Tumor-Infiltrating Immune Cell Landscapes in the Lymph Node Metastasis of Papillary Thyroid Cancer
Abstract
1. Introduction
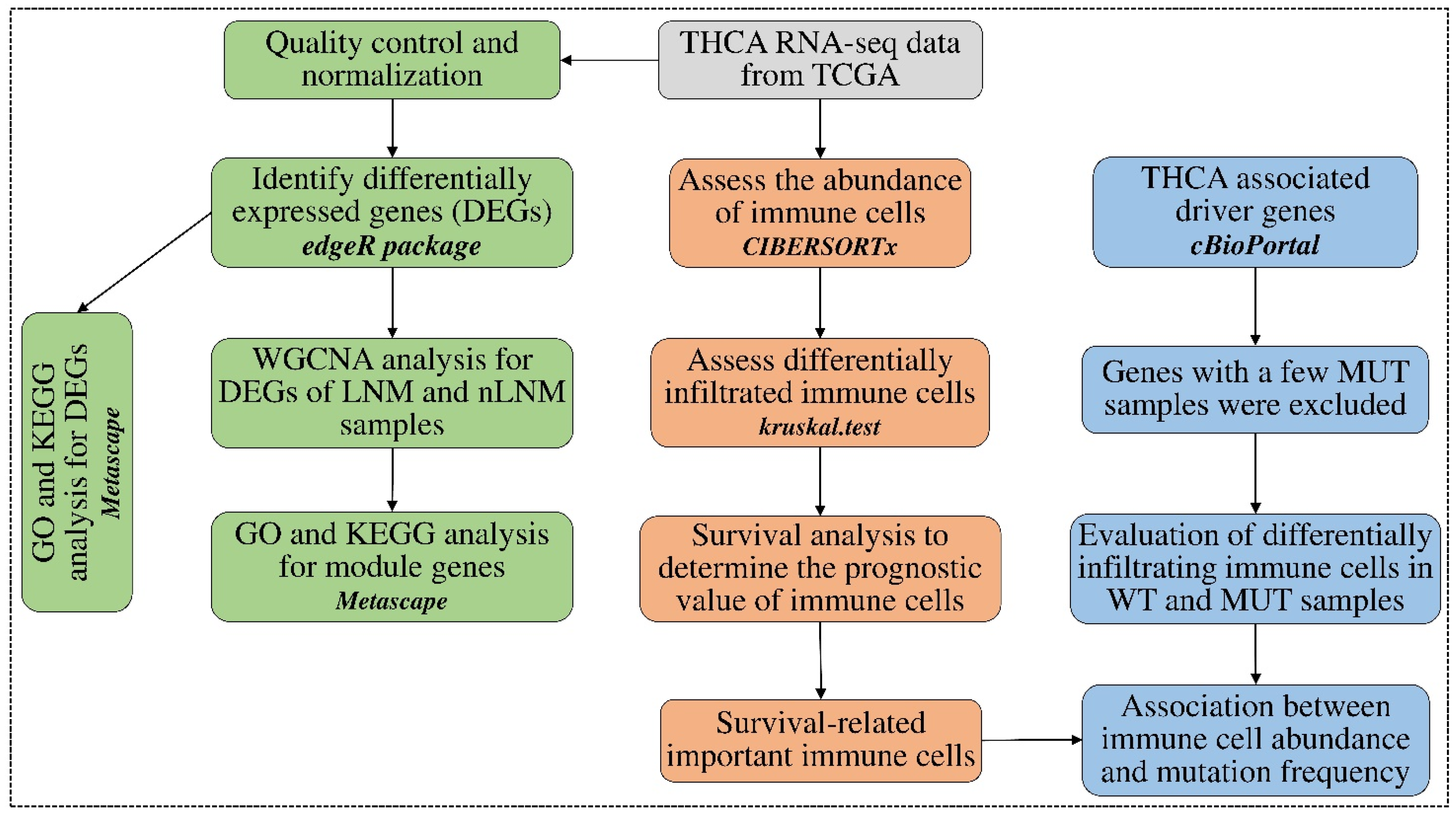
2. Materials and Methods
2.1. Data Sources and Data Pre-Processing
2.2. Estimation of the Abundance of Tumor-Infiltering Immune Cells
2.3. Identification of Differential Abundances of Immune Cells between PTC Groups
2.4. Identifying Survival-Related Tumor-Infiltrating Immune Cells
2.5. Detecting Differentially Expressed Genes between PTC Groups
2.6. Discovering Immune-Related Genes Using WGCNA Analysis
2.7. Correlation between Immune Cell Abundances and Gene Mutation Profiles
3. Results
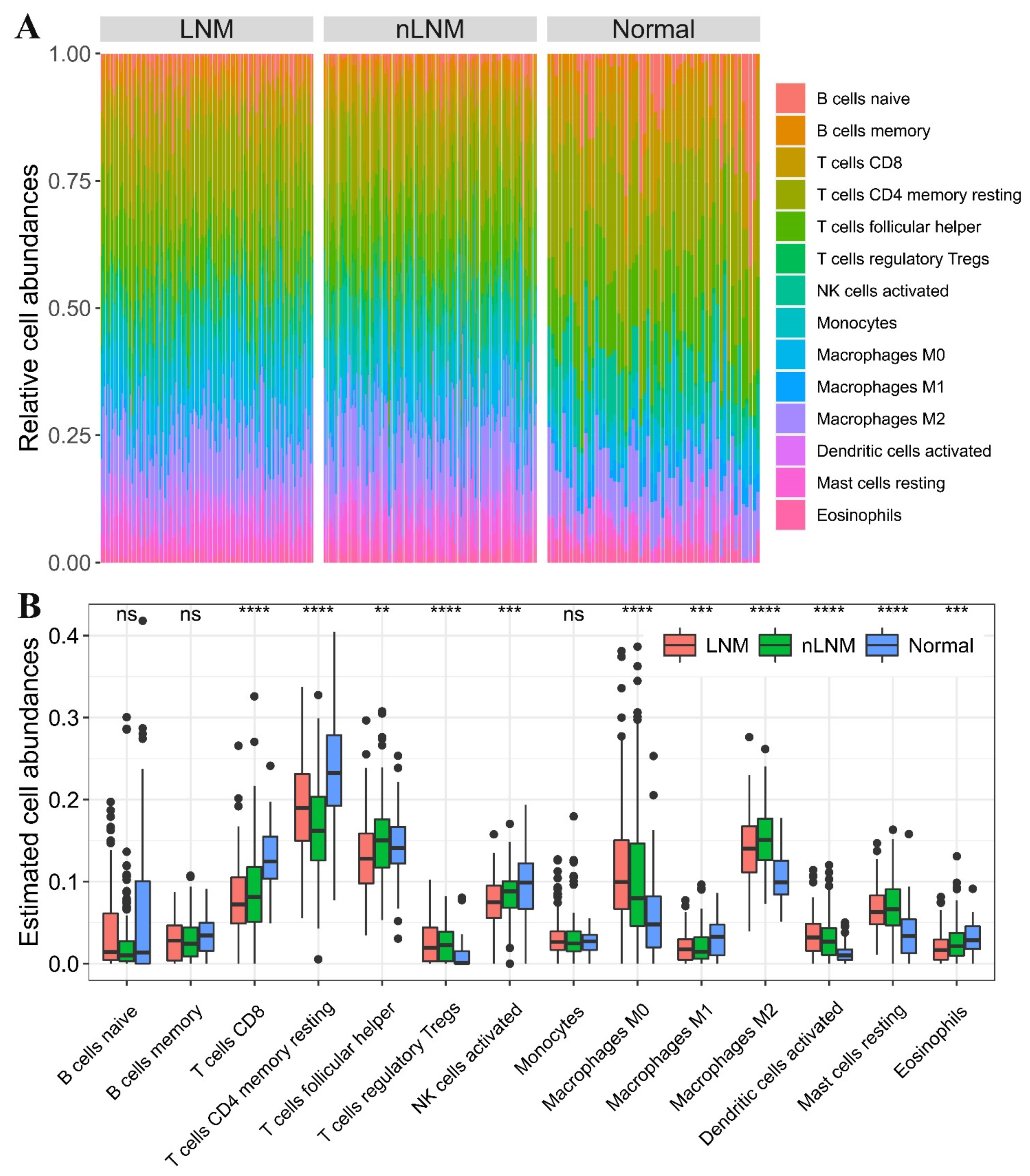
3.1. Tumor-Infiltrating Immune Cell Landscape in PTC
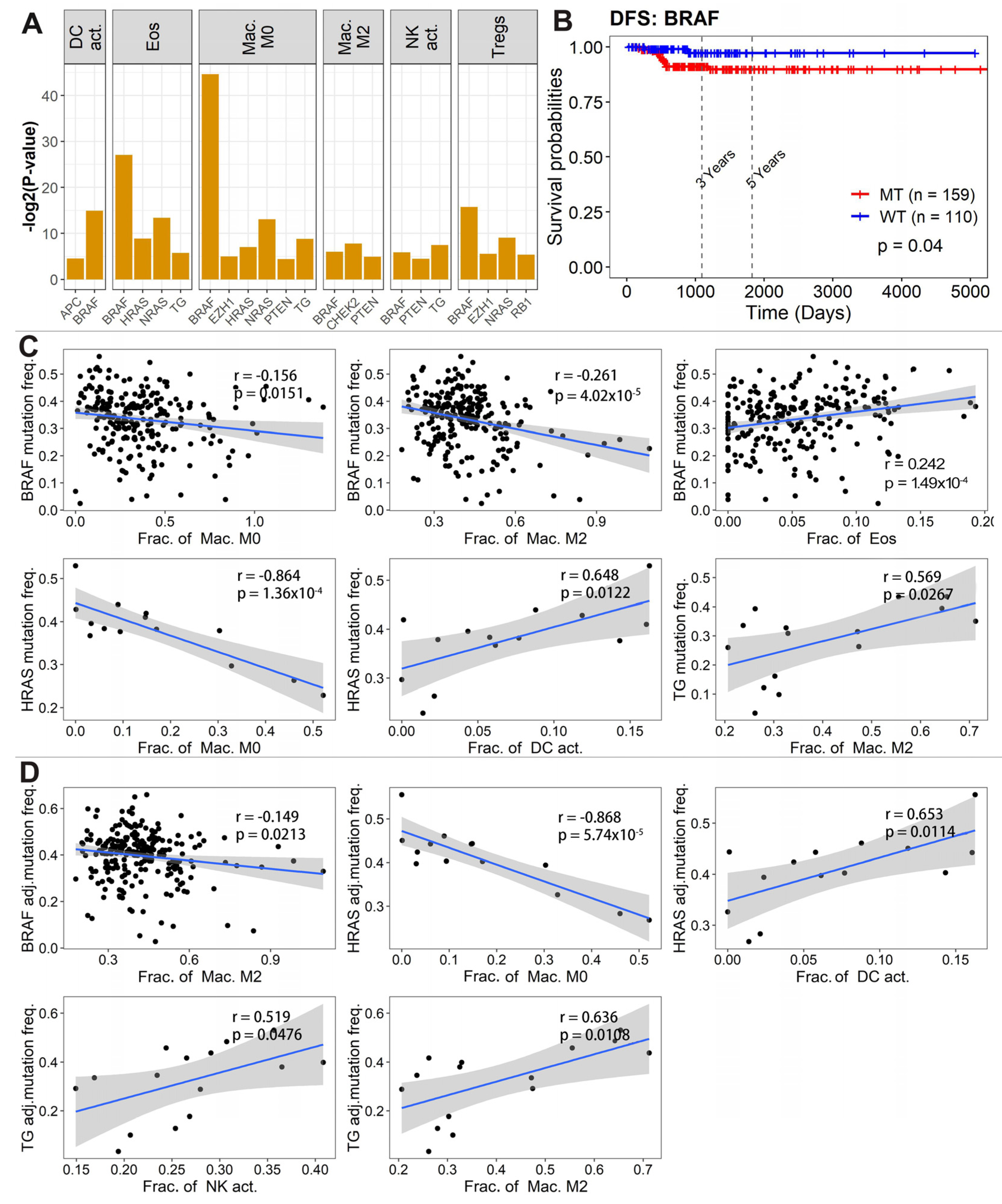
3.2. Tumor-Infiltrating Immune Cells Associated with Prognosis
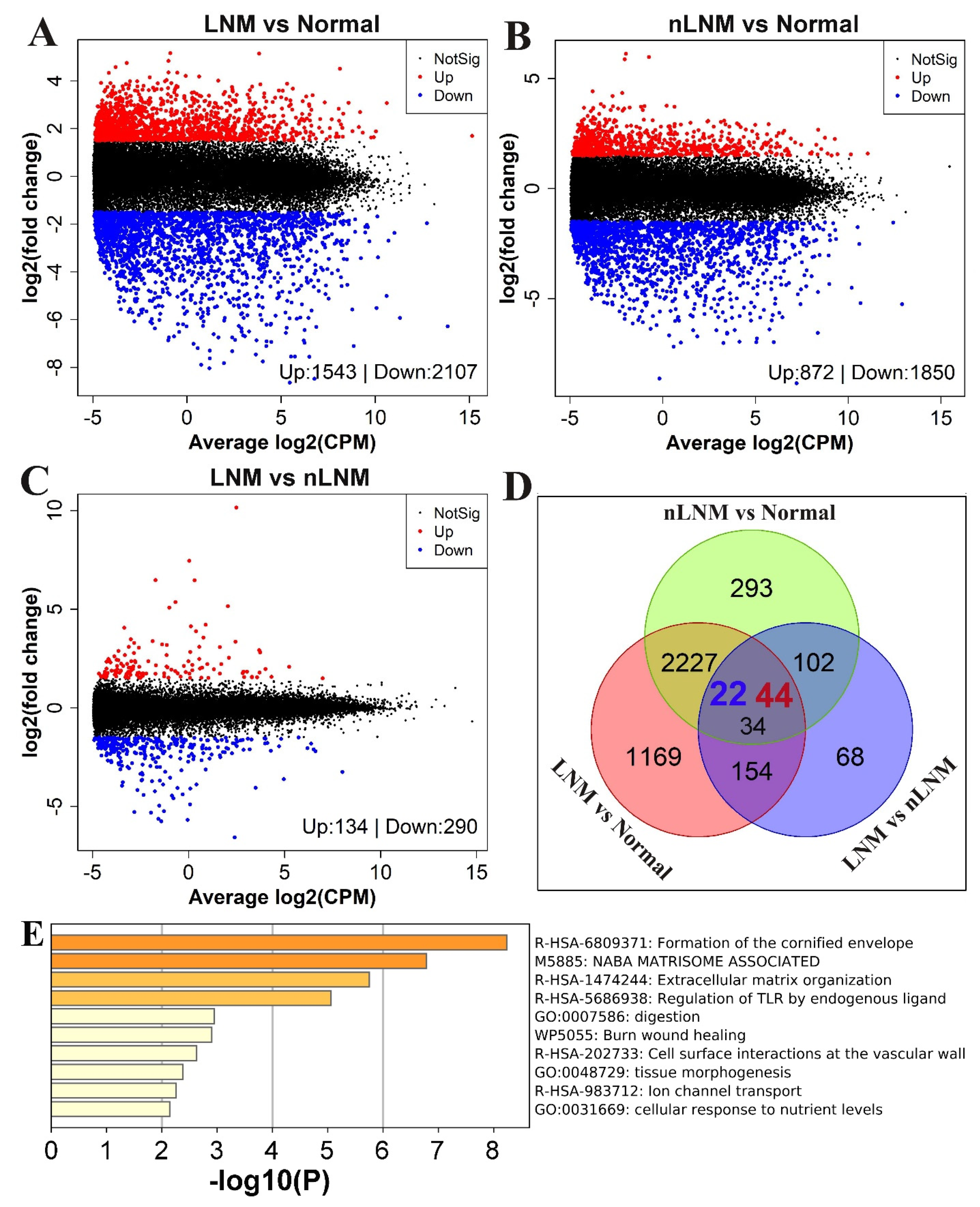
3.3. Genes Associated with LNM
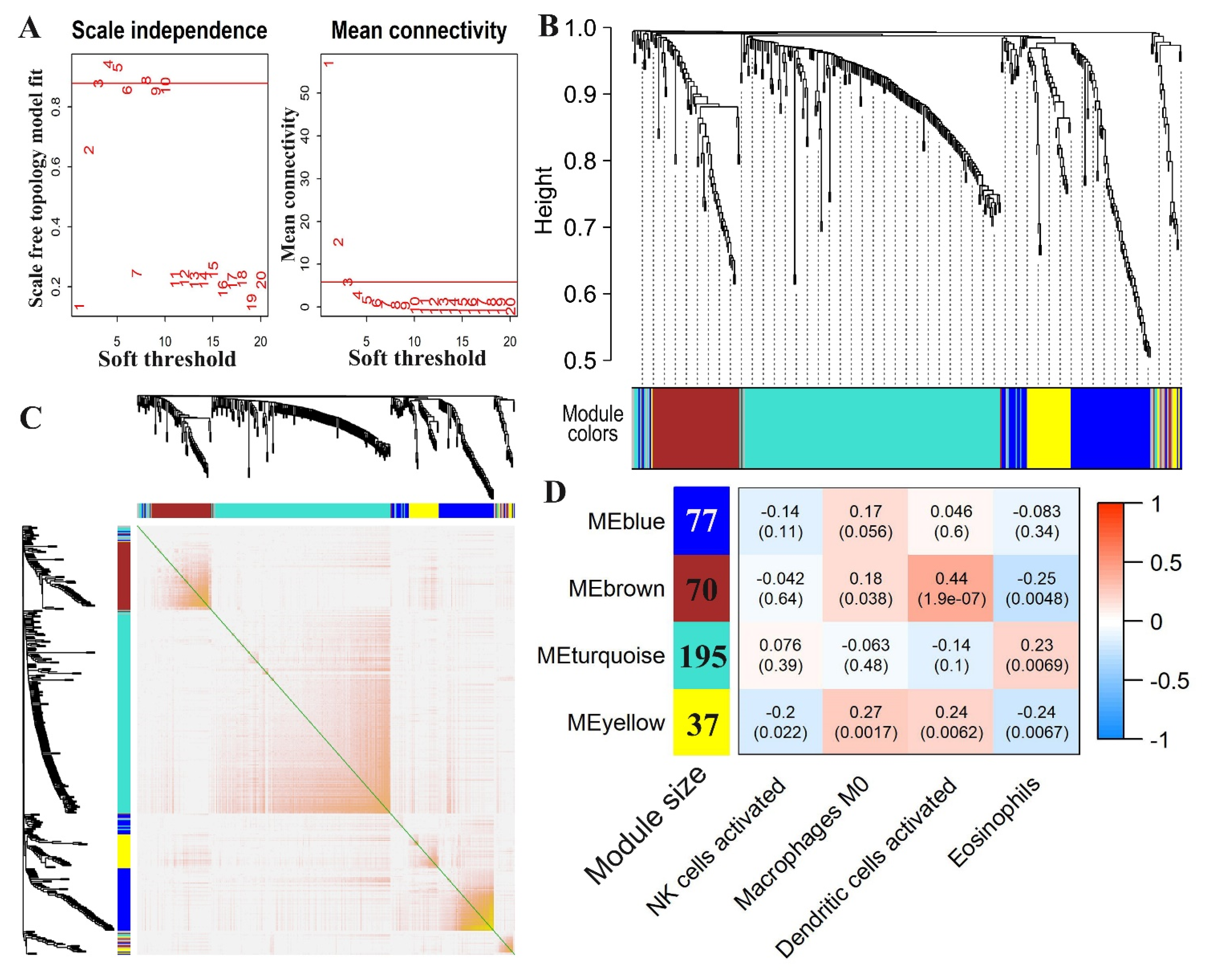
3.4. Co-Expression Module Analysis to Identify Immune Cell-Related Genes
3.5. Immune Cell Abundance Associated with Driver Gene Mutation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPM | Counts per million |
| DEGs | Differentially expressed genes |
| DFS | Disease-free survival |
| DSS | Disease-specific survival |
| HR | Hazard ratio |
| IGF | Insulin-like growth factor |
| IGFBPs | Insulin-like growth factor binding proteins |
| LNM | Lymph node metastasis |
| ME | Module eigengene |
| OS | Overall survival |
| PFS | Progression-free survival |
| PTC | Papillary thyroid cancer |
| TC | Thyroid cancer |
| TCGA | The Cancer Genome Atlas |
| TME | Tumor microenvironment |
| TOM | Topological overlap matrix |
| Tregs | Regulatory T cells |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rahbari, R.; Zhang, L.; Kebebew, E. Thyroid cancer gender disparity. Future Oncol. 2010, 6, 1771–1779. [Google Scholar] [CrossRef]
- Aschebrook-Kilfoy, B.; Ward, M.H.; Sabra, M.M.; Devesa, S.S. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid 2011, 21, 125–134. [Google Scholar] [CrossRef]
- Biersack, H.J.; Grünwald, F. Thyroid Cancer, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Markovina, S.; Grigsby, P.W.; Schwarz, J.K.; DeWees, T.; Moley, J.F.; Siegel, B.A.; Perkins, S.M. Treatment approach, surveillance, and outcome of well-differentiated thyroid cancer in childhood and adolescence. Thyroid 2014, 24, 1121–1126. [Google Scholar] [CrossRef]
- Sun, J.; Shi, R.; Zhang, X.; Fang, D.; Rauch, J.; Lu, S.; Wang, X.; Kasmann, L.; Ma, J.; Belka, C.; et al. Characterization of immune landscape in papillary thyroid cancer reveals distinct tumor immunogenicity and implications for immunotherapy. Oncoimmunology 2021, 10, e1964189. [Google Scholar] [CrossRef]
- Baek, S.K.; Jung, K.Y.; Kang, S.M.; Kwon, S.Y.; Woo, J.S.; Cho, S.H.; Chung, E.J. Clinical risk factors associated with cervical lymph node recurrence in papillary thyroid carcinoma. Thyroid 2010, 20, 147–152. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, Q.; Zhang, H.; Zheng, K.; Wang, R.; Wang, G. Risk Factors for Lymph Node Metastasis in Papillary Thyroid Carcinoma: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 265. [Google Scholar] [CrossRef]
- Daniels, G.H. Follicular Thyroid Carcinoma: A Perspective. Thyroid 2018, 28, 1229–1242. [Google Scholar] [CrossRef]
- Gronlund, M.P.; Jensen, J.S.; Hahn, C.H.; Gronhoj, C.; Buchwald, C.V. Risk Factors for Recurrence of Follicular Thyroid Cancer: A Systematic Review. Thyroid 2021, 31, 1523–1530. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Leboeuf, R.; Martorella, A.J. Papillary thyroid cancer: Monitoring and therapy. Endocrinol. Metab. Clin. N. Am. 2007, 36, 753–778. [Google Scholar] [CrossRef]
- Spill, F.; Reynolds, D.S.; Kamm, R.D.; Zaman, M.H. Impact of the physical microenvironment on tumor progression and metastasis. Curr. Opin. Biotechnol. 2016, 40, 41–48. [Google Scholar] [CrossRef]
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef]
- Alfarouk, K.O.; Muddathir, A.K.; Shayoub, M.E. Tumor acidity as evolutionary spite. Cancers 2011, 3, 408–414. [Google Scholar] [CrossRef]
- Crespo, J.; Sun, H.; Welling, T.H.; Tian, Z.; Zou, W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr. Opin. Immunol. 2013, 25, 214–221. [Google Scholar] [CrossRef]
- Renaude, E.; Kroemer, M.; Loyon, R.; Binda, D.; Borg, C.; Guittaut, M.; Hervouet, E.; Peixoto, P. The Fate of Th17 Cells is Shaped by Epigenetic Modifications and Remodeled by the Tumor Microenvironment. Int. J. Mol. Sci. 2020, 21, 1673. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, Y.; Yang, N.; Gao, Q.; Ding, X.; Kuang, X.; Bao, R.; Zhang, Z.; Sun, C.; Zhou, B.; et al. Profiling of immune features to predict immunotherapy efficacy. Innovation 2022, 3, 100194. [Google Scholar] [CrossRef]
- Institute, N.C. Study Tests Immunotherapy in People with Cancer and Autoimmune Diseases. Available online: https://www.cancer.gov/ (accessed on 26 August 2021).
- Gambardella, C.; Patrone, R.; Di Capua, F.; Offi, C.; Mauriello, C.; Clarizia, G.; Andretta, C.; Polistena, A.; Sanguinetti, A.; Calo, P.; et al. The role of prophylactic central compartment lymph node dissection in elderly patients with differentiated thyroid cancer: A multicentric study. BMC Surg. 2019, 18, 110. [Google Scholar] [CrossRef]
- Na, K.J.; Choi, H. Immune landscape of papillary thyroid cancer and immunotherapeutic implications. Endocr.-Relat. Cancer 2018, 25, 523–531. [Google Scholar] [CrossRef]
- Lamartina, L.; Grani, G.; Durante, C.; Filetti, S. Recent advances in managing differentiated thyroid cancer. F1000Res 2018, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.J. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation—A target for novel cancer therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef]
- Melillo, R.M.; Castellone, M.D.; Guarino, V.; De Falco, V.; Cirafici, A.M.; Salvatore, G.; Caiazzo, F.; Basolo, F.; Giannini, R.; Kruhoffer, M.; et al. The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J. Clin. Investig. 2005, 115, 1068–1081. [Google Scholar] [CrossRef]
- Puxeddu, E.; Knauf, J.A.; Sartor, M.A.; Mitsutake, N.; Smith, E.P.; Medvedovic, M.; Tomlinson, C.R.; Moretti, S.; Fagin, J.A. RET/PTC-induced gene expression in thyroid PCCL3 cells reveals early activation of genes involved in regulation of the immune response. Endocr.-Relat. Cancer 2005, 12, 319–334. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, F.; Shen, X.; Zhu, G.; Liu, R.; Viola, D.; Elisei, R.; Puxeddu, E.; Fugazzola, L.; Colombo, C.; et al. BRAF V600E Status Sharply Differentiates Lymph Node Metastasis-associated Mortality Risk in Papillary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2021, 106, 3228–3238. [Google Scholar] [CrossRef]
- Cunha, L.L.; Nonogaki, S.; Soares, F.A.; Vassallo, J.; Ward, L.S. Immune Escape Mechanism is Impaired in the Microenvironment of Thyroid Lymph Node Metastasis. Endocr. Pathol. 2017, 28, 369–372. [Google Scholar] [CrossRef]
- Newman, A.M.; Steen, C.B.; Liu, C.L.; Gentles, A.J.; Chaudhuri, A.A.; Scherer, F.; Khodadoust, M.S.; Esfahani, M.S.; Luca, B.A.; Steiner, D.; et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol. 2019, 37, 773–782. [Google Scholar] [CrossRef]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Broido, A.D.; Clauset, A. Scale-free networks are rare. Nat. Commun. 2019, 10, 1017. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Xu, M.; Hu, K.; Liu, Y.; Huang, Y.; Liu, S.; Chen, Y.; Wang, D.; Zhou, S.; Zhang, Q.; Mei, N.; et al. Systemic metastasis-targeted nanotherapeutic reinforces tumor surgical resection and chemotherapy. Nat. Commun. 2021, 12, 3187. [Google Scholar] [CrossRef]
- French, J.D. Immunotherapy for advanced thyroid cancers—Rationale, current advances and future strategies. Nat. Rev. Endocrinol. 2020, 16, 629–641. [Google Scholar] [CrossRef]
- Moretti, S.; Menicali, E.; Nucci, N.; Guzzetti, M.; Morelli, S.; Puxeddu, E. Therapy of endocrine disease Immunotherapy of advanced thyroid cancer: From bench to bedside. Eur. J. Endocrinol. 2020, 183, R41–R55. [Google Scholar] [CrossRef]
- Cunha, L.L.; Morari, E.C.; Guihen, A.C.; Razolli, D.; Gerhard, R.; Nonogaki, S.; Soares, F.A.; Vassallo, J.; Ward, L.S. Infiltration of a mixture of different immune cells may be related to molecular profile of differentiated thyroid cancer. Endocr.-Relat.Cancer 2012, 19, L31–L36. [Google Scholar] [CrossRef]
- Yu, H.; Huang, X.; Liu, X.; Jin, H.; Zhang, G.; Zhang, Q.; Yu, J. Regulatory T cells and plasmacytoid dendritic cells contribute to the immune escape of papillary thyroid cancer coexisting with multinodular non-toxic goiter. Endocrine 2013, 44, 172–181. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Varricchi, G.; Marone, G. The immune network in thyroid cancer. Oncoimmunology 2016, 5, e1168556. [Google Scholar] [CrossRef]
- Park, S.J.; Kang, Y.E.; Kim, J.H.; Park, J.L.; Kim, S.K.; Baek, S.W.; Chu, I.S.; Yi, S.; Lee, S.E.; Park, Y.J.; et al. Transcriptomic Analysis of Papillary Thyroid Cancer: A Focus on Immune-Subtyping, Oncogenic Fusion, and Recurrence. Clin. Exp. Otorhinolaryngol. 2022, 15, 183–193. [Google Scholar] [CrossRef]
- Xie, Z.; Li, X.; He, Y.; Wu, S.; Wang, S.; Sun, J.; He, Y.; Lun, Y.; Zhang, J. Immune Cell Confrontation in the Papillary Thyroid Carcinoma Microenvironment. Front. Endocrinol. 2020, 11, 570604. [Google Scholar] [CrossRef]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Gogali, F.; Paterakis, G.; Rassidakis, G.Z.; Kaltsas, G.; Liakou, C.I.; Gousis, P.; Neonakis, E.; Manoussakis, M.N.; Liapi, C. Phenotypical analysis of lymphocytes with suppressive and regulatory properties (Tregs) and NK cells in the papillary carcinoma of thyroid. J. Clin. Endocrinol. Metab. 2012, 97, 1474–1482. [Google Scholar] [CrossRef]
- Grisaru-Tal, S.; Itan, M.; Klion, A.D.; Munitz, A. A new dawn for eosinophils in the tumour microenvironment. Nat. Rev. Cancer 2020, 20, 594–607. [Google Scholar] [CrossRef]
- Jairath, N.K.; Farha, M.W.; Jairath, R.; Harms, P.W.; Tsoi, L.C.; Tejasvi, T. Prognostic value of intratumoral lymphocyte-to-monocyte ratio and M0 macrophage enrichment in tumor immune microenvironment of melanoma. Melanoma Manag. 2020, 7, MMT51. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Roberts, E.W.; Broz, M.L.; Binnewies, M.; Headley, M.B.; Nelson, A.E.; Wolf, D.M.; Kaisho, T.; Bogunovic, D.; Bhardwaj, N.; Krummel, M.F. Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell 2016, 30, 324–336. [Google Scholar] [CrossRef]
- Salmon, H.; Idoyaga, J.; Rahman, A.; Leboeuf, M.; Remark, R.; Jordan, S.; Casanova-Acebes, M.; Khudoynazarova, M.; Agudo, J.; Tung, N.; et al. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity 2016, 44, 924–938. [Google Scholar] [CrossRef]
- Verbeke, H.; Struyf, S.; Berghmans, N.; Van Coillie, E.; Opdenakker, G.; Uyttenhove, C.; Van Snick, J.; Van Damme, J. Isotypic neutralizing antibodies against mouse GCP-2/CXCL6 inhibit melanoma growth and metastasis. Cancer Lett. 2011, 302, 54–62. [Google Scholar] [CrossRef]
- Ma, J.C.; Sun, X.W.; Su, H.; Chen, Q.; Guo, T.K.; Li, Y.; Chen, X.C.; Guo, J.; Gong, Z.Q.; Zhao, X.D.; et al. Fibroblast-derived CXCL12/SDF-1alpha promotes CXCL6 secretion and co-operatively enhances metastatic potential through the PI3K/Akt/mTOR pathway in colon cancer. World J. Gastroenterol. 2017, 23, 5167–5178. [Google Scholar] [CrossRef]
- Li, J.; Tang, Z.; Wang, H.; Wu, W.; Zhou, F.; Ke, H.; Lu, W.; Zhang, S.; Zhang, Y.; Yang, S.; et al. CXCL6 promotes non-small cell lung cancer cell survival and metastasis via down-regulation of miR-515-5p. Biomed. Pharmacother. 2018, 97, 1182–1188. [Google Scholar] [CrossRef]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef]
| Immune Cells | Log Test p | HR | Upper Limit | Lower Limit | Z Score | |
|---|---|---|---|---|---|---|
| OS | NK cells activated | 0.003 | 0.892 | 0.969 | 0.821 | −2.697 |
| Macrophages M0 | 0.040 | 1.018 | 1.034 | 1.002 | 2.233 | |
| Dendritic cells activated | 0.033 | 1.077 | 1.149 | 1.009 | 2.227 | |
| PFS | NK cells activated | 0.039 | 0.963 | 1.000 | 0.927 | −1.970 |
| Macrophages M0 | 0.018 | 1.013 | 1.024 | 1.003 | 2.488 | |
| Eosinophils | 0.023 | 0.933 | 0.993 | 0.875 | −2.162 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amanullah, M.; Pan, M.; Lu, K.; Pan, X.; Lu, Y.; Luo, D.; Liu, P. Tumor-Infiltrating Immune Cell Landscapes in the Lymph Node Metastasis of Papillary Thyroid Cancer. Curr. Oncol. 2023, 30, 2625-2641. https://doi.org/10.3390/curroncol30030200
Amanullah M, Pan M, Lu K, Pan X, Lu Y, Luo D, Liu P. Tumor-Infiltrating Immune Cell Landscapes in the Lymph Node Metastasis of Papillary Thyroid Cancer. Current Oncology. 2023; 30(3):2625-2641. https://doi.org/10.3390/curroncol30030200
Chicago/Turabian StyleAmanullah, Md, Meidie Pan, Kaining Lu, Xiaoqing Pan, Yan Lu, Dingcun Luo, and Pengyuan Liu. 2023. "Tumor-Infiltrating Immune Cell Landscapes in the Lymph Node Metastasis of Papillary Thyroid Cancer" Current Oncology 30, no. 3: 2625-2641. https://doi.org/10.3390/curroncol30030200
APA StyleAmanullah, M., Pan, M., Lu, K., Pan, X., Lu, Y., Luo, D., & Liu, P. (2023). Tumor-Infiltrating Immune Cell Landscapes in the Lymph Node Metastasis of Papillary Thyroid Cancer. Current Oncology, 30(3), 2625-2641. https://doi.org/10.3390/curroncol30030200





