder, W.J.; Van De Poll-Franse, L.V. Most colorectal cancer survivors live a large proportion of their remaining life in good health. Cancer Causes Control 2012, 23, 1421–1428. [Google Scholar] [CrossRef][Green Version]
- Mols, F.; Beijers, T.; Lemmens, V.; Hurk, C.J.V.D.; Vreugdenhil, G.; van de Poll-Franse, L.V. Chemotherapy-Induced Neuropathy and Its Association with Quality of Life Among 2- to 11-Year Colorectal Cancer Survivors: Results from the Population-Based PROFILES Registry. J. Clin. Oncol. 2013, 31, 2699–2707. [Google Scholar] [CrossRef]
- Chen, T.Y.-T.; Wiltink, L.M.; Nout, R.A.; Kranenbarg, E.M.-K.; Laurberg, S.; Marijnen, C.A.; van de Velde, C.J. Bowel Function 14 Years after Preoperative Short-Course Radiotherapy and Total Mesorectal Excision for Rectal Cancer: Report of a Multicenter Randomized Trial. Clin. Color. Cancer 2014, 14, 106–114. [Google Scholar] [CrossRef]
- Thong, M.S.Y.; Koch-Gallenkamp, L.; Jansen, L.; Bertram, H.; Eberle, A.; Holleczek, B.; Waldeyer-Sauerland, M.; Waldmann, A.; Zeissig, S.R.; Brenner, H.; et al. Age-specific health-related quality of life in long-term and very long-term colorectal cancer survivors versus population controls—A population-based study. Acta Oncol. 2019, 58, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.A.; Cameron, L.D.; Brown, P.M.; Whitehead, L.C.; Porter, D.; Ottaway-Parkes, T.; Robinson, E. Time Since Diagnosis as a Predictor of Symptoms, Depression, Cognition, Social Concerns, Perceived Benefits, and Overall Health in Cancer Survivors. Oncol. Nurs. Forum 2010, 37, 331–338. [Google Scholar] [CrossRef]
- Caravati-Jouvenceaux, A.; Launoy, G.; Klein, D.; Henry-Amar, M.; Abeilard, E.; Danzon, A.; Pozet, A.; Velten, M.; Mercier, M. Health-Related Quality of Life Among Long-Term Survivors of Colorectal Cancer: A Population-Based Study. Oncologist 2011, 16, 1626–1636. [Google Scholar] [CrossRef] [PubMed]
- Hart, T.L.; Charles, S.T.; Gunaratne, M.; Baxter, N.N.; Cotterchio, M.; Cohen, Z.; Gallinger, S. Symptom Severity and Quality of Life Among Long-term Colorectal Cancer Survivors Compared with Matched Control Subjects: A Population-Based Study. Dis. Colon Rectum 2018, 61, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Kunitake, H.; Russell, M.M.; Zheng, P.; Yothers, G.; Land, S.R.; Petersen, L.; Fehrenbacher, L.; Giguere, J.K.; Wickerham, D.L.; Ko, C.Y.; et al. Quality of life and symptoms in long-term survivors of colorectal cancer: Results from NSABP protocol LTS-01. J. Cancer Surviv. 2016, 11, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Doege, D.; Thong, M.S.; Weisser, L.; Koch-Gallenkamp, L.; Jansen, L.; Bertram, H.; Eberle, A.; Holleczek, B.; Nennecke, A.; Pritzkuleit, R.; et al. Health-related quality of life in cancer survivors >10 years past diagnosis compared to population controls: A population-based study. Cancers 2021, 13, 2754. [Google Scholar] [CrossRef]
- Arndt, V.; Koch-Gallenkamp, L.; Jansen, L.; Bertram, H.; Eberle, A.; Holleczek, B.; Schmid-Höpfner, S.; Waldmann, A.; Zeissig, S.R.; Brenner, H. Quality of life in long-term and very long-term cancer survivors versus population controls in Germany. Acta Oncol. 2017, 56, 190–197. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. JNCI J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Fayers, P.M.; Aaronson, N.K.; Bjordal, K.; Sullivan, M. EORTC QLQ-C30 Scoring Manual; EORTC: Brussels, Belgium, 1995. [Google Scholar]
- Jansen, L.; Koch, L.; Brenner, H.; Arndt, V. Quality of life among long-term (≥5 years) colorectal cancer survivors—Systematic review. Eur. J. Cancer 2010, 46, 2879–2888. [Google Scholar] [CrossRef]
- Reyes, M.E.; Ye, Y.; Zhou, Y.; Liang, A.; Kopetz, S.; Rodriquez, M.A.; Wu, X.; Hildebrandt, M.A.T. Predictors of health-related quality of life and association with survival may identify colorectal cancer patients at high risk of poor prognosis. Qual. Life Res. 2016, 26, 319–330. [Google Scholar] [CrossRef]
- van Heinsbergen, M.; Janssen-Heijnen, M.L.; Leijtens, J.W.; Slooter, G.D.; Konsten, J.L. Bowel dysfunction after sigmoid resection underestimated: Multicentre study on quality of life after surgery for carcinoma of the rectum and sigmoid. Eur. J. Surg. Oncol. 2018, 44, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Al Rashid, F.; Liberman, A.S.; Charlebois, P.; Stein, B.; Feldman, L.S.; Fiore, J.F., Jr.; Lee, L. The impact of bowel dysfunction on health-related quality of life after rectal cancer surgery: A systematic review. Tech. Coloproctol. 2022, 26, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.Y.S.; Laidsaar-Powell, R.C.; Young, J.M.; Kao, S.C.; Zhang, Y.; Butow, P. Colorectal cancer survivorship: A systematic review and thematic synthesis of qualitative research. Eur. J. Cancer Care 2021, 30, e13421. [Google Scholar] [CrossRef] [PubMed]
- Drury, A.; Payne, S.; Brady, A.-M. Prevalence vs impact: A mixed methods study of survivorship issues in colorectal cancer. Qual. Life Res. 2021, 31, 1117–1134. [Google Scholar] [CrossRef]
- Sun, V.; Grant, M.; Wendel, C.S.; McMullen, C.K.; Bulkley, J.E.; Altschuler, A.; Ramirez, M.; Baldwin, C.M.; Herrinton, L.J.; Hornbrook, M.C.; et al. Dietary and Behavioral Adjustments to Manage Bowel Dysfunction After Surgery in Long-Term Colorectal Cancer Survivors. Ann. Surg. Oncol. 2015, 22, 4317–4324. [Google Scholar] [CrossRef] [PubMed]
- Jansen, F.; Van Uden-Kraan, C.F.; Braakman, J.A.; Van Keizerswaard, P.M.; Witte, B.I.; Leeuw, I.M.V.-D. A mixed-method study on the generic and ostomy-specific quality of life of cancer and non-cancer ostomy patients. Support. Care Cancer 2014, 23, 1689–1697. [Google Scholar] [CrossRef]
- Mols, F.; Lemmens, V.; Bosscha, K.; Broek, W.V.D.; Thong, M.S. Living with the physical and mental consequences of an ostomy: A study among 1-10-year rectal cancer survivors from the population-based PROFILES registry. Psycho-Oncology 2014, 23, 998–1004. [Google Scholar] [CrossRef]
- Näsvall, P.; Dahlstrand, U.; Löwenmark, T.; Rutegård, J.; Gunnarsson, U.; Strigård, K. Quality of life in patients with a permanent stoma after rectal cancer surgery. Qual. Life Res. 2016, 26, 55–64. [Google Scholar] [CrossRef]
- Vonk-Klaassen, S.M.; De Vocht, H.M.; den Ouden, M.E.M.; Eddes, E.H.; Schuurmans, M.J. Ostomy-related problems and their impact on quality of life of colorectal cancer ostomates: A systematic review. Qual. Life Res. 2016, 25, 125–133. [Google Scholar] [CrossRef]
- Dunn, J.; Ng, S.K.; Breitbart, W.; Aitken, J.; Youl, P.; Baade, P.D.; Chambers, S.K. Health-related quality of life and life satisfaction in colorectal cancer survivors: Trajectories of adjustment. Health Qual. Life Outcomes 2013, 11, 46. [Google Scholar] [CrossRef]
- Wong, M.C.; Huang, J.; Lok, V.; Wang, J.; Fung, F.; Ding, H.; Zheng, Z.-J. Differences in Incidence and Mortality Trends of Colorectal Cancer Worldwide Based on Sex, Age, and Anatomic Location. Clin. Gastroenterol. Hepatol. 2020, 19, 955–966.e61. [Google Scholar] [CrossRef]
- Wiltink, L.M.; White, K.; King, M.T.; Rutherford, C. Systematic review of clinical practice guidelines for colorectal and anal cancer: The extent of recommendations for managing long-term symptoms and functional impairments. Support. Care Cancer 2020, 28, 2523–2532. [Google Scholar] [CrossRef] [PubMed]
- German Cancer Society GCA, AWMF. German Guideline Program in Oncology; S3-Guideline Colorectal Cancer, Long Version 2.1, 2019 AWMF Registration Number: 021-007OL; German Cancer Society GCA, AWMF: Berlin, Germany, 2019. [Google Scholar]
- Yde, J.; Larsen, H.M.; Laurberg, S.; Krogh, K.; Moeller, H.B. Chronic diarrhoea following surgery for colon cancer—Frequency, causes and treatment options. Int. J. Colorectal. Dis. 2018, 33, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Larsen, H.M.; Borre, M.; Christensen, P.; Drewes, A.; Laurberg, S.; Krogh, K.; Fassov, J. Clinical evaluation and treatment of chronic bowel symptoms following cancer in the colon and pelvic organs. Acta Oncol. 2019, 58, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Song, M. Chapter Four—Personalized nutrition for colorectal cancer. In Advances in Cancer Research; Berger, F.G., Boland, C.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 109–136. [Google Scholar]
- Borre, M.; Fassov, J.; Juul, T.; Laurberg, S.; Christensen, P.; Bräuner, A.B.; Ussing, O.T.; Lauritzen, M.B.; Drewes, A.M.; Faaborg, P.M.; et al. Diet and bowel symptoms among colon cancer survivors. Acta Oncol. 2022, 61, 1192–1199. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Aziz, N.M.; Rowland, J.H.; Pinto, B.M. Riding the Crest of the Teachable Moment: Promoting Long-Term Health After the Diagnosis of Cancer. J. Clin. Oncol. 2005, 23, 5814–5830. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Turner, J.; Kerin-Ayres, K.; Butler, S.; Deguchi, C.; Khatri, S.; Mo, C.; Warby, A.; Cunningham, I.; Malalasekera, A.; et al. Health concerns of cancer survivors after primary anti-cancer treatment. Support. Care Cancer 2019, 27, 3739–3747. [Google Scholar] [CrossRef] [PubMed]
- Akhtar-Danesh, N.; Logie, K.; Akhtar-Danesh, G.-G.; Finley, C. Uptake of minimally invasive surgery for early stage colorectal cancer and its effect on survival: A population-based study. Surg. Oncol. 2020, 35, 540–546. [Google Scholar] [CrossRef]
- Benz, S.; Barlag, H.; Gerken, M.; Fürst, A.; Klinkhammer-Schalke, M. Laparoscopic surgery in patients with colon cancer: A population-based analysis. Surg. Endosc. 2016, 31, 2586–2595. [Google Scholar] [CrossRef]
- Sussman, J.; Bainbridge, D.; Whelan, T.J.; Brazil, K.; Parpia, S.; Wiernikowski, J.; Schiff, S.; Rodin, G.; Sergeant, M.; Howell, D. Evaluation of a specialized oncology nursing supportive care intervention in newly diagnosed breast and colorectal cancer patients following surgery: A cluster randomized trial. Support. Care Cancer 2017, 26, 1533–1541. [Google Scholar] [CrossRef]
- Thong, M.S.Y.; Jansen, L.; Chang-Claude, J.; Hoffmeister, M.; Brenner, H.; Arndt, V. Association of laparoscopic colectomy versus open colectomy on the long-term health-related quality of life of colon cancer survivors. Surg. Endosc. 2020, 34, 5593–5603. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. From Cancer Patients to Cancer Survivors: Lost in Transition; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Vaz-Luis, I.; Masiero, M.; Cavaletti, G.; Cervantes, A.; Chlebowski, R.; Curigliano, G.; Felip, E.; Ferreira, A.; Ganz, P.; Hegarty, J.; et al. ESMO Expert Consensus Statements on Cancer Survivorship: Promoting high-quality survivorship care and research in Europe. Ann. Oncol. 2022, 33, 1119–1133. [Google Scholar] [CrossRef] [PubMed]
- Muls, A.C.; Watson, L.; Shaw, C.; Andreyev, H.J.N. Managing gastrointestinal symptoms after cancer treatment: A practical approach for gastroenterologists. Front. Gastroenterol. 2012, 4, 57–68. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).