Update on the Management of Stage III NSCLC: Navigating a Complex and Heterogeneous Stage of Disease
Abstract
:1. Introduction
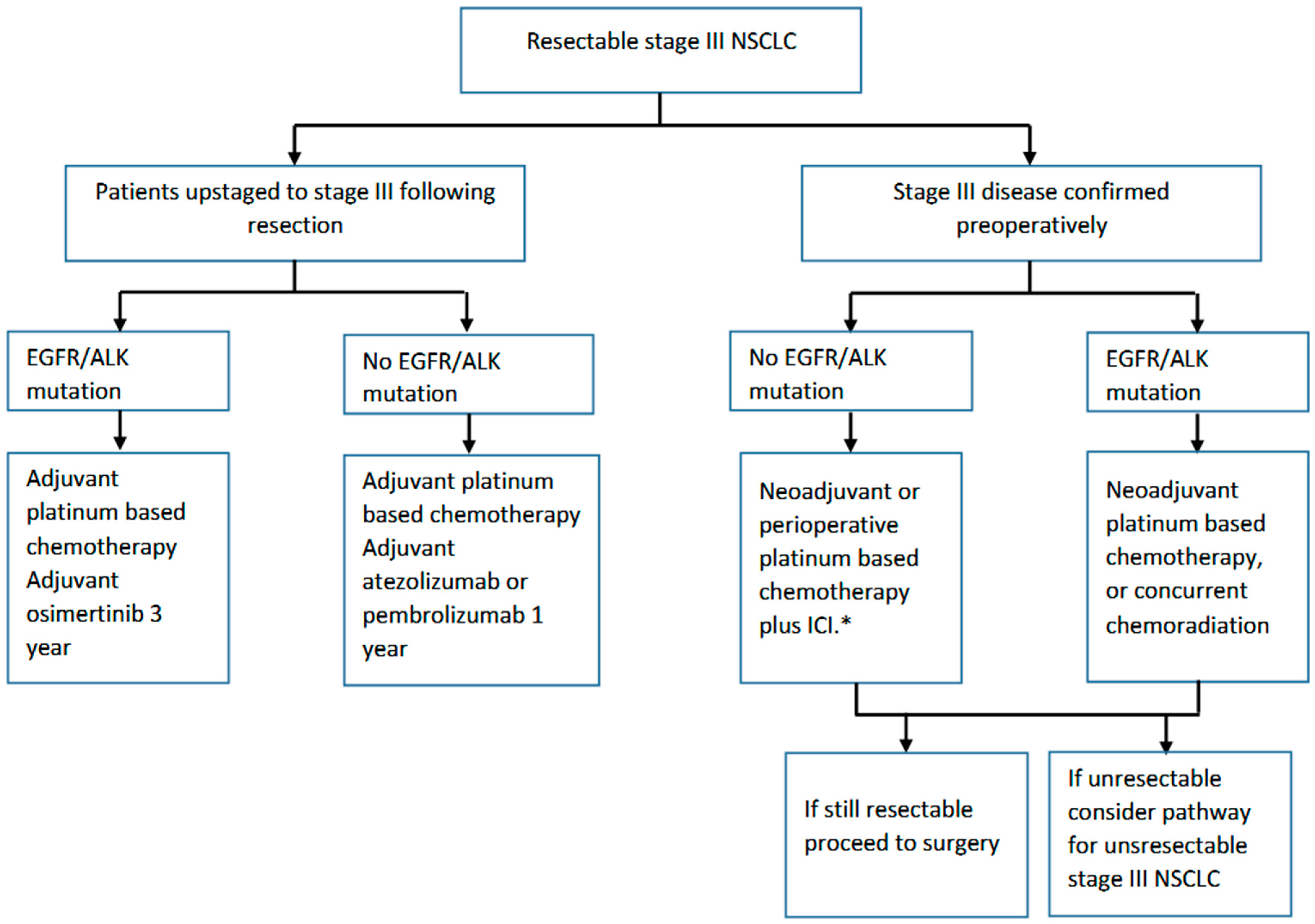
2. Resectable NSCLC
2.1. Adjuvant Systemic Therapy
2.2. Neoadjuvant Systemic Therapy
3. Unresectable NSCLC
3.1. Historical Approach
3.2. Recent Advances
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics: A 2020 Special Report on Lung Cancer; Canadian Cancer Statistics Advisory Committee: Toronto, ON, USA, 2020. [Google Scholar]
- Al-Shamsi, H.O.; Al Farsi, A.; Ellis, P.M. Stage III Non Small-Cell Lung Cancer: Establishing a Benchmark for the Proportion of Patients Suitable for Radical Treatment. Clin. Lung Cancer 2014, 15, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Albain, K.S.; Swann, R.S.; Rusch, V.W.; Turrisi, A.T., 3rd; Shepherd, F.A.; Smith, C.; Chen, Y.; Livingston, R.B.; Feins, R.H.; Gandara, D.R.; et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: A phase III randomised controlled trial. Lancet 2009, 374, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.M. The importance of multidisciplinary team management of patients with non-small-cell lung cancer. Curr. Oncol. 2012, 19 (Suppl. 1), S7–S15. [Google Scholar] [CrossRef] [PubMed]
- Kris, M.G.; Gaspar, L.E.; Chaft, J.E.; Kennedy, E.B.; Azzoli, C.G.; Ellis, P.M.; Lin, S.H.; Pass, H.I.; Seth, R.; Shepherd, F.A.; et al. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA Completely Resected Non–Small-Cell Lung Cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 2960–2974. [Google Scholar] [CrossRef] [PubMed]
- Pisters, K.; Kris, M.G.; Gaspar, L.E.; Ismaila, N. Therapy ftAS, Panel ARTfSItINGE. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I-IIIA Completely Resected Non–Small-Cell Lung Cancer: ASCO Guideline Rapid Recommendation Update. J. Clin. Oncol. 2022, 40, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.E.; Singh, N.; Ismaila, N.; Antonoff, M.B.; Arenberg, D.A.; Bradley, J.; David, E.; Detterbeck, F.; Früh, M.; Gubens, M.A.; et al. Management of Stage III Non–Small-Cell Lung Cancer: ASCO Guideline. J. Clin. Oncol. 2022, 40, 1356–1384. [Google Scholar] [CrossRef]
- Swaminath, A.; Vella, E.T.; Ramchandar, K.; Robinson, A.; Simone, C.; Sun, A.; Ung, Y.C.; Yasufuku, K.; Ellis, P.M. Surgery after Chemoradiotherapy in Patients with Stage III (n2 or N3, Excluding T4) Non-Small-Cell Lung Cancer: A Systematic Review. Curr. Oncol. 2019, 26, 398–404. [Google Scholar] [CrossRef]
- Le Chevalier, T. Adjuvant chemotherapy for resectable non-small-cell lung cancer: Where is it going? Ann. Oncol. 2010, 21, vii196–vii198. [Google Scholar] [CrossRef]
- Lim, E.; Baldwin, D.; Beckles, M.; Duffy, J.; Entwisle, J.; Faivre-Finn, C.; Kerr, K.; Macfie, A.; McGuigan, J.; Padley, S.; et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010, 65 (Suppl. 3), iii1–iii27. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Guidelines Version 3.2023: Non Small Cell Lung Cancer; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2023. [Google Scholar]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.-P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.-Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung Adjuvant Cisplatin Evaluation: A Pooled Analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, P.; Sivajohanathan, D.; Chan, A.; Kulkarni, S.; Ung, Y.; Ellis, P.M. Postoperative Adjuvant Systemic Therapy in Completely Resected Non–Small-Cell Lung Cancer: A Systematic Review. Clin. Lung Cancer 2017, 18, 259–273.e8. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.-Y.; Tribodet, H.; Aubert, D.; Shepherd, F.A.; Rosell, R.; Ding, K.; Veillard, A.-S.; Seymour, L.; Le Chevalier, T.; Spiro, S.; et al. Adjuvant Cisplatin and Vinorelbine for Completely Resected Non-small Cell Lung Cancer: Subgroup Analysis of the Lung Adjuvant Cisplatin Evaluation. J. Thorac. Oncol. 2010, 5, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Kenmotsu, H.; Yamamoto, N.; Yamanaka, T.; Yoshiya, K.; Takahashi, T.; Ueno, T.; Goto, K.; Daga, H.; Ikeda, N.; Sugio, K.; et al. Randomized Phase III Study of Pemetrexed Plus Cisplatin Versus Vinorelbine Plus Cisplatin for Completely Resected Stage II to IIIA Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 2187–2196. [Google Scholar] [CrossRef]
- Gadgeel, S.; Rodríguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Dómine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F.; et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Vicente, D.; Tafreshi, A.; Robinson, A.; Soto Parra, H.; Mazières, J.; Hermes, B.; Cicin, I.; Medgyasszay, B.; Rodríguez-Cid, J.; et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J. Thorac. Oncol. 2020, 15, 1657–1669. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez–Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non–Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Ciuleanu, T.-E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB—IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- O’Brien, M.; Paz-Ares, L.; Marreaud, S.; Dafni, U.; Oselin, K.; Havel, L.; Esteban, E.; Isla, D.; Martinez-Marti, A.; Faehling, M.; et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB—IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): An interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022, 23, 1274–1286. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Man, J.; Lord, S.; Links, M.; Gebski, V.; Mok, T.; Yang, J.C. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2017, 12, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Goss, G.D.; O’Callaghan, C.; Lorimer, I.; Tsao, M.S.; Masters, G.A.; Jett, J.; Edelman, M.J.; Lilenbaum, R.; Choy, H.; Khuri, F.; et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: Results of the NCIC CTG BR19 study. J. Clin. Oncol. 2013, 31, 3320–3326. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.Z.; Wang, Q.; Mao, W.M.; Xu, S.T.; Wu, L.; Wei, Y.C.; Liu, Y.Y.; Chen, C.; Cheng, Y.; Yin, R.; et al. Gefitinib Versus Vinorelbine Plus Cisplatin as Adjuvant Treatment for Stage II-IIIA (N1-N2) EGFR-Mutant NSCLC: Final Overall Survival Analysis of CTONG1104 Phase III Trial. J. Clin. Oncol. 2021, 39, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.; Altorki, N.K.; Eberhardt, W.E.E.; O’Brien, M.E.R.; Spigel, D.R.; Crinò, L.; Tsai, C.-M.; Kim, J.-H.; Cho, E.K.; Hoffman, P.C.; et al. Adjuvant Erlotinib Versus Placebo in Patients With Stage IB-IIIA Non–Small-Cell Lung Cancer (RADIANT): A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2015, 33, 4007–4014. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Mitsudomi, T.; Misumi, T.; Sugio, K.; Tsuboi, M.; Okamoto, I.; Iwamoto, Y.; Sakakura, N.; Sugawara, S.; Atagi, S.; et al. Randomized Phase III Study of Gefitinib Versus Cisplatin Plus Vinorelbine for Patients With Resected Stage II-IIIA Non–Small-Cell Lung Cancer With EGFR Mutation (IMPACT). J. Clin. Oncol. 2022, 40, 231–241. [Google Scholar] [CrossRef]
- Rosell, R.; Gomez-Codina, J.; Camps, C.; Maestre, J.; Padille, J.; Canto, A.; Mate, J.L.; Li, S.; Roig, J.; Olazabal, A.; et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer [see comments]. N. Engl. J. Med. 1994, 330, 153–158. [Google Scholar] [CrossRef]
- Roth, J.A.; Fossella, F.; Komaki, R.; Ryan, M.B.; Putnam, J.B.; Lee, J.S., Jr.; Dhingra, H.; De Caro, L.; Chasen, M.; McGavran, M.; et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. JNCI 1994, 86, 673–680. [Google Scholar] [CrossRef]
- Felip, E.; Rosell, R.; Maestre, J.A.; Rodríguez-Paniagua, J.M.; Morán, T.; Astudillo, J.; Alonso, G.; Borro, J.M.; González-Larriba, J.L.; Torres, A.; et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J. Clin. Oncol. 2010, 28, 3138–3145. [Google Scholar] [CrossRef]
- Eberhardt, W.E.; Pöttgen, C.; Gauler, T.C.; Friedel, G.; Veit, S.; Heinrich, V.; Welter, S.; Budach, W.; Spengler, W.; Kimmich, M.; et al. Phase III Study of Surgery Versus Definitive Concurrent Chemoradiotherapy Boost in Patients With Resectable Stage IIIA(N2) and Selected IIIB Non-Small-Cell Lung Cancer After Induction Chemotherapy and Concurrent Chemoradiotherapy (ESPATUE). J. Clin. Oncol. 2015, 33, 4194–4201. [Google Scholar] [CrossRef]
- Pless, M.; Stupp, R.; Ris, H.-B.; Stahel, R.A.; Weder, W.; Thierstein, S.; Gerard, M.-A.; Xyrafas, A.; Früh, M.; Cathomas, R.; et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: A phase 3 randomised trial. Lancet 2015, 386, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Taube, J.M.; Pardoll, D.M. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 2020, 367, eaax0182. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Othus, M.; Chen, Y.; Wright, G.P., Jr.; Yost, K.J.; Hyngstrom, J.R.; Hu-Lieskovan, S.; Lao, C.D.; Fecher, L.A.; Truong, T.G.; et al. Neoadjuvant-Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. N. Engl. J. Med. 2023, 388, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Rosner, S.; Liu, C.; Forde, P.M.; Hu, C. Association of Pathologic Complete Response and Long-Term Survival Outcomes Among Patients Treated With Neoadjuvant Chemotherapy or Chemoradiotherapy for NSCLC: A Meta-Analysis. JTO Clin. Res. Rep. 2022, 3, 100384. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Chaft, J.E.; William, W.N., Jr.; Rusch, V.; Pisters, K.M.; Kalhor, N.; Pataer, A.; Travis, W.D.; Swisher, S.G.; Kris, M.G. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: Proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014, 15, e42–e50. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Heymach, J.V.; Harpole, D.; Mitsudomi, T.; Taube, J.M.; Galffy, G.; Hochmair, M.; Winder, T.; Zukov, R.; Garbaos, G.; Gao, S.; et al. Abstract CT005: AEGEAN: A phase 3 trial of neoadjuvant durvalumab + chemotherapy followed by adjuvant durvalumab in patients with resectable NSCLC. Cancer Res. 2023, 83 (Suppl. 8), CT005. [Google Scholar] [CrossRef]
- Wakelee, H.; Liberman, M.; Kato, T.; Tsuboi, M.; Lee, S.-H.; Gao, S.; Chen, K.-N.; Dooms, C.; Majem, M.; Eigendorff, E.; et al. Perioperative Pembrolizumab for Early-Stage Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 491–503. [Google Scholar] [CrossRef]
- Provencio, M.; Nadal, E.; González-Larriba, J.L.; Martínez-Martí, A.; Bernabé, R.; Bosch-Barrera, J.; Casal-Rubio, J.; Calvo, V.; Insa, A.; Ponce, S.; et al. Perioperative Nivolumab and Chemotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 504–513. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, W.; Zhang, P.; Wang, W.; Fang, W.; Xing, W.; Chen, Q.; Mei, J.; Yang, L.; Tan, L.; et al. Perioperative toripalimab + platinum-doublet chemotherapy vs chemotherapy in resectable stage II/III non-small cell lung cancer (NSCLC): Interim event-free survival (EFS) analysis of the phase III Neotorch study. J. Clin. Oncol. 2023, 41 (Suppl. 36), 425126. [Google Scholar]
- Cascone, T.; Provencio, M.; Sepesi, B.; Lu, S.; Aanur, N.; Li, S.; Spicer, J. Checkmate 77T: A phase III trial of neoadjuvant nivolumab (NIVO) plus chemotherapy (chemo) followed by adjuvant nivo in resectable early-stage NSCLC. J. Clin. Oncol. 2020, 38 (Suppl. 15), TPS9076. [Google Scholar] [CrossRef]
- Peters, S.; Kim, A.W.; Solomon, B.; Gandara, D.R.; Dziadziuszko, R.; Brunelli, A.; Garassino, M.C.; Reck, M.; Wang, L.; To, I.; et al. IMpower030: Phase III study evaluating neoadjuvant treatment of resectable stage II-IIIB non-small cell lung cancer (NSCLC) with atezolizumab (atezo) + chemotherapy. Ann Oncol. 2019, 30, ii30. [Google Scholar] [CrossRef]
- Tsuboi, M.; Weder, W.; Escriu, C.; Blakely, C.; He, J.; Dacic, S.; Yatabe, Y.; Zeng, L.; Walding, A.; Chaft, J.E. Neoadjuvant osimertinib with/without chemotherapy versus chemotherapy alone for EGFR-mutated resectable non-small-cell lung cancer: NeoADAURA. Future Oncol. 2021, 17, 4045–4055. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.J.; Ohtani, S.; Im, Y.H.; Lee, E.S.; Yokota, I.; Kuroi, K.; Im, S.A.; Park, B.W.; Kim, S.B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2018, 380, 617–628. [Google Scholar] [CrossRef]
- Dillman, R.O.; Seagren, S.L.; Propert, K.J.; Guerra, J.; Eaton, W.L.; Perry, M.C.; Carey, R.W.; Frei, E.F., III; Green, M.R. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N. Engl. J. Med. 1990, 323, 940–945. [Google Scholar] [CrossRef]
- Sause, W.T.; Scott, C.; Taylor, S.; Johnson, D.; Livingston, R.; Komaki, R.; Emami, B.; Curran, W.J.; Byhardt, R.W.; Turrisi, A.T.; et al. Radiation Therapy Oncology Group (RTOG) 88-08 and Eastern Cooperative Oncology Group (ECOG) 4588: Preliminary results of a phase III trial in regionally advanced, unresectable non-small-cell lung cancer. JNCI 1995, 87, 198–205. [Google Scholar] [CrossRef]
- Curran, W.J., Jr.; Paulus, R.; Langer, C.J.; Komaki, R.; Lee, J.S.; Hauser, S.; Movsas, B.; Wasserman, T.; Rosenthal, S.A.; Gore, E.; et al. Sequential vs Concurrent Chemoradiation for Stage III Non–Small Cell Lung Cancer: Randomized Phase III Trial RTOG 9410. JNCI 2011, 103, 1452–1460. [Google Scholar] [CrossRef]
- Furuse, K.; Fukuoka, M.; Kawahara, M.; Nishikawa, H.; Takada, Y.; Kudoh, S.; Katagami, N.; Ariyoshi, Y. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J. Clin. Oncol. 1999, 17, 2692–2699. [Google Scholar] [CrossRef]
- Aupérin, A.; Péchoux, C.L.; Rolland, E.; Curran, W.J.; Furuse, K.; Fournel, P.; Belderbos, J.; Clamon, G.; Ulutin, H.C.; Paulus, R.; et al. Meta-Analysis of Concomitant Versus Sequential Radiochemotherapy in Locally Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2010, 28, 2181–2190. [Google Scholar] [CrossRef]
- Albain, K.S.; Crowley, J.J.; Turrisi, A.T., 3rd; Gandara, D.R.; Farrar, W.B.; Clark, J.I.; Beasley, K.R.; Livingston, R.B. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: A Southwest Oncology Group phase II study, SWOG 9019. J. Clin. Oncol. 2002, 20, 3454–3460. [Google Scholar] [CrossRef]
- Belani, C.P.; Choy, H.; Bonomi, P.; Scott, C.; Travis, P.; Haluschak, J.; Curran, W.J., Jr. Combined Chemoradiotherapy Regimens of Paclitaxel and Carboplatin for Locally Advanced Non–Small-Cell Lung Cancer: A Randomized Phase II Locally Advanced Multi-Modality Protocol. J. Clin. Oncol. 2005, 23, 5883–5891. [Google Scholar] [CrossRef]
- Santana-Davila, R.; Devisetty, K.; Szabo, A.; Sparapani, R.; Arce-Lara, C.; Gore, E.M.; Moran, A.; Williams, C.D.; Kelley, M.J.; Whittle, J. Cisplatin and etoposide versus carboplatin and paclitaxel with concurrent radiotherapy for stage III non-small-cell lung cancer: An analysis of Veterans Health Administration data. J. Clin. Oncol. 2015, 33, 567–574. [Google Scholar] [CrossRef]
- Senan, S.; Brade, A.; Wang, L.-h.; Vansteenkiste, J.; Dakhil, S.; Biesma, B.; Aguillo, M.M.; Aerts, J.; Govindan, R.; Rubio-Viqueira, B.; et al. PROCLAIM: Randomized Phase III Trial of Pemetrexed-Cisplatin or Etoposide-Cisplatin Plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 953–962. [Google Scholar] [CrossRef]
- Hanna, N.; Neubauer, M.; Yiannoutsos, C.; McGarry, R.; Arseneau, J.; Ansari, R.; Reynolds, C.; Govindan, R.; Melnyk, A.; Fisher, W.; et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: The Hoosier Oncology Group and U.S. Oncology. J. Clin. Oncol. 2008, 26, 5755–5760. [Google Scholar] [CrossRef]
- Kelly, K.; Chansky, K.; Gaspar, L.E.; Albain, K.S.; Jett, J.; Ung, Y.C.; Lau, D.H.; Crowley, J.J.; Gandara, D.R. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J. Clin. Oncol. 2008, 26, 2450–2456. [Google Scholar] [CrossRef]
- Perez, C.A.; Stanley, K.; Rubin, P.; Kramer, S.; Brady, L.; Perez-Tamayo, R.; Brown, G.S.; Concannon, J.; Rotman, M.; Seydel, H.G. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung. Preliminary report by the Radiation Therapy Oncology Group. Cancer 1980, 45, 2744–2753. [Google Scholar] [CrossRef]
- Mauguen, A.; Le Péchoux, C.; Saunders, M.I.; Schild, S.E.; Turrisi, A.T.; Baumann, M.; Sause, W.T.; Ball, D.; Belani, C.P.; Bonner, J.A.; et al. Hyperfractionated or accelerated radiotherapy in lung cancer: An individual patient data meta-analysis. J. Clin. Oncol. 2012, 30, 2788–2797. [Google Scholar] [CrossRef]
- Saunders, M.; Dische, S.; Barrett, A.; Harvey, A.; Gibson, D.; Parmar, M. Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small-cell lung cancer: A randomised multicentre trial. Lancet 1997, 350, 161–165. [Google Scholar] [CrossRef]
- Bradley, J.D.; Bae, K.; Graham, M.V.; Byhardt, R.; Govindan, R.; Fowler, J.; Purdy, J.A.; Michalski, J.M.; Gore, E.; Choy, H. Primary analysis of the phase II component of a phase I/II dose intensification study using three-dimensional conformal radiation therapy and concurrent chemotherapy for patients with inoperable non-small-cell lung cancer: RTOG 0117. J. Clin. Oncol. 2010, 28, 2475–2480. [Google Scholar] [CrossRef]
- Bradley, J.D.; Paulus, R.; Komaki, R.; Masters, G.; Blumenschein, G.; Schild, S.; Bogart, J.; Hu, C.; Forster, K.; Magliocco, A.; et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015, 16, 187–199. [Google Scholar]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef]
- Garassino, M.C.; Mazieres, J.; Reck, M.; Chouaid, C.; Bischoff, H.; Reinmuth, N.; Cove-Smith, L.; Mansy, T.; Cortinovis, D.; Migliorino, M.R.; et al. Durvalumab After Sequential Chemoradiotherapy in Stage III, Unresectable NSCLC: The Phase 2 PACIFIC-6 Trial. J. Thorac. Oncol. 2022, 17, 1415–1427. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Spira, A.; Raben, D.; Planchard, D.; Cho, B.C.; Özgüroğlu, M.; Daniel, D.; Villegas, A.; Vicente, D.; Hui, R.; et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann Oncol. 2020, 31, 798–806. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, M.; Jiang, O.; Pan, Y.; Hu, D.; Lin, Q.; Wu, G.; Cui, J.; Chang, J.; Cheng, Y.; et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): Interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2022, 23, 209–219. [Google Scholar]
- Peters, S.; Felip, E.; Dafni, U.; Belka, C.; Guckenberger, M.; Irigoyen, A.; Nadal, E.; Becker, A.; Vees, H.; Pless, M.; et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer—The ETOP NICOLAS trial. Lung Cancer 2019, 133, 83–87. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, L.; Kalhor, N.; Carter, B.W.; Altan, M.; Blumenschein, G.; Byers, L.A.; Fossella, F.; Gibbons, D.L.; Kurie, J.M.; et al. Final efficacy outcomes of atezolizumab with chemoradiation for unresectable NSCLC: The phase II DETERRED trial. Lung Cancer 2022, 174, 112–117. [Google Scholar] [CrossRef]
- Jabbour, S.K.; Lee, K.H.; Frost, N.; Breder, V.; Kowalski, D.M.; Pollock, T.; Levchenko, E.; Reguart, N.; Martinez-Marti, A.; Houghton, B.; et al. Pembrolizumab Plus Concurrent Chemoradiation Therapy in Patients With Unresectable, Locally Advanced, Stage III Non–Small Cell Lung Cancer: The Phase 2 KEYNOTE-799 Nonrandomized Trial. JAMA Oncol. 2021, 7, 1351–1359. [Google Scholar] [CrossRef]
- Durm, G.A.; Mamdani, H.; Althouse, S.K.; Jabbour, S.K.; Ganti, A.K.; Jalal, S.I.; Chesney, J.A.; Naidoo, J.; Hrinczenko, B.; Fidler, M.J.J.; et al. Consolidation nivolumab plus ipilimumab or nivolumab alone following concurrent chemoradiation for patients with unresectable stage III non-small cell lung cancer: BTCRC LUN 16-081. J. Clin. Oncol. 2022, 40 (Suppl. 16), 8509. [Google Scholar] [CrossRef]
- Herbst, R.S.; Majem, M.; Barlesi, F.; Carcereny, E.; Chu, Q.; Monnet, I.; Sanchez-Hernandez, A.; Dakhil, S.; Camidge, D.R.; Winzer, L.; et al. COAST: An Open-Label, Phase II, Multidrug Platform Study of Durvalumab Alone or in Combination With Oleclumab or Monalizumab in Patients With Unresectable, Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 3383–3393. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Mann, H.; Gopinathan, A.; Newton, M.D.; Aggarwal, C. Phase 3 study of durvalumab combined with oleclumab or monalizumab in patients with unresectable stage III NSCLC (PACIFIC-9). J. Clin. Oncol. 2023, 41 (Suppl. 16), TPS8610. [Google Scholar]
- Cortiula, F.; Reymen, B.; Peters, S.; Van Mol, P.; Wauters, E.; Vansteenkiste, J.; De Ruysscher, D.; Hendriks, L.E.L. Immunotherapy in unresectable stage III non-small-cell lung cancer: State of the art and novel therapeutic approaches. Ann Oncol. 2022, 33, 893–908. [Google Scholar] [CrossRef]

| Patient | Treatment | Control | Time on Tx | DFS | OS | Approval Indications | |
|---|---|---|---|---|---|---|---|
| IMpower010 [22] | IB (>4 cm)-IIIA (7th ed.) Any PD-L1 status | Adjuvant chemotherapy (mandatory) Adjuvant atezolizumab × 1 year | Adjuvant chemotherapy | 1 year | 3 y 60% vs. 48% HR 0.66, 95% CI 0.50–0.88 | Stage II-IIIA PD-L1+ | |
| PEARLS/KEYNOTE 091 [23] | IB (>4 cm)-IIIA (7th ed.) Any PD-L1 status | Pembrolizumab × 18 cycles +/− chemotherapy | Placebo | 1 year | 53.6 m vs. 42 m HR 0.76 (0.63–0.91) | NR | Receipt of at least 1 cycle of adjuvant chemotherapy |
| ADAURA [24] | IB-IIIA EGFR (exon 19 del, exon 21 L858R) | Osimertinib +/− chemotherapy | Placebo | 3 years | HR 0.23 | HR 0.49 (0.33–0.73) 85% vs. 73% | Stage IB (tumours > 3 cm) Within 10 weeks of surgery (no chemo) or 26 weeks if adjuvant chemotherapy. |
| Trial | Stage (AJCC 7th ed.) | Treatment | Time on Tx | Comparator | Adjuvant | Time on Tx | EFS | PCR | OS | Surgery | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | C | T | C | T | C | T | C | |||||||
| CHECKMATE 816 [39] | IB (≥4 cm)–IIIA | Nivolumab + Platinum doublet × 3 cycles | 9 weeks | Platinum doublet × 3 cycles | Platinum doublet × 4 cycles | No | 31.6 m HR 0.63 (0.43–0.91) | 20.8 m | 24% | 2.2% | HR 0.53 (0.30–1.07) | 15.6% | 20% | |
| AEGEAN [40] | II-IIIB (N2) | Durvalumab + Platinum doublet × 4 cycles | 12 weeks | Platinum doublet × 4 cycles | Durvalumab × 12 cycles | 1 year | NR vs. 63.3% HR 0.68 (0.53–0.88) | 25.9 m 52.4% (2 years) | 17.3% | 4.3% | NR | NR | 20% | 20% |
| KEYNOTE 671 [41] | II-IIIB (N2) | Pembrolizumab + Cisplatin doublet × 4 cycles | 12 weeks | Cisplatin doublet × 4 cycles | Pembrolizumab × 13 cycles | 1 year | NR 62.4% HR 0.58 (0.46–0.72) | 17 m 40.6% (2 years) | 18.1% | 4% | HR 0.73 (0.54–0.99) | 13.6 | 16% | |
| NADIM2 [42] | IIIA-IIIB | Nivolumab + Carbo + pacli × 3 cycles | 9 weeks | Carbo + pacli | Nivolumab 6 months | 6 months | 85% HR 0.47 (0.25–0.88) | 63.6% | 37% | 7% | HR 0.43 (0.19–0.98) | 93% | 69% | |
| NEOTORCH [43] | II-III | Toripalimab + platinum doublet | 9 weeks | Platinum doublet | Toripalimab 13 cycles | I year | 64.7% HR 0.40 (0.277–0.565) | 38.7% | 48.5% | 8.4% | HR 0.62 (0.38–0.999) | 82% | 73% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sathiyapalan, A.; Baloush, Z.; Ellis, P.M. Update on the Management of Stage III NSCLC: Navigating a Complex and Heterogeneous Stage of Disease. Curr. Oncol. 2023, 30, 9514-9529. https://doi.org/10.3390/curroncol30110689
Sathiyapalan A, Baloush Z, Ellis PM. Update on the Management of Stage III NSCLC: Navigating a Complex and Heterogeneous Stage of Disease. Current Oncology. 2023; 30(11):9514-9529. https://doi.org/10.3390/curroncol30110689
Chicago/Turabian StyleSathiyapalan, Arani, Ziad Baloush, and Peter M. Ellis. 2023. "Update on the Management of Stage III NSCLC: Navigating a Complex and Heterogeneous Stage of Disease" Current Oncology 30, no. 11: 9514-9529. https://doi.org/10.3390/curroncol30110689
APA StyleSathiyapalan, A., Baloush, Z., & Ellis, P. M. (2023). Update on the Management of Stage III NSCLC: Navigating a Complex and Heterogeneous Stage of Disease. Current Oncology, 30(11), 9514-9529. https://doi.org/10.3390/curroncol30110689




