Immunotherapy and Chemotherapy Versus Sleep Disturbances for NSCLC Patients
Abstract
1. Introduction
2. Patients and Methods
Sleep Evaluation Methodology
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parekh, A.; Deokar, K.; Verma, M.; Singhal, S.; Bhatt, M.L.; Katoch, C. The 50-Year Journey of Lung Cancer Screening: A Narrative Review. Cureus 2022, 14, e29381. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Huang, H.; Chen, W.; Petridis, D.; Matthaios, D.; Hohenforst-Schmidt, W.; Tolis, C.; Tsakiridis, K.; Baka, S.; Arnaoutoglou, C.; et al. Radial Endobronchial Ultrasound for Lung Cancer Diagnosis: Tips and Tricks. J. Cancer 2022, 13, 1307–1312. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Kosmidis, C.S.; Hohenforst-Schmidt, W.; Matthaios, D.; Sapalidis, K.; Petridis, D.; Perdikouri, E.I.; Courcoutsakis, N.; Hatzibougias, D.; Arnaoutoglou, C.; et al. Radial-EBUS: CryoBiopsy Versus Conventional Biopsy: Time-Sample and C-Arm. Int. J. Environ. Res. Public Health 2022, 19, 3569. [Google Scholar] [CrossRef]
- Zaric, B.; Stojsic, V.; Carapic, V.; Kovacevic, T.; Stojanovic, G.; Panjkovic, M.; Kioumis, I.; Darwiche, K.; Zarogoulidis, K.; Stratakos, G.; et al. Radial Endobronchial Ultrasound (EBUS) Guided Suction Catheter-Biopsy in Histological Diagnosis of Peripheral Pulmonary Lesions. J. Cancer 2016, 7, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Oikonomidou, R.; Petridis, D.; Alexidis, P.; Matthaios, D.; Boukovinas, I.; Perdikouri, E.I.; Baka, S.; Hohenforst-Schmidt, W.; Huang, H.; Bai, C.; et al. “One Shot” Sample Evaluation of 22G, 22G upgraded, 21G and 19G needle for Endobronchial Ultrasound-EBUS-TBNA. J. Cancer 2022, 13, 2982–2987. [Google Scholar] [CrossRef]
- Oikonomidou, R.; Petridis, D.; Kosmidis, C.; Sapalidis, K.; Hohenforst-Schmidt, W.; Christakidis, V.; Petanidis, S.; Mathaios, D.; Perdikouri, E.I.; Baka, S.; et al. Cryo-Biopsy versus 19G needle versus 22G needle with EBUS-TBNA endoscopy. J. Cancer 2022, 13, 3084–3090. [Google Scholar] [CrossRef]
- Sapalidis, K.; Zarogoulidis, P.; Petridis, D.; Kosmidis, C.; Fyntanidou, B.; Tsakiridis, K.; Maragouli, E.; Amaniti, A.; Giannakidis, D.; Koulouris, C.; et al. EBUS-TNBA 22G samples: Comparison of PD-L1 expression between DAKO and BIOCARE((R)). J. Cancer 2019, 10, 4739–4746. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Huang, H.; Hu, Z.; Wu, N.; Wang, J.; Petridis, D.; Tsakiridis, K.; Matthaios, D.; Kosmidis, C.; Hohenforst-Schmidt, W.; et al. Priority of PET-CT vs. CT Thorax for EBUS-TBNA 22G vs. 19G: Mesothorax Lymphadenopathy. J. Cancer 2021, 12, 5874–5878. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Pan, F.; Cai, H.; Pan, L.; Li, Y.; Li, L.; Li, Y.; Wu, X.; Fan, H. Positron emission tomography imaging of lung cancer: An overview of alternative positron emission tomography tracers beyond F18 fluorodeoxyglucose. Front. Med. 2022, 9, 945602. [Google Scholar] [CrossRef]
- Young, J.S.; Bourgeois, J.A.; Hilty, D.M.; Hardin, K.A. Sleep in hospitalized medical patients, part 2: Behavioral and pharmacological management of sleep disturbances. J. Hosp. Med. 2009, 4, 50–59. [Google Scholar] [CrossRef]
- Uzer, A.; Kurtses Gursoy, B. The mediating roles of depression, anxiety, and psychological pain in the relationship between chronotype and suicide in patients with depressive disorder. Chronobiol. Int. 2022, 39, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, D. Losing sleep over cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 2431–2432. [Google Scholar] [CrossRef]
- Giglio, M.; Preziosa, A.; Mele, R.; Brienza, N.; Grasso, S.; Puntillo, F. Effects of an Intrathecal Drug Delivery System Connected to a Subcutaneous Port on Pain, Mood and Quality of Life in End Stage Cancer Patients: An Observational Study. Cancer Control J. Moffitt Cancer Cent. 2022, 29, 10732748221133752. [Google Scholar] [CrossRef]
- Zarogoulidis, K.; Zarogoulidis, P.; Darwiche, K.; Boutsikou, E.; Machairiotis, N.; Tsakiridis, K.; Katsikogiannis, N.; Kougioumtzi, I.; Karapantzos, I.; Huang, H.; et al. Treatment of non-small cell lung cancer (NSCLC). J. Thorac. Dis. 2013, 5 (Suppl. 4), S389–S396. [Google Scholar]
- Domvri, K.; Zarogoulidis, P.; Darwiche, K.; Browning, R.F.; Li, Q.; Turner, J.F.; Kioumis, I.; Spyratos, D.; Porpodis, K.; Papaiwannou, A.; et al. Molecular Targeted Drugs and Biomarkers in NSCLC, the Evolving Role of Individualized Therapy. J. Cancer 2013, 4, 736–754. [Google Scholar] [CrossRef] [PubMed]
- Domvri, K.; Darwiche, K.; Zarogoulidis, P.; Zarogoulidis, K. Following the crumbs: From tissue samples, to pharmacogenomics, to NSCLC therapy. Transl. Lung Cancer Res. 2013, 2, 256–258. [Google Scholar] [PubMed]
- Lim, J.U.; Kang, H.S.; Shin, A.Y.; Yeo, C.D.; Kim, S.K.; Kim, J.W.; Kim, S.J.; Lee, S.H. Investigation of poor predictive factors in extensive stage small cell lung cancer under etoposide-platinum-atezolizumab treatment. Thorac. Cancer 2022, 13, 3384–3392. [Google Scholar] [CrossRef]
- Economou, N.T.; Ilias, I.; Velentza, L.; Papachatzakis, Y.; Zarogoulidis, P.; Kallianos, A.; Trakada, G. Sleepiness, fatigue, anxiety and depression in Chronic Obstructive Pulmonary Disease and Obstructive Sleep Apnea—Overlap—Syndrome, before and after continuous positive airways pressure therapy. PloS ONE 2018, 13, e0197342. [Google Scholar] [CrossRef]
- Kiss, I.; Kuhn, M.; Hrusak, K.; Buchler, B.; Boublikova, L.; Buchler, T. Insomnia in patients treated with checkpoint inhibitors for cancer: A meta-analysis. Front. Oncol. 2022, 12, 946307. [Google Scholar] [CrossRef]
- Tsara, V.; Serasli, E.; Amfilochiou, A.; Constantinidis, T.; Christaki, P. Greek version of the Epworth Sleepiness Scale. Sleep Breath. 2004, 8, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Kotronoulas, G.C.; Papadopoulou, C.N.; Papapetrou, A.; Patiraki, E. Psychometric evaluation and feasibility of the Greek Pittsburgh Sleep Quality Index (GR-PSQI) in patients with cancer receiving chemotherapy. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2011, 19, 1831–1840. [Google Scholar] [CrossRef]
- Katsarou, Z.; Bostantjopoulou, S.; Hatzizisi, O.; Giza, E.; Soler-Cardona, A.; Kyriazis, G. Immune factors or depression? Fatigue correlates in Parkinson’s disease. Rev. De Neurol. 2007, 45, 725–728. [Google Scholar]
- Williams, N. The MRC breathlessness scale. Occup. Med. 2017, 67, 496–497. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Steiropoulos, P.; Perantoni, E.; Archontogeorgis, K.; Eleftheriadou, E.; Porpodis, K.; Charpidou, A.G.; Angelopoulou, C.; Nena, E.; Zarogoulidis, K.; et al. Subjective sleep quality in lung cancer patients before and after chemotherapy. Thorac. Cancer 2013, 4, 138–142. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Kosmidis, C.; Kesisoglou, I.; Tsakiridis, K.; Hohenforst-Schmidt, W.; Huang, H.; Romanidis, K.; Vagionas, A.; Sapalidis, K. Nutrition and NSCLC; Should We Administer Food Supplements? Curr. Pharm. Des. 2021, 27, 3602–3608. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Darwiche, K.; Huang, H.; Spyratos, D.; Yarmus, L.; Li, Q.; Kakolyris, S.; Syrigos, K.; Zarogoulidis, K. Time recall; future concept of chronomodulating chemotherapy for cancer. Curr. Pharm. Biotechnol. 2013, 14, 632–642. [Google Scholar] [CrossRef]
- Seifen, C.; Huppertz, T.; Matthias, C.; Gouveris, H. Obstructive Sleep Apnea in Patients with Head and Neck Cancer-More than Just a Comorbidity? Medicina 2021, 57, 1174. [Google Scholar] [CrossRef]
- Kiss, I.; Kuhn, M.; Hrusak, K.; Buchler, T. Incidence of fatigue associated with immune checkpoint inhibitors in patients with cancer: A meta-analysis. ESMO Open 2022, 7, 100474. [Google Scholar] [CrossRef]
- Zuo, C.; Hong, Y.; Qiu, X.; Yang, D.; Liu, N.; Sheng, X.; Zhou, K.; Tang, B.; Xiong, S.; Ma, M.; et al. Celecoxib suppresses proliferation and metastasis of pancreatic cancer cells by down-regulating STAT3/NF-kB and L1CAM activities. Pancreatol. Off. J. Int. Assoc. Pancreatol. 2018, 18, 328–333. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, P.; Gao, W.; Zhou, Q.; Feng, T.; Tian, X. Circadian clock: A regulator of the immunity in cancer. Cell Commun. Signal. CCS 2021, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E.; Ganz, P.A.; Irwin, M.R.; Kwan, L.; Breen, E.C.; Cole, S.W. Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 3517–3522. [Google Scholar] [CrossRef]
- Mystakidou, K.; Parpa, E.; Tsilika, E.; Pathiaki, M.; Patiraki, E.; Galanos, A.; Vlahos, L. Sleep quality in advanced cancer patients. J. Psychosom. Res. 2007, 62, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Mystakidou, K.; Parpa, E.; Tsilika, E.; Pathiaki, M.; Gennatas, K.; Smyrniotis, V.; Vassiliou, I. The relationship of subjective sleep quality, pain, and quality of life in advanced cancer patients. Sleep 2007, 30, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Vena, C.; Parker, K.; Allen, R.; Bliwise, D.; Jain, S.; Kimble, L. Sleep-wake disturbances and quality of life in patients with advanced lung cancer. Oncol. Nurs. Forum 2006, 33, 761–769. [Google Scholar] [CrossRef] [PubMed]

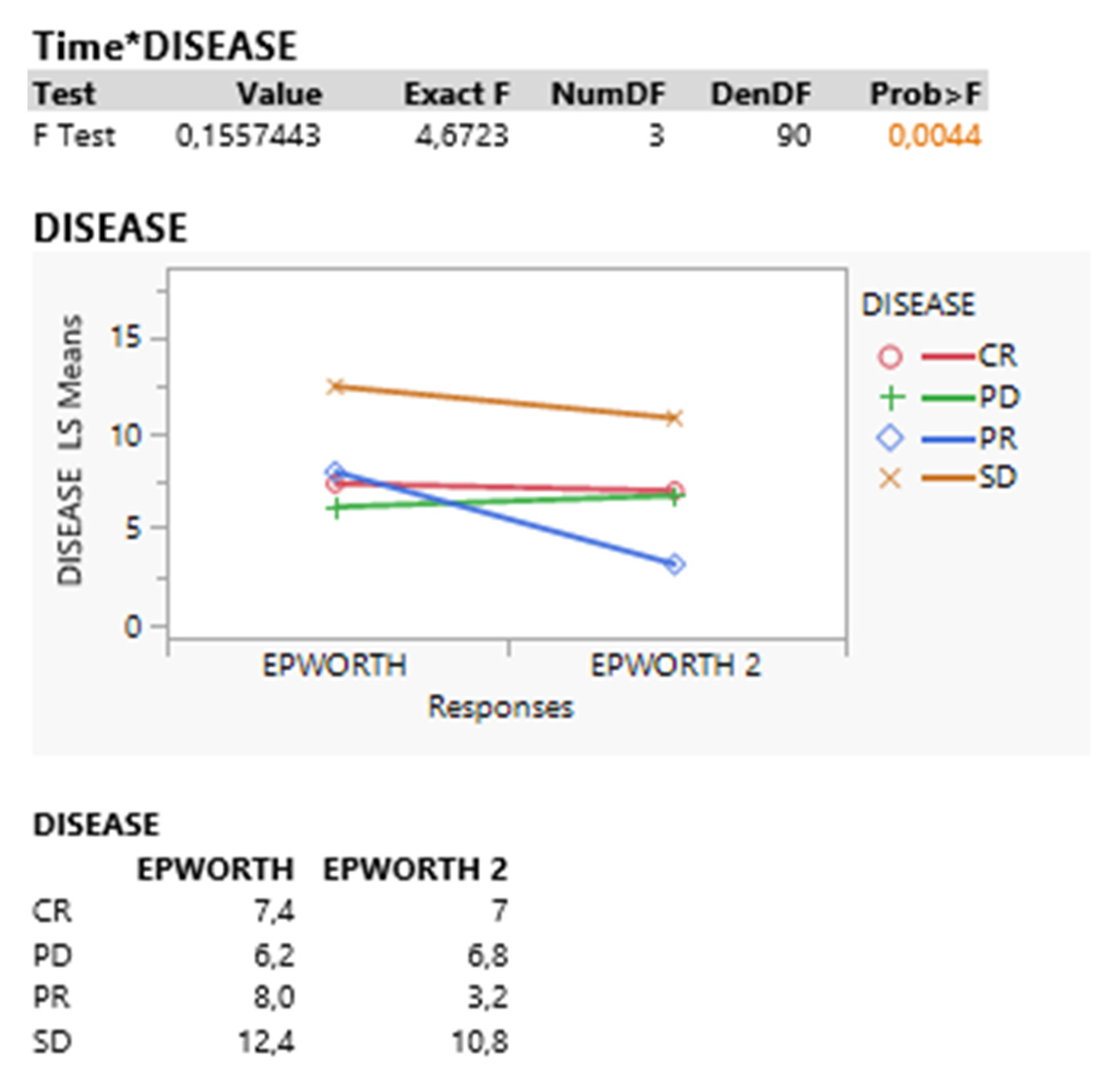
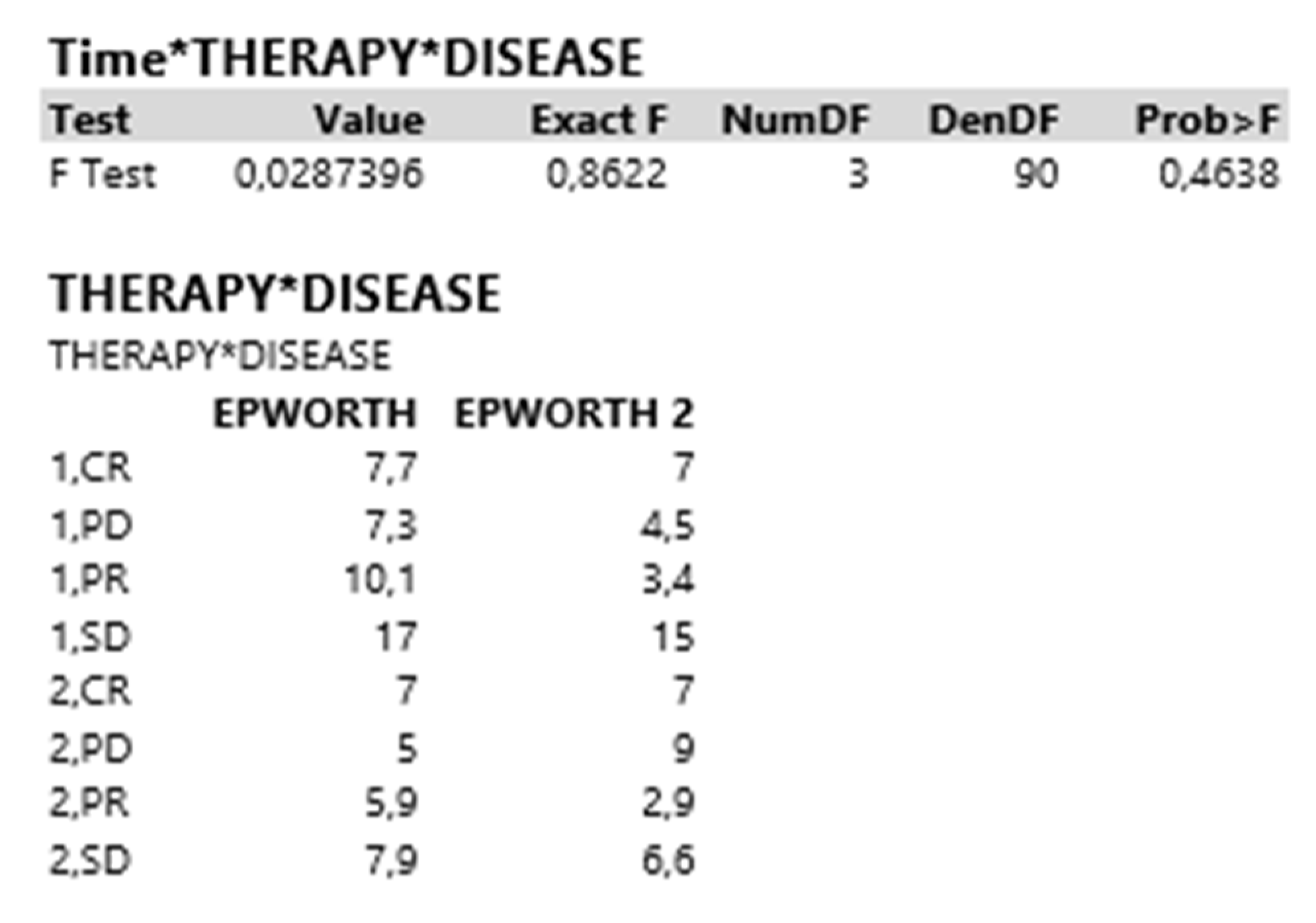
| Test | Value | Exact F | NumDF | DenDF | Prob > F |
| F Test | 0.1935683 | 2.4887 | 7 | 90 | 0.0220 |
| Intercept | |||||
| Test | Value | Exact F | NumDF | DenDF | Prob > F |
| F Test | 1.5755925 | 141.8033 | 1 | 90 | <0.0001 |
| EPWORTH | EPWORTH 2 | ||||
| 8.49117603 | 6.92638889 | ||||
| Test | Value | Exact F | NumDF | DenDF | Prob > F |
| F Test | 0.0447519 | 4.0277 | 1 | 90 | 0.0478 |
| Disease | |||||
| Test | Value | Exact F | NumDF | DenDF | Prob > F |
| F Test | 0.1260081 | 3.7802 | 3 | 90 | 0.0132 |
| Therapy*Disease | |||||
| Test | Value | Exact F | NumDF | DenDF | Prob > F |
| F Test | 0.0741014 | 2.2230 | 3 | 90 | 0.0909 |
| Test | Value | Exact F | NumDF | DenDF | Prob > F |
|---|---|---|---|---|---|
| F Test | 0.1867806 | 2.4015 | 7 | 90 | 0.0267 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarogoulidis, P.; Petridis, D.; Kosmidis, C.; Sapalidis, K.; Nena, L.; Matthaios, D.; Porpodis, K.; Kakavelas, P.; Steiropoulos, P. Immunotherapy and Chemotherapy Versus Sleep Disturbances for NSCLC Patients. Curr. Oncol. 2023, 30, 1999-2006. https://doi.org/10.3390/curroncol30020155
Zarogoulidis P, Petridis D, Kosmidis C, Sapalidis K, Nena L, Matthaios D, Porpodis K, Kakavelas P, Steiropoulos P. Immunotherapy and Chemotherapy Versus Sleep Disturbances for NSCLC Patients. Current Oncology. 2023; 30(2):1999-2006. https://doi.org/10.3390/curroncol30020155
Chicago/Turabian StyleZarogoulidis, Paul, Dimitrios Petridis, Christoforos Kosmidis, Konstantinos Sapalidis, Lila Nena, Dimitrios Matthaios, Konstantinos Porpodis, Paschalis Kakavelas, and Paschalis Steiropoulos. 2023. "Immunotherapy and Chemotherapy Versus Sleep Disturbances for NSCLC Patients" Current Oncology 30, no. 2: 1999-2006. https://doi.org/10.3390/curroncol30020155
APA StyleZarogoulidis, P., Petridis, D., Kosmidis, C., Sapalidis, K., Nena, L., Matthaios, D., Porpodis, K., Kakavelas, P., & Steiropoulos, P. (2023). Immunotherapy and Chemotherapy Versus Sleep Disturbances for NSCLC Patients. Current Oncology, 30(2), 1999-2006. https://doi.org/10.3390/curroncol30020155








