The Psychosocial Impact of the Decision to Undergo Risk-Reducing Salpingo-Oophorectomy Surgery in BRCA Mutation Carriers and the Role of Physician-Patient Communication
Abstract
1. Introduction
2. Clinical Background for the Recommendation of RRSO
2.1. Genomic Susceptibility to Ovarian/Breast Cancer
2.2. Risk-Reducing Measures and RRSO
2.3. Physiological Implications of RRSO
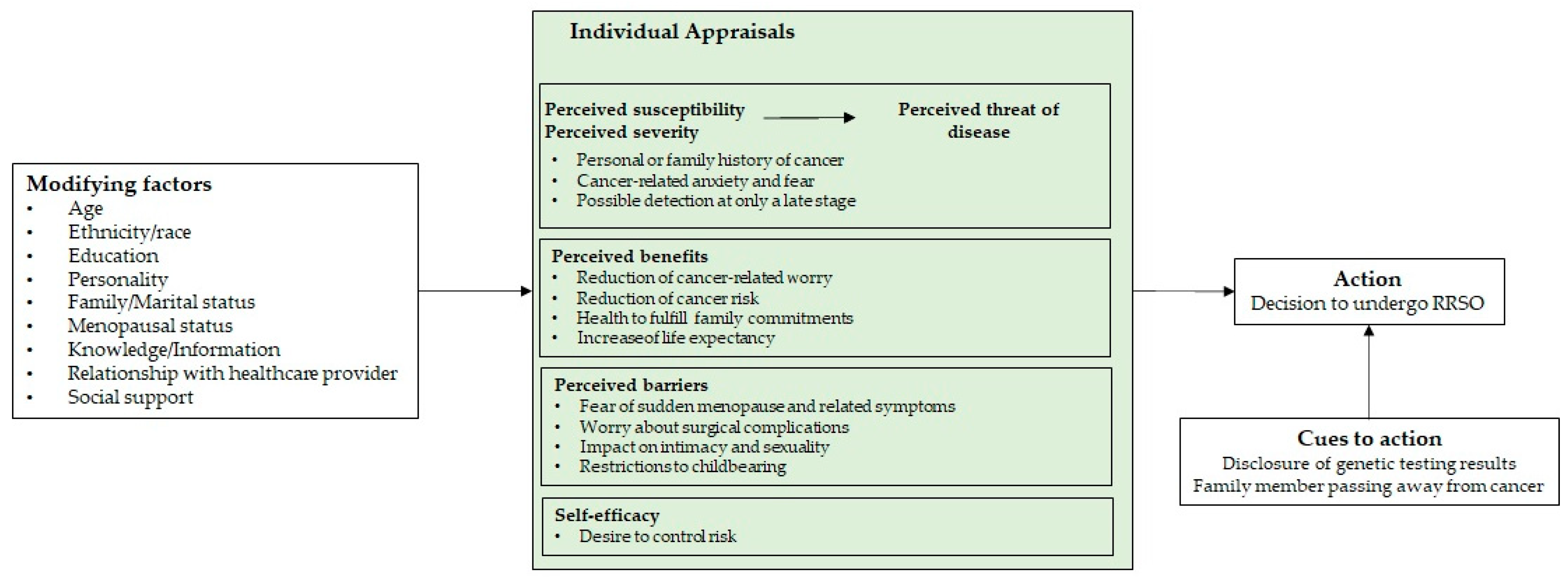
3. The Psychological Process of Deciding to Undergo RRSO
3.1. The Psychosocial Impact of Undergoing RRSO
3.2. The Role of Physician-Patient Communication in the Decision-Making Process and in Post-Surgery Adaptation
4. Recommendations to Improve Clinical Practice
5. Future Research Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walker, M.; Jacobson, M.; Sobel, M. Management of ovarian cancer risk in women with BRCA1/2 pathogenic variants. Can. Med. Assoc. J. 2019, 191, E886–E893. [Google Scholar] [CrossRef] [PubMed]
- González-Santiago, S.; The SEOM Hereditary Cancer Working Group; Cajal, T.R.Y.; Aguirre, E.; Alés-Martínez, J.E.; Andrés, R.; Balmaña, J.; Graña, B.; Herrero, A.; Llort, G.; et al. SEOM clinical guidelines in hereditary breast and ovarian cancer. Clin. Transl. Oncol. 2019, 22, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Samadder, N.J.; Giridhar, K.V.; Baffy, N.; Riegert-Johnson, D.; Couch, F.J. Hereditary Cancer Syndromes—A Primer on Diagnosis and Management: Part 1: Breast-ovarian cancer syndromes. Mayo Clin. Proc. 2019, 94, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.; Mutch, D.G. Hereditary Ovarian Cancer and Risk Reduction. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 31–48. [Google Scholar] [CrossRef]
- Piombino, C.; Cortesi, L.; Lambertini, M.; Punie, K.; Grandi, G.; Toss, A. Secondary Prevention in Hereditary Breast and/or Ovarian Cancer Syndromes Other Than BRCA. J. Oncol. 2020, 2020, e6384190. [Google Scholar] [CrossRef]
- Sabiani, L.; Barrou, J.; Mathis, J.; Eisinger, F.; Bannier, M.; Lambaudie, E.; Houvenaeghel, G. How to manage BRCA mutation carriers? Horm. Mol. Biol. Clin. Investig. 2019, 41, 1–7. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 182 Summary: Hereditary Breast and Ovarian Cancer Syndrome. Obstet. Gynecol. 2017, 130, 657–659. [Google Scholar] [CrossRef]
- AlHilli, M.M.; Al-Hilli, Z. Perioperative Management of Women Undergoing Risk-reducing Surgery for Hereditary Breast and Ovarian Cancer. J. Minim. Invasive Gynecol. 2018, 26, 253–265. [Google Scholar] [CrossRef]
- Kostov, S.; Watrowski, R.; Kornovski, Y.; Dzhenkov, D.; Slavchev, S.; Ivanova, Y.; Yordanov, A. Hereditary Gynecologic Cancer Syndromes – A Narrative Review. OncoTargets Ther. 2022, 15, 381–405. [Google Scholar] [CrossRef]
- Daly, M.B.; Pal, T.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Goggins, M.; Hutton, M.L.; et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 77–102. [Google Scholar] [CrossRef]
- Gaba, F.; Goyal, S.; Marks, D.; Chandrasekaran, D.; Evans, O.; Robbani, S.; Tyson, C.; Legood, R.; Saridogan, E.; McCluggage, W.G.; et al. Surgical decision making in premenopausal BRCA carriers considering risk-reducing early salpingectomy or salpingo-oophorectomy: A qualitative study. J. Med. Genet. 2021, 59, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.F.; Balneaves, L.G.; Bottorff, J.L. Women’s Decision Making about Risk-Reducing Strategies in the Context of Hereditary Breast and Ovarian Cancer: A Systematic Review. J. Genet. Couns. 2009, 18, 578–597. [Google Scholar] [CrossRef] [PubMed]
- Cherry, C.; Ropka, M.; Lyle, J.; Napolitano, L.; Daly, M.B. Understanding the Needs of Women Considering Risk-Reducing Salpingo-oophorectomy. Cancer Nurs. 2013, 36, E33–E38. [Google Scholar] [CrossRef]
- Meadows, R.; Padamsee, T.J.; Paskett, E.D. Distinctive psychological and social experiences of women choosing prophylactic oophorectomy for cancer prevention. Health Care Women Int. 2018, 39, 595–616. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Members of the Guidelines International Network Implementation Working Group; Bernhardsson, S.; Vernooij, R.W.M.; Armstrong, M.J.; Bussières, A.; Brouwers, M.C.; Gagliardi, A.R. Use of theory to plan or evaluate guideline implementation among physicians: A scoping review. Implement. Sci. 2017, 12, 26. [Google Scholar] [CrossRef]
- Jiang, S. Pathways Linking Patient-Centered Communication to Health Improvement: A Longitudinal Study in China. J. Health Commun. 2019, 24, 156–164. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, R. Impact of Physician-Patient Communication in Online Health Communities on Patient Compliance: Cross-Sectional Questionnaire Study. J. Med. Internet Res. 2019, 21, e12891. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Hong, Y.A. Patient-centered communication and emotional well-being in the era of medical violence in China. Health Promot. Int. 2020, 36, 313–320. [Google Scholar] [CrossRef]
- Alexandre, M.; Black, J.; Whicker, M.; Minkin, M.J.; Ratner, E. The management of sexuality, intimacy, and menopause symptoms (SIMS) after prophylactic bilateral salpingo-oophorectomy: How to maintain sexual health in “previvors”. Maturitas 2017, 105, 46–51. [Google Scholar] [CrossRef]
- Carter, J.; Stabile, C.; Gunn, A.; Sonoda, Y. The Physical Consequences of Gynecologic Cancer Surgery and Their Impact on Sexual, Emotional, and Quality of Life Issues. J. Sex. Med. 2013, 10, 21–34. [Google Scholar] [CrossRef]
- Klitzman, R.; Chung, W. The process of deciding about prophylactic surgery for breast and ovarian cancer: Patient questions, uncertainties, and communication. Am. J. Med. Genet. Part A 2009, 152A, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R. Writing narrative style literature reviews. Med. Writ. 2015, 24, 230–235. [Google Scholar] [CrossRef]
- Green, B.N.; Johnson, C.D.; Adams, A. Writing narrative literature reviews for peer-reviewed journals: Secrets of the trade. J. Chiropr. Med. 2006, 5, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Breen, K.; Catchings, A.; Ranganathan, M.; Latham, A.; Goldfrank, D.J.; Grisham, R.N.; Roche, K.L.; Frey, M.K.; Chi, D.S.; et al. Risk-Reducing Bilateral Salpingo-Oophorectomy for Ovarian Cancer: A Review and Clinical Guide for Hereditary Predisposition Genes. JCO Oncol. Pract. 2022, 18, 201–209. [Google Scholar] [CrossRef]
- Vermeulen, R.F.M.; Van Beurden, M.; Korse, C.M.; Kenter, G.G. Impact of risk-reducing salpingo-oophorectomy in premenopausal women. Climacteric 2017, 20, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Altman, A.M.; Hui, J.Y.; Tuttle, T. Quality-of-life implications of risk-reducing cancer surgery. Br. J. Surg. 2018, 105, e121–e130. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, L.C.; Lindor, N.M. The Role of Risk-Reducing Surgery in Hereditary Breast and Ovarian Cancer. N. Engl. J. Med. 2016, 374, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.; Seitz, S.; Kast, K.; Emons, G.; Ortmann, O. Hormone replacement therapy in BRCA mutation carriers and risk of ovarian, endometrial, and breast cancer: A systematic review. J. Cancer Res. Clin. Oncol. 2021, 147, 2035–2045. [Google Scholar] [CrossRef]
- Jacobson, M.; Coakley, N.; Bernardini, M.; Branco, K.-A.; Elit, L.; Ferguson, S.; Kim, R. Risk reduction strategies for BRCA1/2 hereditary ovarian cancer syndromes: A clinical practice guideline. Hered. Cancer Clin. Pract. 2021, 19, 1–7. [Google Scholar] [CrossRef]
- Mau, C.; Untch, M. Prophylactic Surgery: For Whom, When and How. Breast Care 2017, 12, 379–384. [Google Scholar] [CrossRef]
- Singer, C.F. Non-surgical prevention strategies in women with hereditary breast and ovarian cancer syndromes. Horm. Mol. Biol. Clin. Investig. 2020, 41, 20190057. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.L.; Garber, J.E.; Tung, N. Managing hereditary breast cancer risk in women with and without ovarian cancer. Gynecol. Oncol. 2017, 146, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R. Hereditary breast and ovarian cancer (HBOC): Review of its molecular characteristics, screening, treatment, and prognosis. Breast Cancer 2020, 28, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Elezaby, M.; Lees, B.; Maturen, K.E.; Barroilhet, L.; Wisinski, K.B.; Schrager, S.; Wilke, L.G.; Sadowski, E. BRCA Mutation Carriers: Breast and Ovarian Cancer Screening Guidelines and Imaging Considerations. Radiology 2019, 291, 554–569. [Google Scholar] [CrossRef]
- Berger, E.R.; Golshan, M. Surgical Management of Hereditary Breast Cancer. Genes 2021, 12, 1371. [Google Scholar] [CrossRef]
- Sekine, M.; Nishino, K.; Enomoto, T. BRCA Genetic Test and Risk-Reducing Salpingo-Oophorectomy for Hereditary Breast and Ovarian Cancer: State-of-the-Art. Cancers 2021, 13, 2562. [Google Scholar] [CrossRef]
- Marchetti, C.; De Felice, F.; Boccia, S.; Sassu, C.; Di Donato, V.; Perniola, G.; Palaia, I.; Monti, M.; Muzii, L.; Tombolini, V.; et al. Hormone replacement therapy after prophylactic risk-reducing salpingo-oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: A meta-analysis. Crit. Rev. Oncol. 2018, 132, 111–115. [Google Scholar] [CrossRef]
- Heemskerk-Gerritsen, B.A.; Seynaeve, C.; van Asperen, C.J.; Ausems, M.G.; Collee, J.M.; van Doorn, H.C.; Gomez Garcia, E.B.; Kets, C.M.; van Leeuwen, F.E.; Meijers-Heijboer, H.E.; et al. Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: Revisiting the evidence for risk reduction. J. Natl. Cancer Inst. 2015, 107, djv033. [Google Scholar] [CrossRef]
- Cohen, J.V.; Chiel, L.; Boghossian, L.; Jones, M.; Stopfer, J.E.; Powers, J.; Rebbeck, T.R.; Nathanson, K.; Domchek, S.M. Non-cancer endpoints in BRCA1/2 carriers after risk-reducing salpingo-oophorectomy. Fam. Cancer 2011, 11, 69–75. [Google Scholar] [CrossRef]
- Michelsen, T.M.; Tonstad, S.; Pripp, A.H.; Tropé, C.G.; Dørum, A. Coronary Heart Disease Risk Profile in Women Who Underwent Salpingo-Oophorectomy to Prevent Hereditary Breast Ovarian Cancer. Int. J. Gynecol. Cancer 2010, 20, 233–239. [Google Scholar] [CrossRef]
- Michelsen, T.M.; Pripp, A.H.; Tonstad, S.; Tropé, C.G.; Dørum, A. Metabolic syndrome after risk-reducing salpingo-oophorectomy in women at high risk for hereditary breast ovarian cancer: A controlled observational study. Eur. J. Cancer 2009, 45, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Grynberg, M.; Raad, J.; Comtet, M.; Vinolas, C.; Cédrin-Durnerin, I.; Sonigo, C. Fertility preservation in BRCA-mutated women: When and how? Futur. Oncol. 2018, 14, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, A.R.; Ibe, C.N.; Dignam, J.J.; Cummings, S.A.; Verp, M.; White, M.A.; Artioli, G.; Dudlicek, L.; Olopade, O.I. Uptake and timing of bilateral prophylactic salpingo-oophorectomy among BRCA1 and BRCA2 mutation carriers. Anesth. Analg. 2008, 10, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Mai, P.L.; Piedmonte, M.; Han, P.K.; Moser, R.P.; Walker, J.L.; Rodriguez, G.; Boggess, J.; Rutherford, T.J.; Zivanovic, O.; Cohn, D.E.; et al. Factors associated with deciding between risk-reducing salpingo-oophorectomy and ovarian cancer screening among high-risk women enrolled in GOG-0199: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2017, 145, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Padamsee, T.J.; Wills, C.E.; Yee, L.D.; Paskett, E.D. Decision making for breast cancer prevention among women at elevated risk. Breast Cancer Res. 2017, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Hurley, K.E.; Miller, S.M.; Costalas, J.W.; Gillespie, D.; Daly, M.B. Anxiety/Uncertainty Reduction as a Motivation for Interest in Prophylactic Oophorectomy in Women with a Family History of Ovarian Cancer. J. Women’s Health Gend.-Based Med. 2001, 10, 189–199. [Google Scholar] [CrossRef]
- Hochbaum, G.; Rosenstock, I.; Kegels, S. Health Belief Model; United States Public Health Service: Washington, DC, USA, 1952.
- Jones, C.L.; Jensen, J.D.; Scherr, C.L.; Brown, N.R.; Christy, K.; Weaver, J. The Health Belief Model as an Explanatory Framework in Communication Research: Exploring Parallel, Serial, and Moderated Mediation. Health Commun. 2015, 30, 566–576. [Google Scholar] [CrossRef]
- Champion, V.L.; Skinner, C.S. The health belief model. In Health Behavior and Health Education: Theory, Research, and Practice; Glanz, K., Rimer, B.K., Viswanath, K., Eds.; Jossey-Bass: San Francisco, CA, USA, 2008; pp. 45–65. [Google Scholar]
- Green, E.C.; Murphy, E.M.; Gryboski, K. The Health Belief Model. In The Wiley Encyclopedia of Health Psychology; Paul, R., Cohen, L.M., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2021; Volume 2, pp. 211–214. [Google Scholar]
- Herrmann, A.; Hall, A.; Proietto, A. Using the Health Belief Model to explore why women decide for or against the removal of their ovaries to reduce their risk of developing cancer. BMC Women’s Health 2018, 18, 184. [Google Scholar] [CrossRef]
- Gellman, C.; Ezratty, C.; Schwarz, J.; Kolev, V.; Blank, S.V. “It was a no-brainer”: A qualitative study of factors driving previvors’ decision-making when considering risk-reducing salpingectomy with delayed oophorectomy. Gynecol. Oncol. Rep. 2022, 40, 100948. [Google Scholar] [CrossRef]
- Finch, A.; Metcalfe, K.A.; Chiang, J.; Elit, L.; McLaughlin, J.; Springate, C.; Esplen, M.J.; Demsky, R.; Murphy, J.; Rosen, B.; et al. The impact of prophylactic salpingo-oophorectomy on quality of life and psychological distress in women with a BRCA mutation. Psycho-Oncology 2011, 22, 212–219. [Google Scholar] [CrossRef]
- Madalinska, J.B.; Hollenstein, J.; Bleiker, E.; van Beurden, M.; Valdimarsdottir, H.B.; Massuger, L.F.; Gaarenstroom, K.N.; Mourits, M.J.; Verheijen, R.H.; van Dorst, E.B.; et al. Quality-of-Life Effects of Prophylactic Salpingo-Oophorectomy Versus Gynecologic Screening Among Women at Increased Risk of Hereditary Ovarian Cancer. J. Clin. Oncol. 2005, 23, 6890–6898. [Google Scholar] [CrossRef]
- Shigehiro, M.; Kita, M.; Takeuchi, S.; Ashihara, Y.; Arai, M.; Okamura, H. Study on the psychosocial aspects of risk-reducing salpingo-oophorectomy (RRSO) in BRCA1/2 mutation carriers in Japan: A preliminary report. Jpn. J. Clin. Oncol. 2015, 46, 254–259. [Google Scholar] [CrossRef]
- Hickey, I.; Jha, S.; Wyld, L. The psychosexual effects of risk-reducing bilateral salpingo-oophorectomy in female BRCA1/2 mutation carriers: A systematic review of qualitative studies. Gynecol. Oncol. 2020, 160, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Hensley, M.; Barakat, R.; Brown, C.; Chi, D.; Poynor, E.; Offit, K. Quality of life in women at risk for ovarian cancer who have undergone risk-reducing oophorectomy. Gynecol. Oncol. 2003, 89, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Bresser, P.; Seynaeve, C.; Van Gool, A.; Niermeijer, M.; Duivenvoorden, H.; van Dooren, S.; van Geel, A.; Menke-Pluijmers, M.; Klijn, J.; Tibben, A. The course of distress in women at increased risk of breast and ovarian cancer due to an (identified) genetic susceptibility who opt for prophylactic mastectomy and/or salpingo-oophorectomy. Eur. J. Cancer 2007, 43, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.; Moss, K.M.; Brand, A.; Wrede, C.D.; Domchek, S.M.; Meiser, B.; Mishra, G.D.; Joffe, H. What happens after menopause? (WHAM): A prospective controlled study of depression and anxiety up to 12 months after premenopausal risk-reducing bilateral salpingo-oophorectomy. Gynecol. Oncol. 2021, 161, 527–534. [Google Scholar] [CrossRef] [PubMed]
- McFarland, D.C.; Blackler, L.; Banerjee, S.; Holland, J. Communicating About Precision Oncology. JCO Precis. Oncol. 2017, 1, 1–9. [Google Scholar] [CrossRef]
- Bomhof-Roordink, H.; Gärtner, F.R.; Stiggelbout, A.M.; Pieterse, A.H. Key components of shared decision making models: A systematic review. BMJ Open 2019, 9, e031763. [Google Scholar] [CrossRef]
- Bomhof-Roordink, H.; Fischer, M.J.; Van Duijn-Bakker, N.; Baas-Thijssen, M.C.; Van Der Weijden, T.; Stiggelbout, A.M.; Pieterse, A.H. Shared decision making in oncology: A model based on patients’, health care professionals’, and researchers’ views. Psycho-Oncology 2018, 28, 139–146. [Google Scholar] [CrossRef]
- Kane, H.L.; Halpern, M.T.; Squiers, L.B.; Treiman, K.A.; McCormack, L.A. Implementing and evaluating shared decision making in oncology practice. CA Cancer J. Clin. 2014, 64, 377–388. [Google Scholar] [CrossRef]
- Shay, L.A.; Lafata, J.E. Understanding patient perceptions of shared decision making. Patient Educ. Couns. 2014, 96, 295–301. [Google Scholar] [CrossRef]
- Amutio-Kareaga, A.; García-Campayo, J.; Delgado, L.C.; Hermosilla, D.; Martínez-Taboada, C. Improving Communication between Physicians and Their Patients through Mindfulness and Compassion-Based Strategies: A Narrative Review. J. Clin. Med. 2017, 6, 33. [Google Scholar] [CrossRef]
- Baig, L.A.; Violato, C.; Crutcher, R.A. Assessing clinical communication skills in physicians: Are the skills context specific or generalizable. BMC Med. Educ. 2009, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Maly, R.C.; Liu, Y.; Leake, B.; Thind, A.; Diamant, A.L. Treatment-related symptoms among underserved women with breast cancer: The impact of physician–patient communication. Breast Cancer Res. Treat. 2009, 119, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Świątoniowska-Lonc, N.; Polański, J.; Tański, W.; Jankowska-Polańska, B. Impact of satisfaction with physician–patient communication on self-care and adherence in patients with hypertension: Cross-sectional study. BMC Health Serv. Res. 2020, 20, 1046. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S. Pathway Linking Patient-Centered Communication to Emotional Well-Being: Taking into Account Patient Satisfaction and Emotion Management. J. Health Commun. 2017, 22, 234–242. [Google Scholar] [CrossRef]
- Venetis, M.; Robinson, J.D.; Turkiewicz, K.L.; Allen, M. An evidence base for patient-centered cancer care: A meta-analysis of studies of observed communication between cancer specialists and their patients. Patient Educ. Couns. 2009, 77, 379–383. [Google Scholar] [CrossRef]
- Hallowell, N.; kConFab Psychosocial Group on behalf of the kConFab Investigators; Baylock, B.; Heiniger, L.; Butow, P.N.; Patel, D.; Meiser, B.; Saunders, C.; Price, M.A. Looking different, feeling different: Women’s reactions to risk-reducing breast and ovarian surgery. Fam. Cancer 2011, 11, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Trister, R.; Jacobson, M.; Nguyen, P.; Sobel, M.; Allen, L.; Narod, S.A.; Kotsopoulos, J. Patient reported experiences following laparoscopic prophylactic bilateral salpingo-oophorectomy or salpingectomy in an ambulatory care hospital. Fam. Cancer 2020, 20, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zarbo, C.; Brugnera, A.; Frigerio, L.; Celi, C.; Compare, A.; Dessì, V.; Giordano, R.; Malandrino, C.; Sina, F.P.; Strepparava, M.G.; et al. Cancer Anxiety Mediates the Association Between Satisfaction with Medical Communication and Psychological Quality of Life After Prophylactic Bilateral Salpingo-Oophorectomy. Front. Psychol. 2022, 13, 840931. [Google Scholar] [CrossRef]
- Riess, H.; Kelley, J.M.; Bailey, R.; Konowitz, P.M.; Gray, S.T. Improving Empathy and Relational Skills in Otolaryngology Residents: A pilot study. Otolaryngol. Neck Surg. 2010, 144, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Riess, H.; Kelley, J.M.; Bailey, R.W.; Dunn, E.; Phillips, M. Empathy Training for Resident Physicians: A Randomized Controlled Trial of a Neuroscience-Informed Curriculum. J. Gen. Intern. Med. 2012, 27, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Defining Sexual Health: Report of a Technical Consultation on Sexual Health, 28–31 January 2002, Geneva; Sexual Health Document Series; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Nash, Z.; Menon, U. Ovarian cancer screening: Current status and future directions. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 65, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.K.; Gupta, V.K.; Naumann, R.W. Ovarian cancer: Screening and future directions. Int. J. Gynecol. Cancer 2019, 29, 195–200. [Google Scholar] [CrossRef] [PubMed]
| Gene | Ovarian Cancer Cumulative Risk (by Age ≥ 70) | Breast Cancer Cumulative Risk (by Age ≥ 70) |
|---|---|---|
| MLH1 | 4–20% | Unknown |
| MSH2 | 8–38% | Unknown |
| MSH6 | 1–13% | Unknown |
| PALB2 | Unknown | 35% |
| ATM | Unknown | 33% |
| STK11 | 18–21% | 45–50% |
| BRIP1 | 7–10% | Unknown |
| RAD51C/D | 5–12% | Unknown |
| BRCA1 | 45–60% | 65–80% |
| BRCA2 | 11–35% | 50–70% |
| Risk-Reducing Measure | Recommended Implementation |
|---|---|
| Screening | |
| Breast examinations (mammography, MRI) | >age 25 |
| Pelvic ultrasound testing + serum biomarkers | >age 35 |
| Risk-reducing agents | |
| Oral contraceptives | More data needed |
| Chemoprevention | More data needed |
| Risk-reducing surgeries | |
| Mastectomy (RRM) | No age defined |
| Salpingo-oophorectomy (RRSO) | >age 35–40 (BRCA1) >age 40–45 (BRCA2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves-Nogueira, A.C.; Melo, D.; Carona, C.; Figueiredo-Dias, M. The Psychosocial Impact of the Decision to Undergo Risk-Reducing Salpingo-Oophorectomy Surgery in BRCA Mutation Carriers and the Role of Physician-Patient Communication. Curr. Oncol. 2023, 30, 2429-2440. https://doi.org/10.3390/curroncol30020185
Alves-Nogueira AC, Melo D, Carona C, Figueiredo-Dias M. The Psychosocial Impact of the Decision to Undergo Risk-Reducing Salpingo-Oophorectomy Surgery in BRCA Mutation Carriers and the Role of Physician-Patient Communication. Current Oncology. 2023; 30(2):2429-2440. https://doi.org/10.3390/curroncol30020185
Chicago/Turabian StyleAlves-Nogueira, Ana C., Daniela Melo, Carlos Carona, and Margarida Figueiredo-Dias. 2023. "The Psychosocial Impact of the Decision to Undergo Risk-Reducing Salpingo-Oophorectomy Surgery in BRCA Mutation Carriers and the Role of Physician-Patient Communication" Current Oncology 30, no. 2: 2429-2440. https://doi.org/10.3390/curroncol30020185
APA StyleAlves-Nogueira, A. C., Melo, D., Carona, C., & Figueiredo-Dias, M. (2023). The Psychosocial Impact of the Decision to Undergo Risk-Reducing Salpingo-Oophorectomy Surgery in BRCA Mutation Carriers and the Role of Physician-Patient Communication. Current Oncology, 30(2), 2429-2440. https://doi.org/10.3390/curroncol30020185





