Continuous Real-Time Neuropsychological Testing during Resection Phase in Left and Right Prefrontal Brain Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Pre- and Post-Surgery Neuropsychological Assessment
2.3. Real-Time Neuropsychological Testing (RTNT) in Prefrontal Areas
2.4. MRI Structural Data
2.5. Statistical Analysis
3. Results
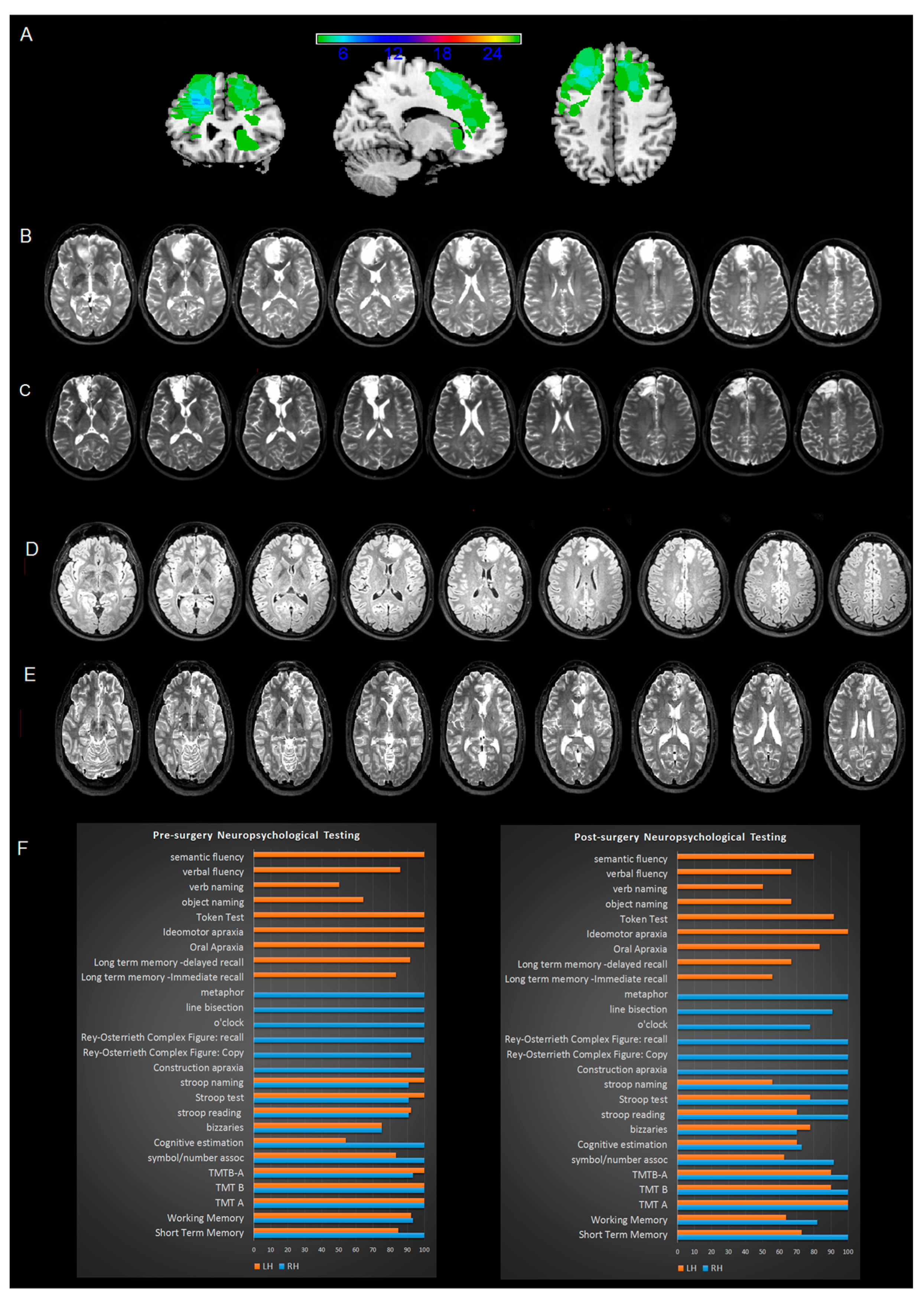
3.1. MRI Analysis
3.2. Pre-Surgery Neuropsychological Assessment
3.3. DES Mapping
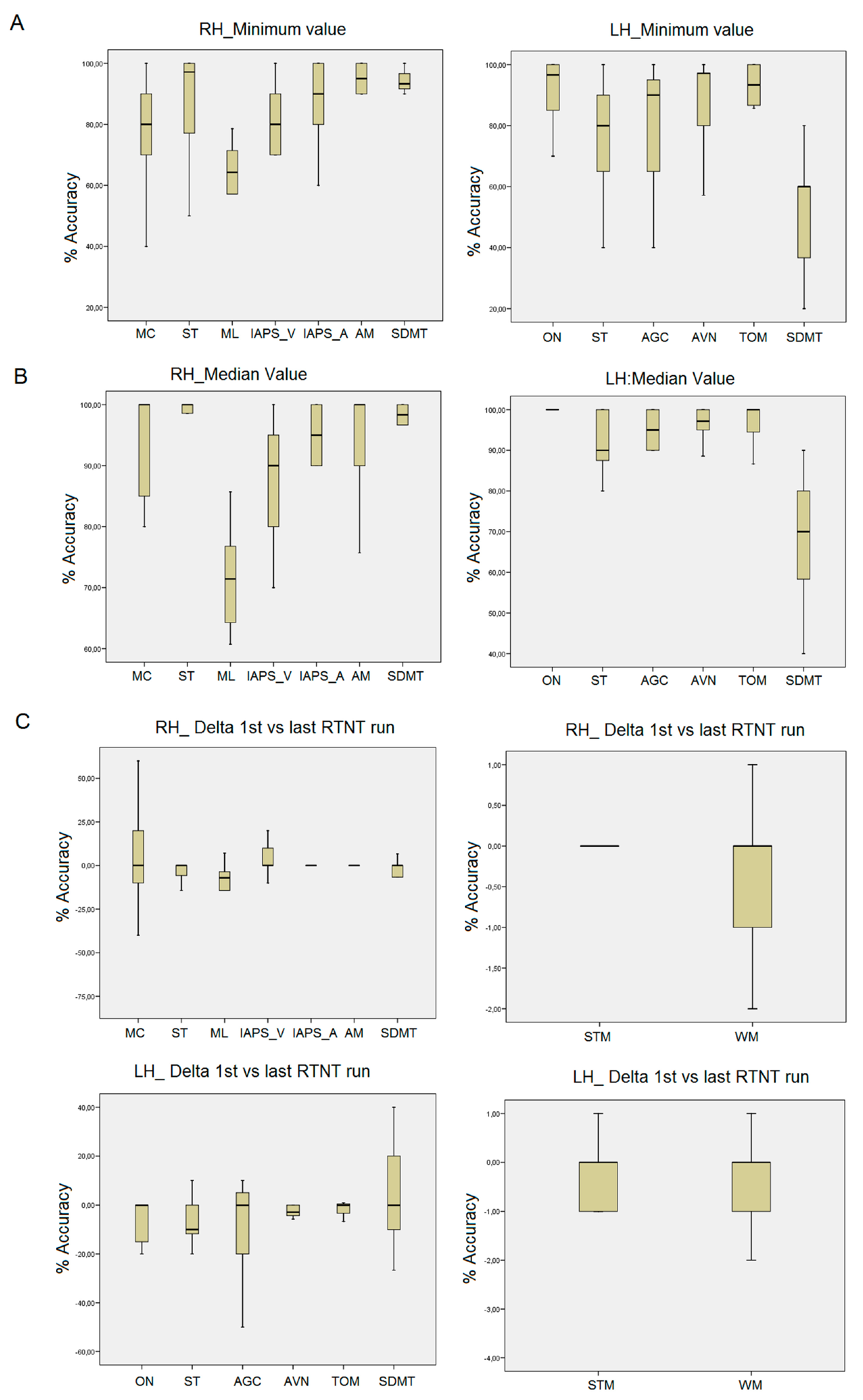
3.4. RTNT Results
3.5. Right Frontal RTNT
3.6. Left Frontal RTNT
3.7. Short-Term Memory and Working Memory
3.8. Narrative Language
3.9. Other Intraoperative Qualitative Observations
3.10. Extent of Resection
3.11. Post-Surgery Neuropsychological Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burgess, P.W.; Scott, S.K.; Frith, C.D. The role of the rostral frontal cortex (area 10) in prospective memory: A lateral versus medial dissociation. Neuropsychologia 2003, 41, 906–918. [Google Scholar] [CrossRef]
- Norman, D.; Shallice, T. Attention to action: Willed and automatic control of behaviour. In Consciousness and Self Regulation; Davidson, R.J., Schwartz, G.E., Shapiro, D., Eds.; Plenum Press: New York, NY, USA, 1986; pp. 1–18. [Google Scholar]
- Luu, P.; Flaisch, T.; Tucker, D.M. Medial frontal cortex in action monitoring. J. Neurosci. 2000, 20, 464–469. [Google Scholar] [CrossRef]
- Zoccolotti, P. Le funzioni esecutive: Quadri clinici e ipotesi interpretative. In Le Funzioni Esecutive; Cantagallo, A., Spitoni, G.F., Antonucci, G., Eds.; Valutazione e Riabilitazione Carocci: Rome, Italy, 2010. [Google Scholar]
- Satoer, D.; Visch-Brink, E.; Dirven, C.; Vincent, A. Glioma surgery in eloquent areas: Can we preserve cognition? Acta Neurochir. 2016, 158, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Knouse, L.E.; Barkley, R.A.; Murphy, K.R. Does executive functioning (EF) predict depression in clinic-referred adults? EF tests vs. rating scales. J. Affect. Disord. 2013, 145, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Ruis, C. Monitoring cognition during awake brain surgery in adults: A systematic review. J. Clin. Exp. Neuropsychol. 2018, 40, 1081–1104. [Google Scholar] [CrossRef]
- Wager, M.; Du Boisgueheneuc, F.; Pluchon, C.; Bouyer, C.; Stal, V.; Bataille, B.; Guillevin, M.C.; Gil, R. Intraoperative monitoring of an aspect of executive functions: Administration of the Stroop test in 9 adult patients during awake surgery for resection of frontal glioma. Neurosurgery 2013, 72, ons169–ons181. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, G.; Sciortino, T.; Rossi, M.; Leonetti, A.; Fornia, L.; Nibali, M.C.; Casarotti, A.; Pessina, F.; Riva, M.; Cerri, G.; et al. Preserving executive functions in nondominant frontal lobe glioma surgery: An intraoperative tool. J. Neurosurg. 2018, 131, 474–480. [Google Scholar] [CrossRef]
- Erez, Y.; Assem, M.; Coelho, P.; Romero-Garcia, R.; Owen, M.; McDonald, A.; Woodberry, E.; Morris, R.C.; Price, S.J.; Suckling, J.; et al. Intraoperative mapping of executive function using electrocorticography for patients with low-grade gliomas. Acta Neurochir. 2021, 163, 1299–1309. [Google Scholar] [CrossRef]
- Prat-Acín, R.; Galeano-Senabre, I.; López-Ruiz, P.; Ayuso-Sacido, A.; Espert-Tortajada, R. Intraoperative brain mapping of language, cognitive functions, and social cognition in awake surgery of low-grade gliomas located in the right non-dominant hemisphere. Clin. Neurol. Neurosurg. 2021, 200, 106363. [Google Scholar] [CrossRef]
- Vilasboas, T.; Herbet, G.; Duffau, H. Challenging the Myth of Right Nondominant Hemisphere: Lessons from Corticosubcortical Stimulation Mapping in Awake Surgery and Surgical Implications. World Neurosurg. 2017, 103, 449–456. [Google Scholar] [CrossRef]
- Nakajima, R.; Kinoshita, M.; Miyashita, K.; Okita, H.; Genda, R.; Yahata, T.; Hayashi, Y.; Nakada, M. Damage of the right dorsal superior longitudinal fascicle by awake surgery for glioma causes persistent visuospatial dysfunction. Sci. Rep. 2017, 7, 17158. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, G.; Howells, H.; Sciortino, T.; Leonetti, A.; Rossi, M.; Nibali, M.C.; Gay, L.G.; Fornia, L.; Bellacicca, A.; Vigano’, L.; et al. Frontal pathways in cognitive control: Direct evidence from intraoperative stimulation and diffusion tractography. Brain 2019, 142, 2451–2465. [Google Scholar] [PubMed]
- Yordanova, Y.N.; Duffau, H.; Herbet, G. Neural pathways subserving face-based mentalizing. Brain Struct. Funct. 2017, 222, 3087–3105. [Google Scholar] [CrossRef]
- Barberis, M.; Poisson, I.; Facque, V.; Letrange, S.; Prevost-Tarabon, C.; Houdart, E.; Froelich, S.; Levy, R.; Mandonnet, E. Group-level stability but individual variability of neurocognitive status after awake resections of right frontal IDH-mutated glioma. Sci. Rep. 2022, 12, 6126. [Google Scholar] [CrossRef] [PubMed]
- Rutten, G.M.; Landers, M.; De Baene, W.; Meijerink, T.; van der Hek, S.; Verheul, J. Executive functional deficits during electrical stimulation of the right frontal aslant tract. Brain Imaging Behav. 2021, 15, 2731–2735. [Google Scholar] [CrossRef]
- Gao, W.; Lin, W. Frontal parietal control network regulates the anti-correlated default and dorsal attention networks. Hum. Brain Mapp. 2012, 33, 192–202. [Google Scholar] [CrossRef]
- Mandonnet, E. Should Complex Cognitive Functions Be Mapped with Direct Electrostimulation in Wide-Awake Surgery? A Commentary. Front. Neurol. 2021, 12, 721038. [Google Scholar] [CrossRef]
- Skrap, M.; Marin, D.; Ius, T.; Fabbro, F.; Tomasino, B. Brain mapping: A novel intraoperative neuropsychological approach. J. Neurosurg. 2016, 125, 877–887. [Google Scholar] [CrossRef]
- Giovagnoli, A.R.; Del Pesce, M.; Mascheroni, S.; Simoncelli, M.; Laiacona, M.; Capitani, E. Trail making test: Normative values from 287 normal adult controls. Ital. J. Neurol. Sci. 1996, 17, 305–309. [Google Scholar] [CrossRef]
- Della Sala, S.; MacPherson, S.E.; Phillips, L.H.; Sacco, L.; Spinnler, H. How many camels are there in Italy? Cognitive estimates standardised on the Italian population. Neurol. Sci. 2003, 24, 10–15. [Google Scholar] [CrossRef]
- Brugnolo, A.; De Carli, F.; Accardo, J.; Amore, M.; Bosia, L.E.; Bruzzaniti, C.; Cappa, S.F.; Cocito, L.; Colazzo, G.; Ferrara, M.; et al. An updated Italian normative dataset for the Stroop color word test (SCWT). Neurol. Sci. 2016, 37, 365–372. [Google Scholar] [CrossRef]
- Orsini, A.; Laicardi, C. WAIS-R. Contributo Alla Taratura Italiana; Giunti Os: Firenze, Italy, 1997. [Google Scholar]
- Monaco, M.; Costa, A.; Caltagirone, C.; Carlesimo, G.A. Erratum to: Forward and backward span for verbal and visuo-spatial data: Standardization and normative data from an Italian adult population. Neurol Sci. 2015, 36, 45–347. [Google Scholar] [CrossRef] [PubMed]
- Tomasino, B.; Marin, D.; Ius, T.; Skrap, M. Neuropsychology in Insular Lesions Prior-During and After Brain Surgery. In Island of Reil (Insula) in the Human Brain; Turgut, M., Yurttaş, C., Tubbs, R., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Tomasino, B.; Guarracino, I.; Ius, T.; Budai, R.; Skrap, M. Real-Time Neuropsychological Testing of sensorimotor cognition during awake surgery in Precentral and Postsomatosensory areas. World Neurosurg. 2022, 164, e599–e610. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Basso, A.; Capitani ELaiacona, M. Raven’s coloured progressive matrices: Normative values on 305 adult normal controls. Funct. Neurol. 1987, 2, 189–194. [Google Scholar] [PubMed]
- Novelli, G.; Papagno, C.; Capitani, E.; Laiacona, M. Tre test clinici di ricerca e produzione lessicale. Taratura su sogetti normali [Three clinical tests to research and rate the lexical performance of normal subjects]. Arch. Psicol. Neurol. Psichiatr. 1986, 47, 477–506. [Google Scholar]
- Mondini, S. Esame Neuropsicologico Breve 2: Una Batteria di Test per lo Screening Neuropsicologico; Cortina: Milan, Italy, 2011. [Google Scholar]
- Papagno, C. Comprehension of metaphors and idioms in patients with Alzheimer’s disease: A longitudinal study. Brain 2001, 124, 1450–1460. [Google Scholar] [CrossRef]
- Miceli, G.; Laudanna, A.; Burani, C.; Capasso, R. Batteria per L’analisi dei Deficit Afasici: BADA [BADA: A Battery for the Assessment of Aphasic Disorders]; CEPSAG: Rome, Italy, 1994. [Google Scholar]
- De Renzi, E.; Motti, F.; Nichelli, P. Imitating gestures. A quantitative approach to ideomotor apraxia. Arch. Neurol. 1980, 37, 6–10. [Google Scholar] [CrossRef]
- De Renzi, E.; Piezcuro, A.; Vignolo, L.A. Oral Apraxia and Aphasia. Cortex 1966, 2, 50–73. [Google Scholar] [CrossRef]
- De Renzi, E.; Faglioni, P. Normative data and screening power of a shortened version of the Token Test. Cortex 1978, 14, 41–49. [Google Scholar] [CrossRef]
- Spinnler, H.; Tognoni, G. Standardizzazione e taratura di test neuropsicologici [Italian normative values and standardization of neuropsychological tests]. Ital. J. Neurol. Sci. 1987, 6, S1–S120. [Google Scholar]
- Wilson, B.; Cockburn, J.; Halligan, P. Development of a behavioral test of visuospatial neglect. Arch. Phys. Med. Rehabil. 1987, 68, 98–102. [Google Scholar]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, F.; Venneri, A. Rey-Osterrieth complex figure: Normative values in an Italian population sample. Neurol. Sci. 2002, 22, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Prior, M.; Sartori, G.; Marchi, S. Cognizione Sociale e Comportamento: Uno Strumento per la Misurazione; Domeneghini Editore: Padova, Italy, 2003. [Google Scholar]
- Tavano, A.; Côté, H.; Ferré, P.; Ska, B.; Joanette, Y. Protocollo MEC-Protocollo Montréal per la Valutazione delle Abilità Comunicative 2013; Springer: Milan, Italy, 2013. [Google Scholar]
- Nocentini, U.; Giordano, A.; Vincenzo, S.D.; Panella, M.; Pasqualetti, P. The Symbol Digit Modalities Test--Oral version: Italian normative data. Funct. Neurol. 2006, 21, 93–96. [Google Scholar]
- Bisiach, E.; Ricci, R.; Lualdi, M.; Colombo, M.R. Perceptual and response bias in unilateral neglect: Two modified versions of the Milner landmark task. Brain Cogn. 1998, 37, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. International affective picture system (IAPS): Technical manual and affective ratings. NIMH Cent. Study Emot. Atten. 1997, 1, 3. [Google Scholar]
- Baird, A.; Dewar, B.K.; Critchley, H.; Gilbert, S.J.; Dolan, R.J.; Cipolotti, L. Cognitive functioning after medial frontal lobe damage including the anterior cingulate cortex: A preliminary investigation. Brain Cogn. 2006, 60, 166–175. [Google Scholar] [CrossRef]
- Kaleita, T.A.; Wellisch, D.K.; Cloughesy, T.F.; Ford, J.M.; Freeman, D.; Belin, T.R.; Goldman, J. Prediction of neurocognitive outcome in adult brain tumor patients. J. Neuro-Oncol. 2004, 67, 245–253. [Google Scholar] [CrossRef]
- Nakajima, R.; Kinoshita, M.; Okita, H.; Liu, Z.; Nakada, M. Preserving Right Pre-motor and Posterior Prefrontal Cortices Contribute to Maintaining Overall Basic Emotion. Front. Hum. Neurosci. 2021, 15, 612890. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Wang, Y.; Jiang, T. The Influence of Frontal Lobe Tumors and Surgical Treatment on Advanced Cognitive Functions. World Neurosurg. 2016, 91, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Karaduman, A.; Göksun, T.; Chatterjee, A. Narratives of focal brain injured individuals: A macro-level analysis. Neuropsychologia 2017, 99, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Mar, R.A. The neuropsychology of narrative: Story comprehension, story production and their interrelation. Neuropsychologia 2004, 42, 1414–1434. [Google Scholar] [CrossRef] [PubMed]
- Zalla, T.; Phipps, M.; Grafman, J. Story processing in patients with damage to the prefrontal cortex. Cortex 2002, 38, 215–231. [Google Scholar] [CrossRef] [PubMed]
| Function | Test | LH/RH Protocol |
|---|---|---|
| Handedness | Oldfield [29] | LH and RH |
| Abstract reasoning | Raven Matrices [30] | LH and RH |
| Psychomotor speed and selective attention, attention shifting and cognitive flexibility | Trail Making Tests A and B [21] | LH and RH |
| Reasoning, development and application of appropriate strategies, and response plausibility | Cognitive Estimations [22] | LH and RH |
| Inhibition of cognitive interference that occurs during automatic processing | Stroop Test [23] | LH and RH |
| Motor speed, attention, and visuo-perceptual functions | Digit Symbol Substitution Test [24] | LH and RH |
| Short-term memory | Digit span forward [25] | LH and RH |
| Working memory | Digit span backward [25] | LH and RH |
| Selection of words meeting certain constraints and repetition avoidance, both based on executive control processes | Verbal and Semantic fluency [31] | LH |
| Visuo-spatial planning | O’Clock Test [32] | RH |
| Analytical thinking in the verbal domain | Comprehension of metaphors and idioms [33] | RH |
| Object and verb naming | Battery for the analysis of language disorders [34] | LH |
| Imitation | Ideomotor apraxia [35] and oro-facial [36] | LH |
| Verbal comprehension | Token Test [37] | LH |
| Visuo-spatial processing | Figure copy [38] | RH |
| Visuo-spatial attention | Line Bisection [39] | RH |
| Visuo-spatial memory | Rey-Osterrieth Complex Figure Test [40] | RH |
| RTNT Test | LH/RH Protocol | Assessed Abilities | Tests Identically Used Intraoperatively vs. Pre- and Post-Surgery | |
|---|---|---|---|---|
| Object Naming task (ON) [34] | LH | The patient is presented with a series of black and white line drawings and asked to name the corresponding object associated with it. | lexical access | V |
| Stroop test (ST) [23] | LH and RH | The patient is asked to read the names of colors, name the colors of squares, and say the color of the ink with which the name of the words is written while inhibiting reading of the name itself (e.g., say “red” in response to the word “green” written in red ink). | selective attention, inhibition of irrelevant information | V |
| Auditory Grammar Comprehension (AGC) [34] | LH | The neuropsychologist reads a sentence, and the patient is required to say which image, choosing between two options, represents the meaning of the sentence. Sentences are reversible items, e.g., active or passive forms with a transitive verb and two nouns, e.g., the horse is chasing the girls (#1: a picture depicts the horse chasing the girls, #2: the girls are chasing the horse). | ability to understand semantically reversible sentences (active or passive) by choosing between two images, divided attention | Indirectly tested by analyzing the Token test performance |
| Short-term memory (STM) [25] | LH and RH | The patients is asked to repeat each digit sequence in the same order as it is read. | verbal short term memory and attention | V |
| Working memory (WM) [25] | LH and RH | The patient is asked to repeat each digit sequence by reversing its order. | working memory and attention | V |
| Verbal fluency (VF) [31] | LH | The patient is asked to produce as many words as she/he can in one minute by maintaining a given criteria (the first letter is given by the neuropsychologist). Each RTNT run has a different letter and lasts one minute. | lexical phonologically access speed and verbal monitoring | V |
| Action Verb Naming (AVN) [34] | LH | Action verb naming was monitored using the oral verb naming task. Patients were presented with a series of black and white line drawings and asked to name the corresponding verb associated with it. | lexical access | V |
| Theory Of Mind (TOM) [41] | LH | Short stories with one character are verbally presented. At the end of each story, the patient is asked to say which emotion the character is feeling, e.g., “Maria has to make a speech at work. She is standing in the room in front of everyone and cannot remember a word of what she has to say. Everyone is staring at her. How will Maria feel in this situation?”. Each RTNT run includes 5 items. | social cognition, emotion processing | Not tested |
| Narrative language (NL) [42] | LH and RH | Black and white pictures depicting a short story are shown. Each is divided into 4 vignettes following a time line. The patient is asked to tell the story depicted. One picture is presented for each RTNT run. | lexical access, verbal monitoring | Indirectly tested by analyzing the patient’s speech during the clinical interview |
| Symbol Digit Modalities Test (SDMT) [43] | LH and RH | The patient is asked to verbally substitute a symbol with a corresponding digit. A grid reporting symbol–digit correspondences is shown on the top of the image. A row of 15 symbols is shown below, and the patient is asked to say the corresponding number (digits 1 to 9). | selective attention, working memory processing speed | V |
| Metaphor comprehension (MC) [33] | RH | The patient reads a metaphor and has to explain its meaning in his/her own words (literally). Each RTNT run includes 5 metaphors. | abstract language | V |
| Milner Landmark Test (LT) [44] | RH | Lines, which are divided into two segments of different length, colored in red and black are shown in the center of the screen. The patient is required to decide which segment (red or black) is the longest (or alternatively the shortest), according to the instruction. | visual attention | Indirectly tested by analyzing the visuospatial attention tasks |
| International Affective Picture System (IAPS) [45] | RH | Emotionally charged (negatively or positively) images or neutrals are presented. For each image, the patient is asked to indicate on a Likert scale (from 1 to 9) how he/she feels when looking at that image. For each image, there are 2 answers: pleasure (happy vs. unhappy scale) and arousal (excited vs. calm dimension). | emotional processing, monitoring internal emotional states and arousal | Not tested |
| Attentional Matrices (AT) [38] | RH | Matrices, composed by a series of numbers and a given target number, are presented. The patient is asked to report how many times the target number(s) appears within the different rows. To facilitate visual search, the test was adapted so that both the target number and the row are underlined in red. Each RTNT run includes 1 attentional matrix. | selective attention, working memory, | Indirectly tested by analyzing performance on the TMT, Stroop test, and SDMT |
| Area | % N > 0 | MaxX | MaxY | MaxZ | % |
|---|---|---|---|---|---|
| Frontal_Sup_RH | 82 | 29 | 25 | 33 | 50 |
| Frontal_Mid_RH | 93 | 30 | 25 | 33 | 50 |
| Frontal_Sup_LH | 74 | −13 | 34 | 38 | 40 |
| Frontal_Mid_LH | 55 | −26 | 16 | 43 | 40 |
| Superior_corona_radiata RH | 68 | 23 | 12 | 24 | 42.85 |
| Anterior_corona_radiata RH | 94 | 25 | 13 | 24 | 42.85 |
| Superior_corona_radiata LH | 38 | −24 | 13 | 28 | 26.66 |
| Anterior_corona_radiata LH | 99 | −17 | 34 | 10 | 26.66 |
| RTNT_Task | Median Value during the First RTNT Run (Baseline) | Mean | Sd | Median | 95% Confidence INTERVAL Lower | 95% Confidence INTERVAL Upper |
|---|---|---|---|---|---|---|
| RH patients | ||||||
| MC | 90 | 93.33 | 8.87 | 100 | 87.69 | 98.97 |
| ST | 100 | 97.44 | 5.66 | 100 | 94.17 | 100.71 |
| ML | 78.57 | 71.74 | 7.73 | 71.42 | 66.55 | 76.94 |
| IAPS_V | 90 | 88.26 | 9.43 | 90 | 82.57 | 93.96 |
| IAPS_A | 100 | 91.73 | 11.15 | 95 | 84.99 | 98.46 |
| AM | 100 | 94.98 | 8.13 | 100 | 88.75 | 100.38 |
| SDMT | 100 | 96.66 | 5.63 | 98.66 | 91.95 | 101.37 |
| STM | 5 | 5.35 | 0.66 | 5 | 4.97 | 5.73 |
| WM | 4 | 3.8 | 0.60 | 4 | 3.33 | 4.27 |
| LH patients | ||||||
| ON | 100 | 97.91 | 4.98 | 100 | 85.4 | 101.08 |
| ST | 100 | 90.90 | 11.13 | 90 | 65.31 | 98.39 |
| AGC | 90 | 91.36 | 12.46 | 95 | 67.32 | 99.73 |
| STM | 6 | 4.45 | 1.29 | 4 | 3.58 | 5.32 |
| WM | 3 | 2.7 | 0.67 | 3 | 2.21 | 3.18 |
| VF | 12 | 10.45 | 3.61 | 9.5 | 7.86 | 13.03 |
| AVN | 100 | 96.62 | 3.95 | 97.14 | 73.93 | 99.27 |
| TOM | 90 | 96.95 | 4.98 | 10 | 87.92 | 101.12 |
| SDMT | 100 | 68.09 | 18.34 | 70 | 31.68 | 85.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomasino, B.; Guarracino, I.; Ius, T.; Skrap, M. Continuous Real-Time Neuropsychological Testing during Resection Phase in Left and Right Prefrontal Brain Tumors. Curr. Oncol. 2023, 30, 2007-2020. https://doi.org/10.3390/curroncol30020156
Tomasino B, Guarracino I, Ius T, Skrap M. Continuous Real-Time Neuropsychological Testing during Resection Phase in Left and Right Prefrontal Brain Tumors. Current Oncology. 2023; 30(2):2007-2020. https://doi.org/10.3390/curroncol30020156
Chicago/Turabian StyleTomasino, Barbara, Ilaria Guarracino, Tamara Ius, and Miran Skrap. 2023. "Continuous Real-Time Neuropsychological Testing during Resection Phase in Left and Right Prefrontal Brain Tumors" Current Oncology 30, no. 2: 2007-2020. https://doi.org/10.3390/curroncol30020156
APA StyleTomasino, B., Guarracino, I., Ius, T., & Skrap, M. (2023). Continuous Real-Time Neuropsychological Testing during Resection Phase in Left and Right Prefrontal Brain Tumors. Current Oncology, 30(2), 2007-2020. https://doi.org/10.3390/curroncol30020156




