Bone Metastases from Intrahepatic Cholangiocarcinoma Confer Worse Prognosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Follow-Up
2.2. Statistical Analysis
3. Results
3.1. Population Characteristics
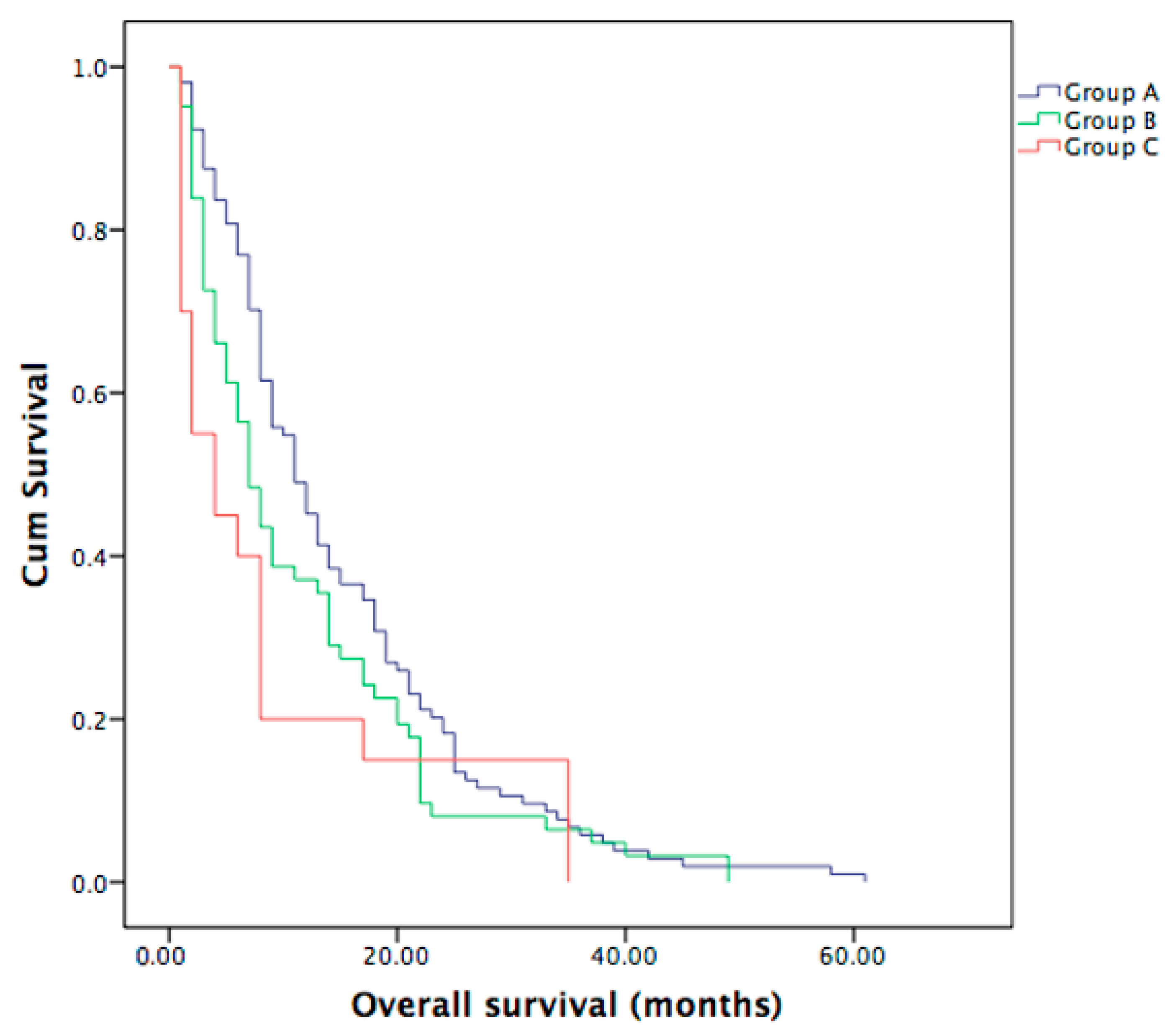
3.2. Overall Survival in Metastatic ICC Patients
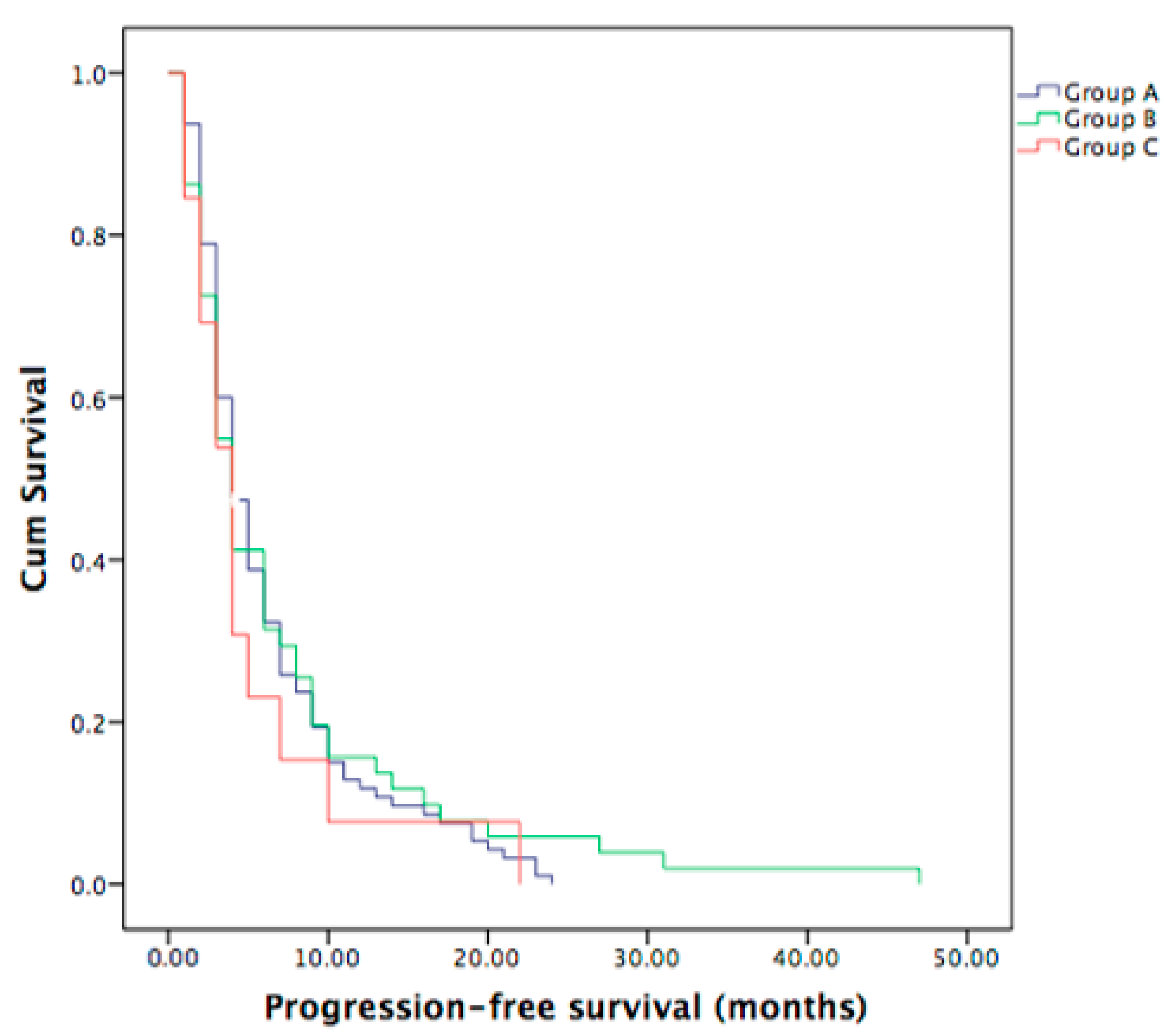
3.3. Progression-Free Survival in Metastatic ICC Patients
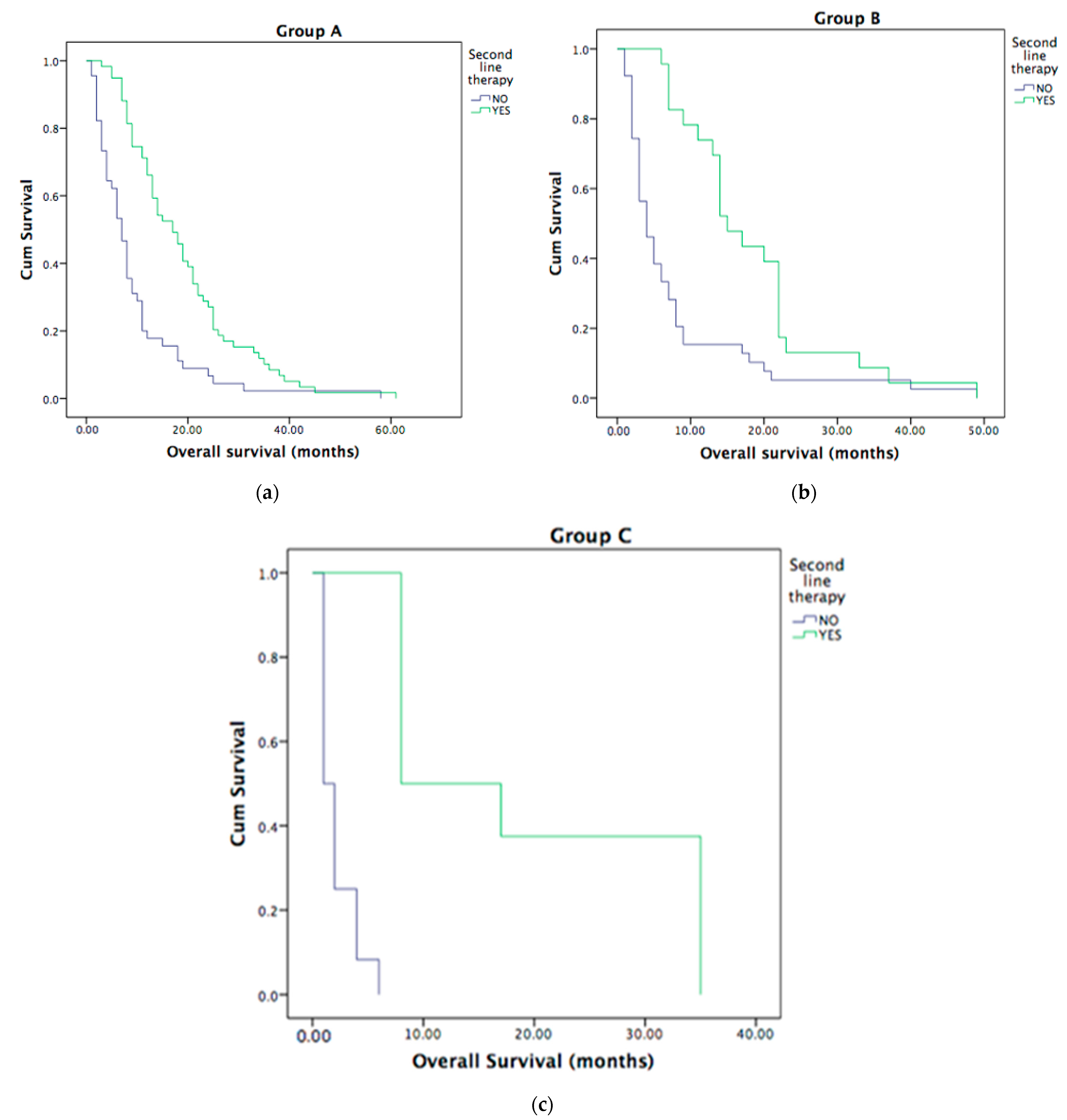
3.4. Second-Line Treatment in Metastatic ICC Patients
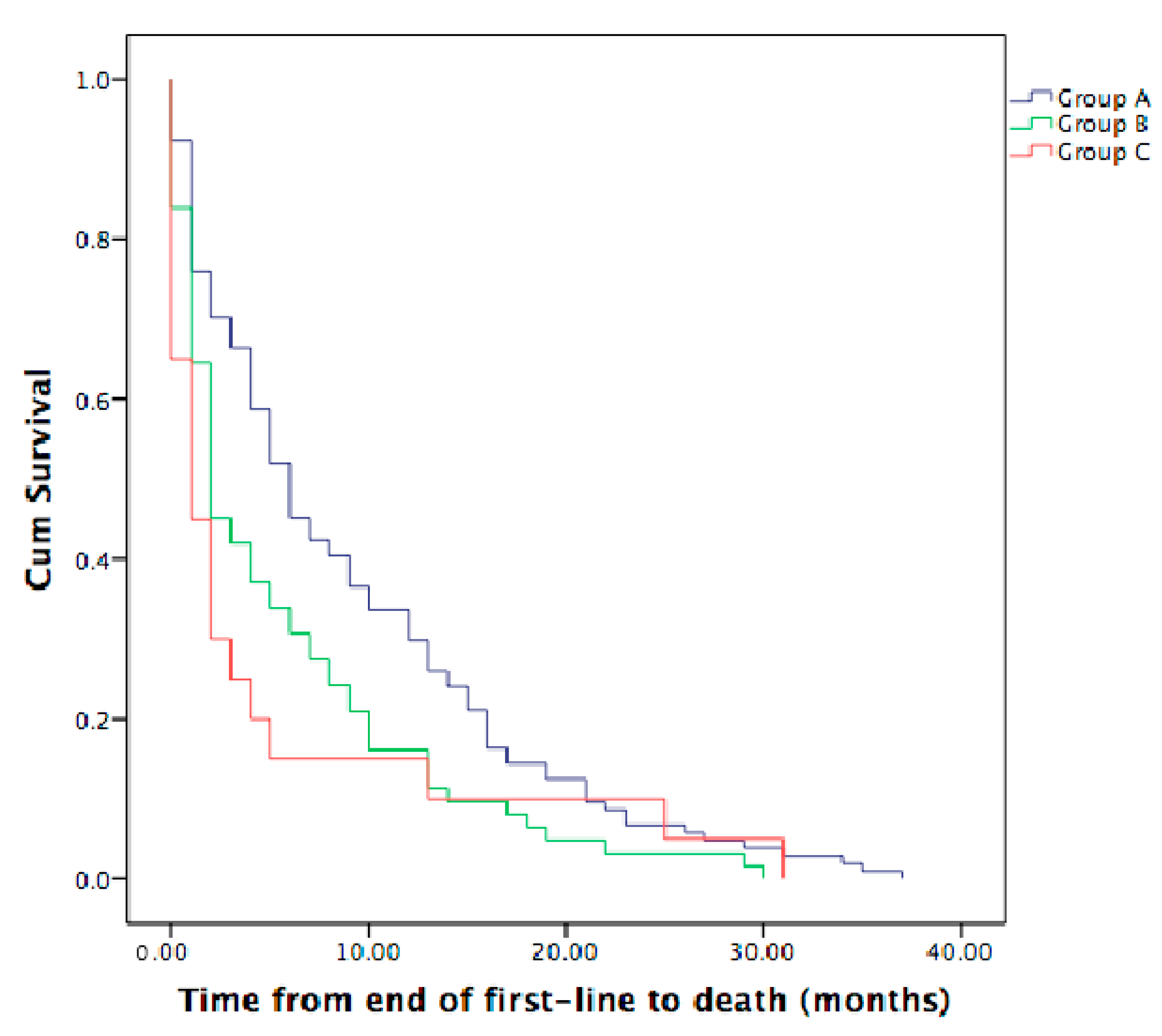
3.5. Time from the End of First-Line Therapy to Death in Metastatic ICC Patients
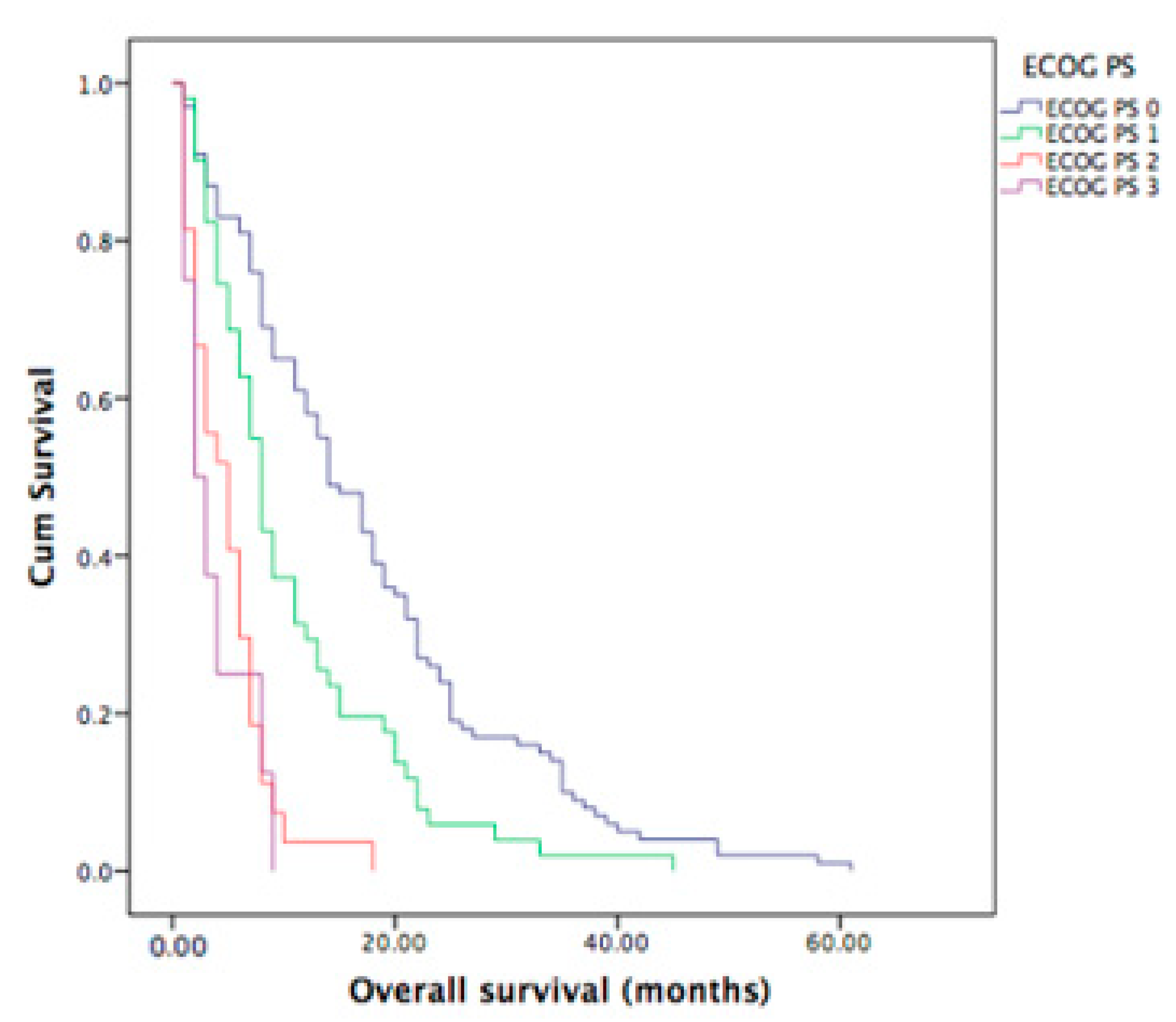
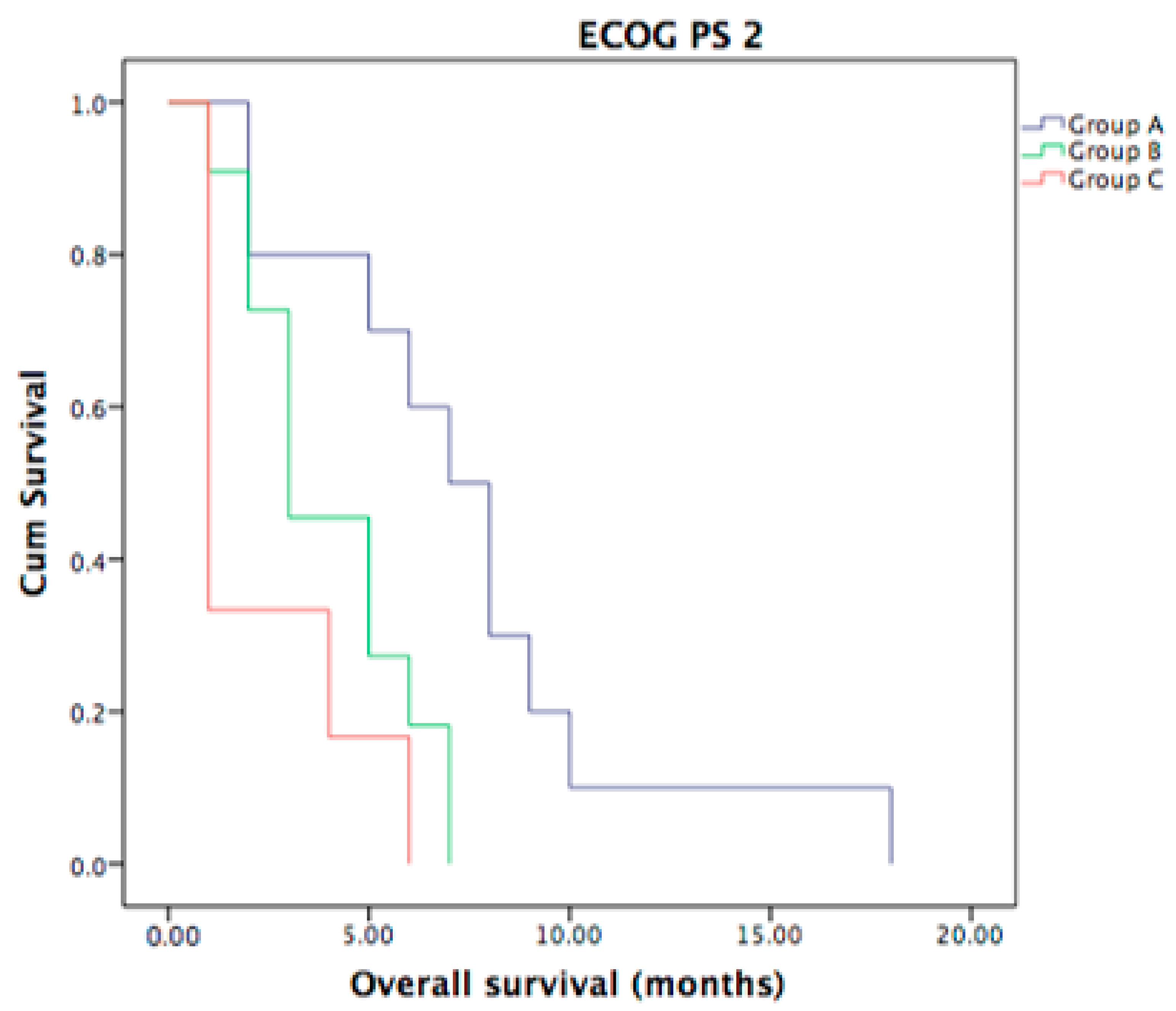
3.6. ECOG PS of Metastatic ICC Patients
3.7. Univariate and Multivariate Prognostic Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamarca, A.; Edeline, J.; Goyal, L. How I Treat Biliary Tract Cancer. ESMO Open 2022, 7. [Google Scholar] [CrossRef] [PubMed]
- Tovoli, F.; Garajovà, I. Pattern of Progression of Intrahepatic Cholangiocarcinoma Impacts on Survival: Implications for Second-Line Trials. Dig. Liver Dis. 2021, 53, S19. [Google Scholar] [CrossRef]
- Massironi, S.; Pilla, L.; Elvevi, A. New and Emerging Systemic Therapeutic Options for Advanced Cholangiocarcinoma. Cells 2020, 9, 688. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. A Phase 3 Randomized, Double-Blind, Placebo-Controlled Study of Durvalumab in Combination with Gemcitabine plus Cisplatin (GemCis) in Patients (Pts) with Advanced Biliary Tract Cancer (BTC): TOPAZ-1. J. Clin. Oncol. 2022, 40, 378. [Google Scholar] [CrossRef]
- Hahn, F.; Müller, L.; Mähringer-Kunz, A.; Tanyildizi, Y.; Dos Santos, D.P.; Düber, C.; Galle, P.R.; Weinmann, A.; Kloeckner, R. Distant Metastases in Patients with Intrahepatic Cholangiocarcinoma: Does Location Matter? A Retrospective Analysis of 370 Patients. J. Oncol. 2020, 2020, 7195373. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Du, Q.; Ye, J.; Wang, B.; Chen, Y. Prognostic Value of Site-Specific Metastases for Patients with Advanced Intrahepatic Cholangiocarcinoma: A SEER Database Analysis. Medicine 2019, 98, e18191. [Google Scholar] [CrossRef]
- Izquierdo-Sanchez, L.; Lamarca, A.; La Casta, A.; Buettner, S.; Utpatel, K.; Klümpen, H.J.; Adeva, J.; Vogel, A.; Lleo, A.; Fabris, L.; et al. Cholangiocarcinoma Landscape in Europe: Diagnostic, Prognostic and Therapeutic Insights from the ENSCCA Registry. J. Hepatol. 2022, 76, 1109–1121. [Google Scholar] [CrossRef]
- Frega, G.; Garajová, I. Brain Metastases from Biliary Tract Cancer: A Monocentric Retrospective Analysis of 450 Patients. Oncology 2018, 94, 7–11. [Google Scholar] [CrossRef]
- Yan, X.; Wang, P. Site-Specific Metastases of Intrahepatic Cholangiocarcinoma and Its Impact on Survival: A Population-Based Study. Future Oncol. 2019, 15, 2125–2137. [Google Scholar] [CrossRef] [PubMed]
- Elvevi, A.; Laffusa, A.; Scaravaglio, M.; Rossi, R.E.; Longarini, R.; Stagno, A.M.; Cristoferi, L.; Ciaccio, A.; Cortinovis, D.L.; Invernizzi, P.; et al. Clinical treatment of cholangiocarcinoma: An updated comprehensive review. Ann. Hepatol. 2022, 27, 100737. [Google Scholar] [CrossRef]
- Ohaegbulam, K.C.; Koethe, Y.; Fung, A.; Mayo, S.C.; Grossberg, A.J.; Chen, E.Y.; Sharzehi, K.; Kardosh, A.; Farsad, K.; Rocha, F.G.; et al. The multidisciplinary management of cholangiocarcinoma. Cancer 2023, 129, 184–214. [Google Scholar] [CrossRef] [PubMed]
- Krenzien, F.; Nevermann, N.; Krombholz, A.; Benzing, C.; Haber, P.; Fehrenbach, U.; Lurje, G.; Pelzer, U.; Pratschke, J.; Schmelzle, M.; et al. Treatment of Intrahepatic Cholangiocarcinoma-A Multidisciplinary Approach. Cancers 2022, 14, 362. [Google Scholar] [CrossRef]
- Macias, R.I.R.; Cardinale, V.; Kendall, T.J.; A Avila, M.; Guido, M.; Coulouarn, C.; Braconi, C.; Frampton, A.E.; Bridgewater, J.; Overi, D.; et al. Clinical relevance of biomarkers in cholangiocarcinoma: Critical revision and future directions. Gut 2022, 71, 1669–1683. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma: Evolving Concepts and Therapeutic Strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, T.; Wu, M.; Shen, F. Intrahepatic Cholangiocarcinoma: Epidemiology, Risk Factors, Diagnosis and Surgical Management. Cancer Lett. 2016, 379, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next Horizon in Mechanisms and Management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Florio, A.A.; Ferlay, J.; Znaor, A.; Ruggieri, D.; Alvarez, C.S.; Laversanne, M.; Bray, F.; McGlynn, K.A.; Petrick, J.L. Global Trends in Intrahepatic and Extrahepatic Cholangiocarcinoma Incidence from 1993 to 2012. Cancer 2020, 126, 2666–2678. [Google Scholar] [CrossRef] [PubMed]
- Thammaroj, P.; Chimcherd, A.; Chowchuen, P.; Panitchote, A.; Sumananont, C.; Wongsurawat, N. Imaging features of bone metastases from cholangiocarcinoma. Eur. J. Radiol. 2020, 129, 109118. [Google Scholar] [CrossRef]
- Ioffe, D.; Phull, P.; Dotan, E. Optimal Management of Patients with Advanced or Metastatic Cholangiocarcinoma: An Evidence-Based Review. Cancer Manag. Res. 2021, 13, 8085–8098. [Google Scholar] [CrossRef]
| Group A (Patients with Liver Metastases Only) | Group B (Patients Other than Group A/C) | Group C (Patients with Bone Metastases) | p | |
|---|---|---|---|---|
| Number of patients | 104 | 62 | 20 | |
| Sex | ||||
| Male | 56 (54%) | 36 (58%) | 11 (55%) | |
| Female | 48 (46%) | 26 (42%) | 9 (45%) | NS (p = 0.87) |
| Age | ||||
| more than median age | 46 (44%) | 34 (55%) | 12 (60%) | |
| less than median age | 58 (56%) | 28 (45%) | 8 (40%) | NS (p = 0.18) |
| ECOG PS | ||||
| 0 | 61 (59%) | 28 (45%) | 11 (55%) | |
| 1 | 29 (28%) | 20 (32%) | 2 (10%) | |
| 2 | 10 (9%) | 11 (18%) | 6 (30%) | |
| 3 | 4 (4%) | 3 (5%) | 1 (5%) | NS (p = 0.14) |
| First-line Gemcitabine-based chemotherapy | 104 (100%) | 62 (100%) | 20 (100%) |
| Variable | HR | 95.0% CI for HR | p | |
|---|---|---|---|---|
| Lower | Upper | |||
| ECOG PS | 2.377 | 1.752 | 3.226 | <0.001 |
| Second line therapy | 2.613 | 1.939 | 3.523 | <0.001 |
| Groups | ||||
| Group B | 1.286 | 0.937 | 1.765 | 0.120 |
| Group C | 1.693 | 1.043 | 2.748 | 0.033 |
| Variable | HR | 95.0% CI for HR | p | |
|---|---|---|---|---|
| Lower | Upper | |||
| ECOG PS | 2.258 | 1.637 | 3.113 | <0.001 |
| Second line therapy | 2.526 | 1.843 | 3.463 | <0.001 |
| Groups | ||||
| Group B | 1.151 | 0.835 | 1.587 | 0.391 |
| Group C | 2.296 | 1.399 | 3.769 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garajová, I.; Gelsomino, F.; Salati, M.; Leonardi, F.; De Lorenzo, S.; Granito, A.; Tovoli, F. Bone Metastases from Intrahepatic Cholangiocarcinoma Confer Worse Prognosis. Curr. Oncol. 2023, 30, 2613-2624. https://doi.org/10.3390/curroncol30030199
Garajová I, Gelsomino F, Salati M, Leonardi F, De Lorenzo S, Granito A, Tovoli F. Bone Metastases from Intrahepatic Cholangiocarcinoma Confer Worse Prognosis. Current Oncology. 2023; 30(3):2613-2624. https://doi.org/10.3390/curroncol30030199
Chicago/Turabian StyleGarajová, Ingrid, Fabio Gelsomino, Massimiliano Salati, Francesco Leonardi, Stefania De Lorenzo, Alessandro Granito, and Francesco Tovoli. 2023. "Bone Metastases from Intrahepatic Cholangiocarcinoma Confer Worse Prognosis" Current Oncology 30, no. 3: 2613-2624. https://doi.org/10.3390/curroncol30030199
APA StyleGarajová, I., Gelsomino, F., Salati, M., Leonardi, F., De Lorenzo, S., Granito, A., & Tovoli, F. (2023). Bone Metastases from Intrahepatic Cholangiocarcinoma Confer Worse Prognosis. Current Oncology, 30(3), 2613-2624. https://doi.org/10.3390/curroncol30030199







