Re-Evaluating Chemotherapy Dosing Strategies for Ovarian Cancer: Impact of Sarcopenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Statistical Analysis
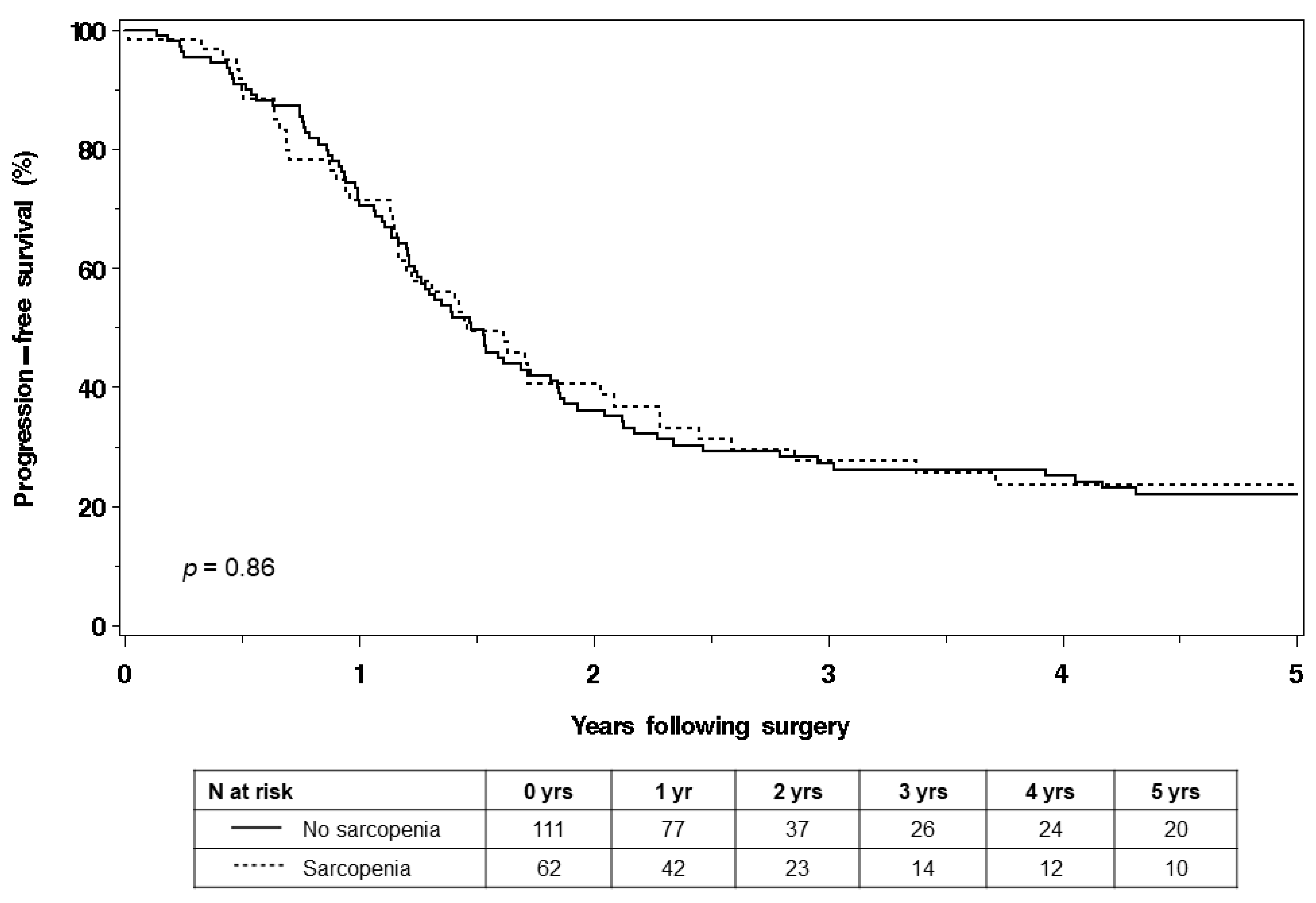
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.M.; Jordan, S.J. Epidemiology of epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- von Haehling, S.; Morley, J.E.; Anker, S.D. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J. Cachexia Sarcopenia Muscle 2010, 1, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Rangel, E.L.; Rios-Diaz, A.J.; Uyeda, J.W.; Castillo-Angeles, M.; Cooper, Z.; Olufajo, O.A.; Salim, A.; Sodickson, A.D. Sarcopenia increases risk of long-term mortality in elderly patients undergoing emergency abdominal surgery. J. Trauma Acute Care Surg. 2017, 83, 1179–1186. [Google Scholar] [CrossRef]
- Joglekar, S.; Nau, P.N.; Mezhir, J.J. The impact of sarcopenia on survival and complications in surgical oncology: A review of the current literature. J. Surg. Oncol. 2015, 112, 503–509. [Google Scholar] [CrossRef]
- Caan, B.J.; Cespedes Feliciano, E.M.; Prado, C.M.; Alexeeff, S.; Kroenke, C.H.; Bradshaw, P.; Quesenberry, C.P.; Weltzien, E.K.; Castillo, A.L.; Olobatuyi, T.A.; et al. Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol. 2018, 4, 798–804. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, Y.S.; Park, I.; Ahn, H.K.; Cho, E.K.; Jeong, Y.M. Prognostic Significance of CT-Determined Sarcopenia in Patients with Small-Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 1795–1799. [Google Scholar] [CrossRef]
- Fukushima, H.; Yokoyama, M.; Nakanishi, Y.; Tobisu, K.; Koga, F. Sarcopenia as a prognostic biomarker of advanced urothelial carcinoma. PLoS ONE 2015, 10, e0115895. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, Y.S.; Kim, E.Y.; Jin, W. Prognostic significance of CT-determined sarcopenia in patients with advanced gastric cancer. PLoS ONE 2018, 13, e0202700. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Takano, M.; Miyamoto, M.; Yajima, I.; Shimizu, Y.; Aizawa, Y.; Suguchi, Y.; Moriiwa, M.; Aoyama, T.; Soyama, H.; et al. Psoas muscle volume as a predictor of peripheral neurotoxicity induced by primary chemotherapy in ovarian cancers. Cancer Chemother. Pharmacol. 2017, 80, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Rutten, I.J.; van Dijk, D.P.; Kruitwagen, R.F.; Beets-Tan, R.G.; Olde Damink, S.W.; van Gorp, T. Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J. Cachexia Sarcopenia Muscle 2016, 7, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Bronger, H.; Hederich, P.; Hapfelmeier, A.; Metz, S.; Noel, P.B.; Kiechle, M.; Schmalfeldt, B. Sarcopenia in Advanced Serous Ovarian Cancer. Int. J. Gynecol. Cancer 2017, 27, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Rutten, I.J.; Ubachs, J.; Kruitwagen, R.F.; van Dijk, D.P.; Beets-Tan, R.G.; Massuger, L.F.; Olde Damink, S.W.; Van Gorp, T. The influence of sarcopenia on survival and surgical complications in ovarian cancer patients undergoing primary debulking surgery. Eur. J. Surg. Oncol. 2017, 43, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, M.; Ratain, M.J. Body surface area as a determinant of pharmacokinetics and drug dosing. Investig. New Drugs 2001, 19, 171–177. [Google Scholar] [CrossRef]
- Ekhart, C.; Rodenhuis, S.; Schellens, J.H.; Beijnen, J.H.; Huitema, A.D. Carboplatin dosing in overweight and obese patients with normal renal function, does weight matter? Cancer Chemother. Pharmacol. 2009, 64, 115–122. [Google Scholar] [CrossRef]
- Aletti, G.D.; Dowdy, S.C.; Podratz, K.C.; Cliby, W.A. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am. J. Obstet. Gynecol. 2007, 197, 676.e1–676.e7. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Dale, D.C.; Crawford, J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: A nationwide study of community practices. J. Clin. Oncol. 2003, 21, 4524–4531. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Dale, D.C.; Friedberg, J.; Crawford, J.; Fisher, R.I. Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin’s lymphoma: A nationwide study. J. Clin. Oncol. 2004, 22, 4302–4311. [Google Scholar] [CrossRef]
- Narasimhulu, D.M.; McGree, M.E.; Weaver, A.L.; Jatoi, A.; LeBrasseur, N.K.; Glaser, G.E.; Langstraat, C.L.; Block, M.S.; Kumar, A. Frailty is a determinant of suboptimal chemotherapy in women with advanced ovarian cancer. Gynecol. Oncol. 2020, 158, 646–652. [Google Scholar] [CrossRef] [PubMed]
- van Warmerdam, L.J.; Rodenhuis, S.; ten Bokkel Huinink, W.W.; Maes, R.A.; Beijnen, J.H. The use of the Calvert formula to determine the optimal carboplatin dosage. J. Cancer Res. Clin. Oncol. 1995, 121, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Ubachs, J.; Ziemons, J.; Minis-Rutten, I.J.G.; Kruitwagen, R.; Kleijnen, J.; Lambrechts, S.; Olde Damink, S.W.M.; Rensen, S.S.; Van Gorp, T. Sarcopenia and ovarian cancer survival: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 1165–1174. [Google Scholar] [CrossRef]
- Tan, B.H.; Brammer, K.; Randhawa, N.; Welch, N.T.; Parsons, S.L.; James, E.J.; Catton, J.A. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur. J. Surg. Oncol. 2015, 41, 333–338. [Google Scholar] [CrossRef]
- Palmela, C.; Velho, S.; Agostinho, L.; Branco, F.; Santos, M.; Santos, M.P.; Oliveira, M.H.; Strecht, J.; Maio, R.; Cravo, M.; et al. Body Composition as a Prognostic Factor of Neoadjuvant Chemotherapy Toxicity and Outcome in Patients with Locally Advanced Gastric Cancer. J. Gastric. Cancer 2017, 17, 74–87. [Google Scholar] [CrossRef]
- Shachar, S.S.; Deal, A.M.; Weinberg, M.; Nyrop, K.A.; Williams, G.R.; Nishijima, T.F.; Benbow, J.M.; Muss, H.B. Skeletal Muscle Measures as Predictors of Toxicity, Hospitalization, and Survival in Patients with Metastatic Breast Cancer Receiving Taxane-Based Chemotherapy. Clin. Cancer Res. 2017, 23, 658–665. [Google Scholar] [CrossRef]
- Blauwhoff-Buskermolen, S.; Versteeg, K.S.; de van der Schueren, M.A.; den Braver, N.R.; Berkhof, J.; Langius, J.A.; Verheul, H.M. Loss of Muscle Mass During Chemotherapy Is Predictive for Poor Survival of Patients With Metastatic Colorectal Cancer. J. Clin. Oncol. 2016, 34, 1339–1344. [Google Scholar] [CrossRef]
- Rollins, K.E.; Tewari, N.; Ackner, A.; Awwad, A.; Madhusudan, S.; Macdonald, I.A.; Fearon, K.C.; Lobo, D.N. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin. Nutr. 2016, 35, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.; Goh, V.; Davies, A.; Gossage, J.; Mitchell-Hay, R.; Hynes, O.; Maisey, N.; Ross, P.; Gaya, A.; Landau, D.B.; et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur. Radiol. 2014, 24, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Baracos, V.E.; McCargar, L.J.; Reiman, T.; Mourtzakis, M.; Tonkin, K.; Mackey, J.R.; Koski, S.; Pituskin, E.; Sawyer, M.B. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin. Cancer Res. 2009, 15, 2920–2926. [Google Scholar] [CrossRef]
- Prado, C.M.; Baracos, V.E.; McCargar, L.J.; Mourtzakis, M.; Mulder, K.E.; Reiman, T.; Butts, C.A.; Scarfe, A.G.; Sawyer, M.B. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin. Cancer Res. 2007, 13, 3264–3268. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Baracos, V.E.; Xiao, J.; Birdsell, L.; Stuyckens, K.; Park, Y.C.; Parekh, T.; Sawyer, M.B. The association between body composition and toxicities from the combination of Doxil and trabectedin in patients with advanced relapsed ovarian cancer. Appl. Physiol. Nutr. Metab. 2014, 39, 693–698. [Google Scholar] [CrossRef]
- Daly, L.E.; Prado, C.M.; Ryan, A.M. A window beneath the skin: How computed tomography assessment of body composition can assist in the identification of hidden wasting conditions in oncology that profoundly impact outcomes. Proc. Nutr. Soc. 2018, 77, 135–151. [Google Scholar] [CrossRef]
- Mir, O.; Coriat, R.; Blanchet, B.; Durand, J.P.; Boudou-Rouquette, P.; Michels, J.; Ropert, S.; Vidal, M.; Pol, S.; Chaussade, S.; et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS ONE 2012, 7, e37563. [Google Scholar] [CrossRef]
- Massicotte, M.H.; Borget, I.; Broutin, S.; Baracos, V.E.; Leboulleux, S.; Baudin, E.; Paci, A.; Deroussent, A.; Schlumberger, M.; Antoun, S. Body composition variation and impact of low skeletal muscle mass in patients with advanced medullary thyroid carcinoma treated with vandetanib: Results from a placebo-controlled study. J. Clin. Endocrinol. Metab. 2013, 98, 2401–2408. [Google Scholar] [CrossRef]
- Antoun, S.; Lanoy, E.; Albiges-Sauvin, L.; Escudier, B. Clinical implications of body composition assessment by computed tomography in metastatic renal cell carcinoma. Expert. Rev. Anticancer Ther. 2014, 14, 279–288. [Google Scholar] [CrossRef]
- Canetta, R.; Rozencweig, M.; Carter, S.K. Carboplatin: The clinical spectrum to date. Cancer Treat. Rev. 1985, 12 (Suppl. A), 125–136. [Google Scholar] [CrossRef]
- Hanna, R.K.; Poniewierski, M.S.; Laskey, R.A.; Lopez, M.A.; Shafer, A.; Van Le, L.; Crawford, J.; Dale, D.C.; Gehrig, P.A.; Secord, A.A.; et al. Predictors of reduced relative dose intensity and its relationship to mortality in women receiving multi-agent chemotherapy for epithelial ovarian cancer. Gynecol. Oncol. 2013, 129, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Repetto, L.; Pace, M.; Mammoliti, S.; Bruzzone, M.; Chiara, S.; Oliva, C.; Guido, T.; Conte, P.F.; Campora, E.; Rubagotti, A.; et al. The impact of received dose intensity on the outcome of advanced ovarian cancer. Eur. J. Cancer 1993, 29, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Fauci, J.M.; Whitworth, J.M.; Schneider, K.E.; Subramaniam, A.; Zhang, B.; Frederick, P.J.; Kilgore, L.C.; Straughn, J.M., Jr. Prognostic significance of the relative dose intensity of chemotherapy in primary treatment of epithelial ovarian cancer. Gynecol. Oncol. 2011, 122, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Levolger, S.; van Vugt, J.L.; de Bruin, R.W.; JN, I.J. Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br. J. Surg. 2015, 102, 1448–1458. [Google Scholar] [CrossRef]
- Kumar, A.; Moynagh, M.R.; Multinu, F.; Cliby, W.A.; McGree, M.E.; Weaver, A.L.; Young, P.M.; Bakkum-Gamez, J.N.; Langstraat, C.L.; Dowdy, S.C.; et al. Muscle composition measured by CT scan is a measurable predictor of overall survival in advanced ovarian cancer. Gynecol. Oncol. 2016, 142, 311–316. [Google Scholar] [CrossRef]
- Polen-De, C.; Fadadu, P.; Weaver, A.L.; Moynagh, M.; Takahashi, N.; Jatoi, A.; LeBrasseur, N.K.; McGree, M.; Cliby, W.; Kumar, A. Quality is more important than quantity: Pre-operative sarcopenia is associated with poor survival in advanced ovarian cancer. Int. J. Gynecol. Cancer 2022, 32, 1289–1296. [Google Scholar] [CrossRef]

| Characteristic | Total n = 173 | No Sarcopenia n = 111 (64.2%) | Sarcopenia n = 62 (35.8%) | p † |
|---|---|---|---|---|
| Age at chemotherapy (years), mean (SD) | 63.6 (10.5) | 61.4 (11.0) | 67.5 (8.2) | <0.01 |
| BMI at chemotherapy (kg/m2), mean (SD) | 26.2 (5.8) | 28.0 (5.8) | 23.1 (4.0) | <0.01 |
| Skeletal muscle area (cm2), mean (SD) | 108.3 (18.7) | 117.5 (15.4) | 91.6 (10.9) | <0.01 |
| Skeletal muscle index (cm2/m2), mean (SD) | 40.9 (7.3) | 44.8 (5.7) | 33.8 (3.5) | <0.01 |
| Skeletal muscle density (HU), mean (SD) | 33.8 (9.9) | 33.7 (10.3) | 34.1 (9.2) | 0.78 |
| ASA score, n (%) | 0.64 | |||
| <3 | 102 (59.0) | 64 (57.7) | 38 (61.3) | |
| ≥3 | 71 (41.0) | 47 (42.3) | 24 (38.7) | |
| Preoperative albumin (g/dL), n (%) | 0.40 | |||
| ≥3.5 | 133 (76.9) | 82 (73.9) | 51 (82.3) | |
| <3.5 | 21 (12.1) | 16 (14.4) | 5 (8.1) | |
| Not available | 19 (11.0) | 13 (11.7) | 6 (9.7) | |
| FIGO grade, n (%) | 0.16 | |||
| 1–2 | 9 (5.2) | 8 (7.2) | 1 (1.6) | |
| 3 | 164 (94.8) | 103 (92.8) | 61 (98.4) | |
| FIGO stage, n (%) | 0.32 | |||
| IIIC | 132 (76.3) | 82 (73.9) | 50 (80.6) | |
| IV | 41 (23.7) | 29 (26.1) | 12 (19.4) | |
| Histology, n (%) | 0.99 | |||
| Non-serous | 13 (7.5) | 8 (7.2) | 5 (8.1) | |
| Serous | 160 (92.5) | 103 (92.8) | 57 (91.9) | |
| Surgical complexity, n (%) | <0.01 | |||
| Low | 21 (12.1) | 14 (12.6) | 7 (11.3) | |
| Intermediate | 77 (44.5) | 61 (55.0) | 16 (25.8) | |
| High | 75 (43.4) | 36 (32.4) | 39 (62.9) | |
| Residual disease, n (%) | 0.55 | |||
| Microscopic | 98 (56.6) | 65 (58.6) | 33 (53.2) | |
| Measurable (≤1 cm) | 64 (37.0) | 38 (34.2) | 26 (41.9) | |
| Suboptimal (>1 cm) | 11 (6.4) | 8 (7.2) | 3 (4.8) |
| Characteristic | Total n = 116 | No Sarcopenia n = 78 | Sarcopenia n = 38 | p † |
|---|---|---|---|---|
| Carboplatin RDI (%), mean (SD) | 86.5 (21.0) | 89.4 (18.8) | 80.4 (24.1) | 0.03 |
| Paclitaxel RDI (%), mean (SD) | 100.1 (27.9) | 104.1 (28.8) | 91.9 (24.4) | 0.03 |
| Carboplatin RDI ≥ 85%, n (%) | 70 (60.3) | 50 (64.1) | 20 (52.6) | 0.24 |
| Paclitaxel RDI ≥ 85%, n (%) | 93 (80.2) | 65 (83.3) | 28 (73.7) | 0.22 |
| Characteristic | Total n = 173 | No Sarcopenia n = 111 | Sarcopenia n = 62 | p † |
|---|---|---|---|---|
| Febrile neutropenia, n (%) | 0.99 | |||
| No | 166 (96.0) | 106 (95.5) | 60 (96.8) | |
| Yes | 7 (4.0) | 5 (4.5) | 2 (3.2) | |
| Severe neutropenia (grade 3–5), n (%) | 0.27 | |||
| No | 88 (50.9) | 53 (47.7) | 35 (56.5) | |
| Yes | 85 (49.1) | 58 (52.3) | 27 (43.5) | |
| Dose delay, n (%) | 0.35 | |||
| No | 92 (53.2) | 62 (55.9) | 30 (48.4) | |
| Yes | 81 (46.8) | 49 (44.1) | 32 (51.6) | |
| Dose reduction, n (%) | 0.85 | |||
| No | 46 (26.6) | 29 (26.1) | 17 (27.4) | |
| Yes | 127 (73.4) | 82 (73.9) | 45 (72.6) | |
| Use of Neupogen or Neulasta during treatment, n (%) | 0.31 | |||
| No | 126 (72.8) | 78 (70.3) | 48 (77.4) | |
| Yes | 47 (27.2) | 33 (29.7) | 14 (22.6) | |
| Completed at least six cycles, n (%) | 0.02 | |||
| No | 15 (8.7) | 5 (4.5) | 10 (16.1) | |
| Yes | 158 (91.3) | 106 (95.5) | 52 (83.9) |
| Characteristic | Carboplatin RDI < 85%, No Sarcopenia n = 28 | Carboplatin RDI < 85% and Sarcopenia n = 18 | Carboplatin RDI ≥ 85%, No Sarcopenia n = 50 | Carboplatin RDI ≥ 85%, Sarcopenia n = 20 | p † |
|---|---|---|---|---|---|
| Age (years), mean (SD) | 63.5 (11.0) | 67.4 (10.4) | 60.8 (10.6) | 68.4 (7.8) | 0.01 |
| BMI (kg/m2), mean (SD) | 30.9 (6.9) | 23.6 (4.7) | 26.7 (4.6) | 22.4 (2.8) | <0.01 |
| ASA score, n (%) | 0.16 | ||||
| <3 | 16 (57.1) | 10 (55.6) | 30 (60.0) | 17 (85.0) | |
| ≥3 | 12 (42.9) | 8 (44.4) | 20 (40.0) | 3 (15.0) | |
| Preoperative albumin (g/dL), n (%) | 0.68 | ||||
| ≥3.5 | 20 (71.4) | 16 (88.9) | 38 (76.0) | 17 (85.0) | |
| <3.5 | 6 (21.4) | 1 (5.6) | 6 (12.0) | 1 (5.0) | |
| Not available | 2 (7.1) | 1 (5.6) | 6 (12.0) | 2 (10.0) | |
| Surgical complexity, n (%) | 0.02 | ||||
| Low | 3 (10.7) | 2 (11.1) | 7 (14.0) | 2 (10.0) | |
| Intermediate | 18 (64.3) | 5 (27.8) | 28 (56.0) | 5 (25.0) | |
| High | 7 (25.0) | 11 (61.1) | 15 (30.0) | 13 (65.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, R.; Polen-De, C.; McGree, M.; Fought, A.; Kumar, A. Re-Evaluating Chemotherapy Dosing Strategies for Ovarian Cancer: Impact of Sarcopenia. Curr. Oncol. 2023, 30, 9501-9513. https://doi.org/10.3390/curroncol30110688
Shah R, Polen-De C, McGree M, Fought A, Kumar A. Re-Evaluating Chemotherapy Dosing Strategies for Ovarian Cancer: Impact of Sarcopenia. Current Oncology. 2023; 30(11):9501-9513. https://doi.org/10.3390/curroncol30110688
Chicago/Turabian StyleShah, Rushi, Clarissa Polen-De, Michaela McGree, Angela Fought, and Amanika Kumar. 2023. "Re-Evaluating Chemotherapy Dosing Strategies for Ovarian Cancer: Impact of Sarcopenia" Current Oncology 30, no. 11: 9501-9513. https://doi.org/10.3390/curroncol30110688
APA StyleShah, R., Polen-De, C., McGree, M., Fought, A., & Kumar, A. (2023). Re-Evaluating Chemotherapy Dosing Strategies for Ovarian Cancer: Impact of Sarcopenia. Current Oncology, 30(11), 9501-9513. https://doi.org/10.3390/curroncol30110688






