A Planned Multidisciplinary Surgical Approach to Treat Primary Pelvic Malignancies
Abstract
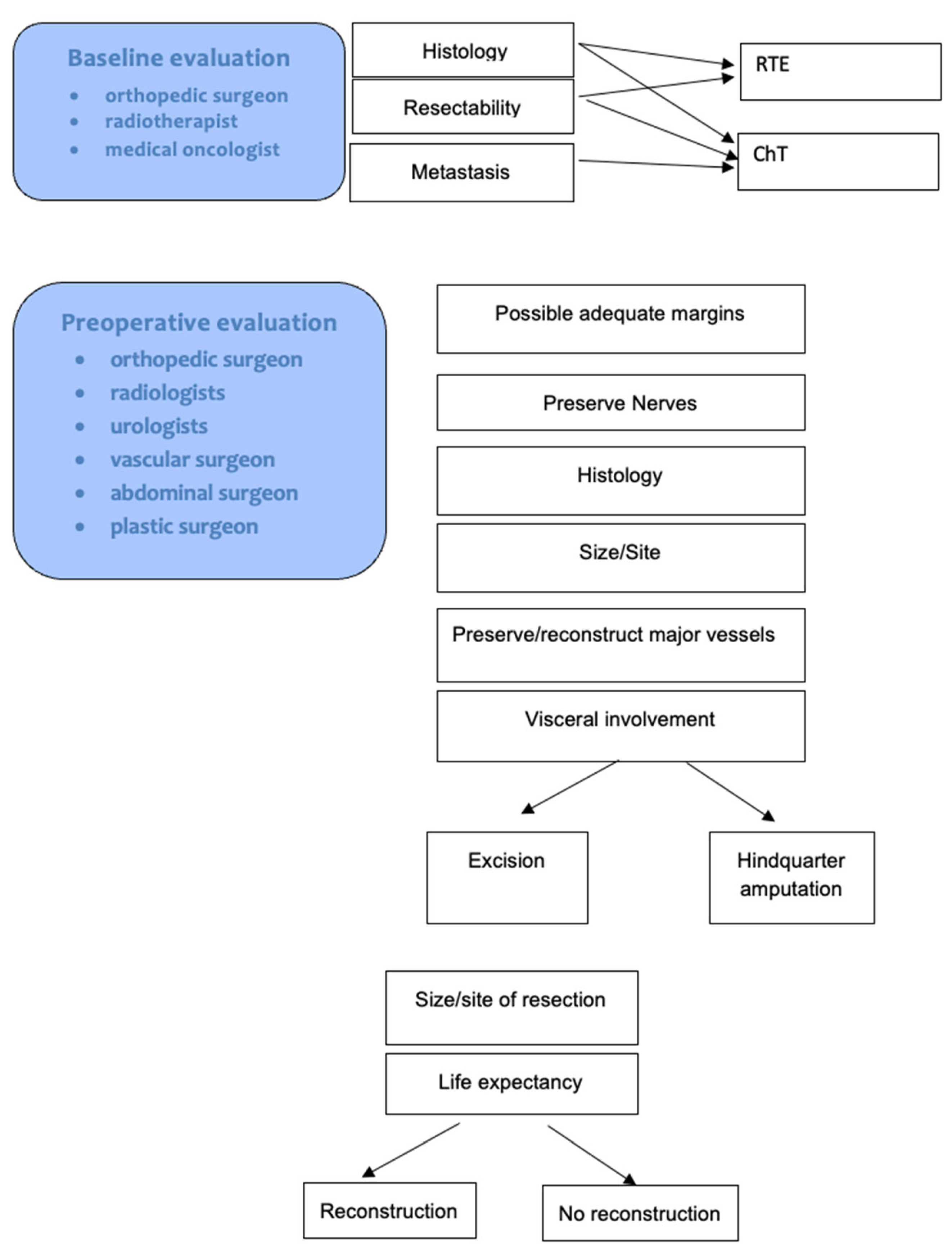
1. Introduction
2. Materials and Methods
3. Results
| Patient | Chemotherapy | Radiotherapy | Surgery | Additional Surgery | Multidisciplinarity | Margins | Resection | Surgery Time (Min) | Transfusion Rate (Blood Units) | Postop Length of Stay (Days) | Reconstruction |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | Adjuvant | No | HA | Nephrectomy Abdominopelvic amputation | Urologist Abdominal surgeon Vascular surgeon | Wide | P1 + P2 + P3 | 298 | 7 | 21 | No |
| #2 | Adjuvant | No | HA | Nephrectomy | Urologist Vascular surgeon | Marginal | P1 + P2 + P3 | 203 | 3 | 15 | No |
| #3 | No | Neoadjuvant | HA | Bladder reconstruction | Urologist | Wide | P1 + P2 + P3 | 192 | 2 | 17 | No |
| #4 | No | Neoadjuvant | excision | Ureter reconstruction Vascular bypass | Urologist Vascular surgeon | Wide | ST excision | 155 | 3 | 22 | No |
| #5 | No | No | excision | Nephrectomy Abdominopelvic amputation | Urologist Abdominal surgeon Vascular surgeon | Wide | P1 | 321 | 6 | 19 | Custom made prosthesis |
| #6 | No | No | excision | Vascular bypass | Vascular surgeon | Marginal | P1 + P2 | 237 | 3 | 16 | Custom made prosthesis |
| #7 | No | No | HA | Abdminopelvic amputation Free flap | Urologist Vascular surgeon Plastic surgeon | Wide | P1 + P2 + P3 | 470 | 7 | 28 | No |
| #8 | No | No | HA | Nephrectomy | Urologist Vascular surgeon | Marginal | P3 | 281 | 4 | 18 | No |
| #9 | Neo + adjuvant | No | HA | Cava vein trombectomy | Neurosurgery Urologist Abdominal surgeon Vascular surgeon | Wide | P1 + P2 + P3 + P4 | 465 | 16 | 39 | No |
| #10 | Neo + adjuvant | No | excision | Bladder reconstruction | Urologist Vascular surgeon | Wide | P1 | 220 | 5 | 18 | Custom made prosthesis |
| #11 | No | Neoadjuvant | excision | Vascular bypass | Vascular surgeon | Wide | ST excision | 164 | 2 | 15 | No |
| #12 | No | No | excision | Urologist Vascular surgeon | Wide | P2 + P3 | 178 | 4 | 13 | Custom made prosthesis | |
| #13 | Neo + adjuvant | No | excision | Bladder reconstruction | Urologist Abdominal surgeon Vascular surgeon | Marginal | P1 | 267 | 4 | 23 | Custom made prosthesis |
| #14 | No | No | HA | Free flap Nephrectomy | Urologist Abdominal surgeon Vascular surgeon Plastic surgeon | Wide | P1 + P2 + P3 | 521 | 5 | 31 | No |
| #15 | Neo + adjuvant | No | excision | Bladder reconstruction | Neurosurgery Urologist Abdominal surgeon Vascular surgeon | Wide | P1 + P4 | 240 | 6 | 33 | Custom made prosthesis |
| #16 | No | Neoadjuvant | excision | Abdominopelvic amputation Free flap | Urologist Abdominal surgeon Vascular surgeon Plastic surgeon | Wide | ST excision | 558 | 4 | 63 | No |
| #17 | Neo + adjuvant | No | excision | Vascular bypass | Vascular surgeon | Marginal | P3 | 190 | 2 | 24 | No |
| Patient | Local Recurrence | Follow Up (Months) | Status | Complications |
|---|---|---|---|---|
| #1 | Yes | 61 | DOD | Wound dehiscence |
| #2 | Yes | 59 | DOD | DVT |
| #3 | No | 43 | NED | Wound dehiscence |
| #4 | Yes | 36 | NED | Seroma DVT |
| #5 | No | 21 | NED | Prosthesis infection |
| #6 | No | 49 | DOD | Bypass occlusion |
| #7 | No | 55 | NED | Wound dehiscence |
| #8 | No | 18 | NED | Wound dehiscence DVT |
| #9 | No | 13 | AWD | |
| #10 | Yes | 25 | DOD | |
| #11 | No | 29 | NED | Wound dehiscence |
| #12 | No | 15 | NED | Seroma |
| #13 | No | 14 | AWD | Bladder fistula Prosthesis infection |
| #14 | Yes | 17 | NED | Wound dehiscence |
| #15 | No | 19 | NED | |
| #16 | No | 45 | DOD | |
| #17 | No | 33 | NED | Bypass occlusion |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keyzer-Dekker, C.M.; Houtkamp, R.G.; Peterse, J.L.; Van Coevorden, F. Adult pelvic sarcomas: A heterogeneous collection of sarcomas? Sarcoma 2004, 8, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Parry, M.C.; Laitinen, M.; Albergo, J.; Jeys, L.; Carter, S.; Gaston, C.L.; Sumathi, V.; Grimer, R.J. Osteosarcoma of the pelvis. Bone Joint J. 2016, 98, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Mullen, J.T.; van Houdt, W. Soft tissue tumors of the pelvis: Technical and histological considerations. J. Surg. Oncol. 2018, 117, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Smolle, M.A.; Szkandera, J.; Andreou, D.; Palmerini, E.; Bergovec, M.; Leithner, A. Treatment options in unresectable soft tissue and bone sarcoma of the extremities and pelvis—A systematic literature review. EFORT Open Rev. 2020, 5, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Abdelfatah, E.; Guzzetta, A.A.; Nagarajan, N.; Wolfgang, C.L.; Pawlik, T.M.; Choti, M.A.; Schulick, R.; Montgomery, E.A.; Meyer, C.; Thornton, K.; et al. Long-term outcomes in treatment of retroperitoneal sarcomas: A 15 year single-institution evaluation of prognostic features. J. Surg. Oncol. 2016, 114, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sarkar, D.; Prasad, S.; Gupta, V.; Ghosala, P.; Kaman, L.; Yadav, T.D.; Ganesamoni, R.; Singh, S.K. Large pelvic masses of obscure origin: Urologist’s perspective. Urol. Int. 2012, 88, 215–224. [Google Scholar] [CrossRef]
- Ferrari, S.; Palmerini, E.; Fabbri, N.; Staals, E.; Ferrari, C.; Alberghini, M.; Picci, P. Osteosarcoma of the pelvis: A monoinstitutional experience in patients younger than 41 years. Tumori J. 2012, 98, 702–708. [Google Scholar] [CrossRef]
- Laitinen, M.; Parry, M.; Albergo, J.I.; Jeys, L.; Sumathi, V.; Grimer, R. Outcome of Pelvic Bone Sarcomas in Children. J. Pediatr. Orthop. 2018, 38, 537–542. [Google Scholar] [CrossRef]
- Jawad, M.U.; Haleem, A.A.; Scully, S.P. Malignant sarcoma of the pelvic bones: Treatment outcomes and prognostic factors vary by histopathology. Cancer 2011, 117, 1529–1541. [Google Scholar] [CrossRef]
- Bacalbasa, N.; Balescu, I. Pelvic exenteration--reconsidering the procedure. J. Med. Life 2015, 8, 146–149. [Google Scholar]
- Wagner, J.R.; Russo, P. Urologic complications of major pelvic surgery. Semin. Surg. Oncol. 2000, 18, 216–228. [Google Scholar] [CrossRef]
- Smit, N.; Lawonn, K.; Kraima, A.; DeRuiter, M.; Sokooti, H.; Bruckner, S.; Eisemann, E.; Vilanova, A. PelVis: Atlas-based Surgical Planning for Oncological Pelvic Surgery. IEEE Trans. Vis. Comput. Graph. 2017, 23, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, R.; Beppu, Y.; Tobisu Ki, K.; Moriya, Y.; Uchiyama, K.; Kito, M.; Umeda, T.; Hasegawa, T.; Shimoda, T. A multidisciplinary approach to the treatment of malignant pelvic bone tumors: Results with eight consecutive patients. J. Orthop. Sci. 2000, 5, 449–456. [Google Scholar] [CrossRef]
- Sagebiel, T.L.; Faria, S.C.; Balachandran, A.; Butler, C.E.; Garvey, P.B.; Bhosale, P.R. Pelvic reconstruction with pedicled thigh flaps: Indications, surgical techniques, and postoperative imaging. AJR Am. J. Roentgenol. 2014, 202, 593–601. [Google Scholar] [CrossRef]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef] [PubMed]
- Marina, N.; Bielack, S.; Whelan, J.; Smeland, S.; Krailo, M.; Sydes, M.R.; Butterfass-Bahloul, T.; Calaminus, G.; Bernstein, M. International collaboration is feasible in trials for rare conditions: The EURAMOS experience. Cancer Treat Res. 2009, 152, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Whelan, J.S.; Bielack, S.S.; Marina, N.; Smeland, S.; Jovic, G.; Hook, J.M.; Krailo, M.; Anninga, J.; Butterfass-Bahloul, T.; Böhling, T.; et al. EURAMOS-1, an international randomised study for osteosarcoma: Results from pre-randomisation treatment. Ann. Oncol. 2015, 26, 407–414. [Google Scholar] [CrossRef]
- Sambri, A.; Caldari, E.; Montanari, A.; Fiore, M.; Cevolani, L.; Ponti, F.; D’Agostino, V.; Bianchi, G.; Miceli, M.; Spinnato, P.; et al. Vascular Proximity Increases the Risk of Local Recurrence in Soft-Tissue Sarcomas of the Thigh-A Retrospective MRI Study. Cancers 2021, 13, 6325. [Google Scholar] [CrossRef]
- Fujiwara, T.; Medellin, M.R.; Sambri, A.; Tsuda, Y.; Balko, J.; Sumathi, V.; Gregory, J.; Jeys, L.; Abudu, A. Preoperative surgical risk stratification in osteosarcoma based on the proximity to the major vessels. Bone Joint J. 2019, 101, 1024–1031. [Google Scholar] [CrossRef]
- Enneking, W.F.; Dunham, W.K. Resection and reconstruction for primary neoplasms involving the innominate bone. J. Bone Joint Surg. Am. 1978, 60, 731–746. [Google Scholar] [CrossRef]
- De Paolis, M.; Sambri, A.; Zucchini, R.; Frisoni, T.; Spazzoli, B.; Taddei, F.; Donati, D.M. Custom-made 3D-Printed Prosthesis in Periacetabular Resections Through a Novel Ileo-adductor Approach. Orthopedics 2022, 45, e110–e114. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Medellin Rincon, M.R.; Sambri, A.; Tsuda, Y.; Clark, R.; Stevenson, J.; Parry, M.C.; Grimer, R.J.; Jeys, L. Limb-salvage reconstruction following resection of pelvic bone sarcomas involving the acetabulum. Bone Joint J. 2021, 103, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Enneking, W.F.; Spanier, S.S.; Goodman, M.A. The Classic: A system for the surgical staging of musculoskeletal sarcoma. Clin. Orthop. Relat. Res. 2003, 415, 4–18. [Google Scholar] [CrossRef]
- Picci, P.; Bacci, G.; Campanacci, M.; Gasparini, M.; Pilotti, S.; Cerasoli, S.; Bertoni, F.; Guerra, A.; Capanna, R.; Albisinni, U. Histologic evaluation of necrosis in osteosarcoma induced by chemotherapy. Regional mapping of viable and nonviable tumor. Cancer 1985, 56, 1515–1521. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Angelini, A.; Piazza, M.; Pagliarini, E.; Trovarelli, G.; Spertino, A.; Ruggieri, P. The Orthopedic-Vascular Multidisciplinary Approach Improves Patient Safety in Surgery for Musculoskeletal Tumors: A Large-Volume Center Experience. J. Pers. Med. 2021, 11, 462. [Google Scholar] [CrossRef]
- Houdek, M.T.; Wellings, E.P.; Moran, S.L.; Bakri, K.; Dozois, E.J.; Mathis, K.L.; Yaszemski, M.J.; Sim, F.H.; Rose, P.S. Outcome of Sacropelvic Resection and Reconstruction Based on a Novel Classification System. J. Bone Joint Surg. Am. 2020, 102, 1956–1965. [Google Scholar] [CrossRef]
- Brown, K.G.; Solomon, M.J.; Latif, E.R.; Koh, C.E.; Vasilaras, A.; Eisinger, D.; Sved, P. Urological complications after cystectomy as part of pelvic exenteration are higher than that after cystectomy for primary bladder malignancy. J. Surg. Oncol. 2017, 115, 307–311. [Google Scholar] [CrossRef]
- Bothwell, W.N.; Bleicher, R.J.; Dent, T.L. Prophylactic ureteral catheterization in colon surgery. A five-year review. Dis. Colon. Rectum. 1994, 37, 330–334. [Google Scholar] [CrossRef]
- Kuno, K.; Menzin, A.; Kauder, H.H.; Sison, C.; Gal, D. Prophylactic ureteral catheterization in gynecologic surgery. Urology 1998, 52, 1004–1008. [Google Scholar] [CrossRef]
- Selzman, A.A.; Spirnak, J.P. Iatrogenic ureteral injuries: A 20-year experience in treating 165 injuries. J. Urol. 1996, 155, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Manzur, M.F.; Ham, S.W.; Elsayed, R.; Abdoli, S.; Simcox, T.; Han, S.; Rowe, V.; Weaver, F.A. Vascular surgery: An essential hospital resource in modern health care. J. Vasc. Surg. 2017, 65, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Mogannam, A.C.; Chavez de Paz, C.; Sheng, N.; Patel, S.; Bianchi, C.; Chiriano, J.; Teruya, T.; Abou-Zamzam, A.M. Early vascular consultation in the setting of oncologic resections: Benefit for patients and a continuing source of open vascular surgical training. Ann. Vasc. Surg. 2015, 29, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Awad, N.; Lackman, R.; McMackin, K.; Kim, T.W.; Lombardi, J.; Caputo, F. Multidisciplinary Approach to Treatment of Soft Tissue Sarcomas Requiring Complex Oncologic Resections. Ann. Vasc. Surg. 2018, 53, 212–216. [Google Scholar] [CrossRef]
- McGoldrick, N.P.; Butler, J.S.; Lavelle, M.; Sheehan, S.; Dudeney, S.; O’Toole, G.C. Resection and reconstruction of pelvic and extremity soft tissue sarcomas with major vascular involvement: Current concepts. World J. Orthop. 2016, 7, 293–300. [Google Scholar] [CrossRef]
- Jurado, M.; Chiva, L.; Tinelli, G.; Alcazar, J.L.; Chi, D.S. The role of oncovascular surgery in gynecologic oncology surgery. Int. J. Gynecol. Cancer 2022, 32, 553–559. [Google Scholar] [CrossRef]
- Stiles, Z.E.; Lohman, R.F.; Mann, G.N. Plastic Surgery Reconstruction of Sarcoma Resection Defects: Form and Function. Surg. Clin. North Am. 2022, 102, 583–599. [Google Scholar] [CrossRef]
- Rust, D.J.; Kato, T.; Yoon, S.S. Treatment for local control of retroperitoneal and pelvis sarcomas: A review of the literature. Surg. Oncol. 2022, 43, 101814. [Google Scholar] [CrossRef]
- Gronchi, A.; Strauss, D.C.; Miceli, R.; Bonvalot, S.; Swallow, C.J.; Hohenberger, P.; Van Coevorden, F.; Rutkowski, P.; Callegaro, D.; Hayes, A.J.; et al. Variability in Patterns of Recurrence After Resection of Primary Retroperitoneal Sarcoma (RPS): A Report on 1007 Patients From the Multi-institutional Collaborative RPS Working Group. Ann. Surg. 2016, 263, 1002–1009. [Google Scholar] [CrossRef]
- Tan, M.C.; Brennan, M.F.; Kuk, D.; Agaram, N.P.; Antonescu, C.R.; Qin, L.X.; Moraco, N.; Crago, A.M.; Singer, S. Histology-based Classification Predicts Pattern of Recurrence and Improves Risk Stratification in Primary Retroperitoneal Sarcoma. Ann. Surg. 2016, 263, 593–600. [Google Scholar] [CrossRef]
- Bonvalot, S.; Rivoire, M.; Castaing, M.; Stoeckle, E.; Le Cesne, A.; Blay, J.Y.; Laplanche, A. Primary retroperitoneal sarcomas: A multivariate analysis of surgical factors associated with local control. J. Clin. Oncol. 2009, 27, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.; Park, S.Z.; Donohue, J.H.; Nagorney, D.M.; Kay, P.A.; Nasciemento, A.G.; Schleck, C.D.; Ilstrup, D.M. Operative management of primary retroperitoneal sarcomas: A reappraisal of an institutional experience. Ann. Surg. 2004, 239, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Bonvalot, S.; Miceli, R.; Berselli, M.; Causeret, S.; Colombo, C.; Mariani, L.; Bouzaiene, H.; Le Péchoux, C.; Casali, P.G.; Le Cesne, A.; et al. Aggressive surgery in retroperitoneal soft tissue sarcoma carried out at high-volume centers is safe and is associated with improved local control. Ann. Surg. Oncol. 2010, 17, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Donati, D.; Giacomini, S.; Gozzi, E.; Ferrari, S.; Sangiorgi, L.; Tienghi, A.; DeGroot, H.; Bertoni, F.; Bacchini, P.; Bacci, G.; et al. Osteosarcoma of the pelvis. Eur. J. Surg. Oncol. 2004, 30, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Ahn, S.; Min, S.K. Oncovascular Surgery: Essential Roles of Vascular Surgeons in Cancer Surgery. Vasc. Spec. Int. 2019, 35, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, M.M.; Carrère, S.; Delmond, L.; Mehta, S.; Rouanet, P.; Canaud, L.; Alric, P.; Quénet, F. Oncovascular compartmental resection for retroperitoneal soft tissue sarcoma with vascular involvement. J. Vasc. Surg. 2016, 64, 1033–1041. [Google Scholar] [CrossRef]
- Leithead, C.C.; Matthews, T.C.; Pearce, B.J.; Novak, Z.; Patterson, M.; Passman, M.A.; Jordan, W.D. Analysis of emergency vascular surgery consults within a tertiary health care system. J. Vasc. Surg. 2016, 63, 177–181. [Google Scholar] [CrossRef]
- Angelini, A.; Tiengo, C.; Sonda, R.; Berizzi, A.; Bassetto, F.; Ruggieri, P. One-Stage Soft Tissue Reconstruction Following Sarcoma Excision: A Personalized Multidisciplinary Approach Called Orthoplasty. J. Pers. Med. 2020, 10, 278. [Google Scholar] [CrossRef]
| Patient | Sex | Age (Years) | Histology | Location | Metastasis at Diagnosis |
|---|---|---|---|---|---|
| #1 | F | 61 | Myxoid Grade 2 CS | Ilium | Lungs |
| #2 | M | 74 | Grade 2 CS | Ilium + acetabulum | Lungs |
| #3 | M | 66 | Dedifferentiated LS | Iliac fossa | No |
| #4 | M | 66 | Pleomorphic sarcoma | Iliac fossa | No |
| #5 | M | 58 | Grade 3 CS | Ilium + acetabulum | No |
| #6 | M | 63 | Dedifferentiated CS | Ilium + acetabulum | No |
| #7 | M | 47 | Grade 2 CS | Ilium | No |
| #8 | M | 63 | Grade 3 CS | Pubis | No |
| #9 | F | 21 | Osteoblastic OS | Ilium + pubis + sacrum | Neoplastic thrombus |
| #10 | F | 33 | Osteoblastic OS | Ilium | LN |
| #11 | M | 71 | Dedifferentiated LS | Iliac fossa | No |
| #12 | M | 66 | Grade 2 CS | Pubis + acetabulum | No |
| #13 | F | 32 | Chondroblastic OS | Ilium | Lungs |
| #14 | F | 43 | Grade 3 CS | Ilium + acetabulum | No |
| #15 | M | 61 | Secondary OS | Ilium + sacrum | No |
| #16 | F | 57 | Pleomorphic sarcoma | Iliac fossa | No |
| #17 | M | 41 | Osteoblastic OS | Pubis | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sambri, A.; Fiore, M.; Rottoli, M.; Bianchi, G.; Pignatti, M.; Bortoli, M.; Ercolino, A.; Ancetti, S.; Perrone, A.M.; De Iaco, P.; et al. A Planned Multidisciplinary Surgical Approach to Treat Primary Pelvic Malignancies. Curr. Oncol. 2023, 30, 1106-1115. https://doi.org/10.3390/curroncol30010084
Sambri A, Fiore M, Rottoli M, Bianchi G, Pignatti M, Bortoli M, Ercolino A, Ancetti S, Perrone AM, De Iaco P, et al. A Planned Multidisciplinary Surgical Approach to Treat Primary Pelvic Malignancies. Current Oncology. 2023; 30(1):1106-1115. https://doi.org/10.3390/curroncol30010084
Chicago/Turabian StyleSambri, Andrea, Michele Fiore, Matteo Rottoli, Giuseppe Bianchi, Marco Pignatti, Marta Bortoli, Amelio Ercolino, Stefano Ancetti, Anna Myriam Perrone, Pierandrea De Iaco, and et al. 2023. "A Planned Multidisciplinary Surgical Approach to Treat Primary Pelvic Malignancies" Current Oncology 30, no. 1: 1106-1115. https://doi.org/10.3390/curroncol30010084
APA StyleSambri, A., Fiore, M., Rottoli, M., Bianchi, G., Pignatti, M., Bortoli, M., Ercolino, A., Ancetti, S., Perrone, A. M., De Iaco, P., Cipriani, R., Brunocilla, E., Donati, D. M., Gargiulo, M., Poggioli, G., & De Paolis, M. (2023). A Planned Multidisciplinary Surgical Approach to Treat Primary Pelvic Malignancies. Current Oncology, 30(1), 1106-1115. https://doi.org/10.3390/curroncol30010084








