Current Update on PET/MRI in Gynecological Malignancies—A Review of the Literature
Abstract
1. Introduction
2. Technical Background
3. Cervical Cancer
3.1. Epidemiology
3.2. Classification
3.3. Imaging
4. Endometrial Cancer
4.1. Epidemiology
4.2. Classification
4.3. Imaging
5. Ovarian Cancer
5.1. Epidemiology
5.2. Classification
5.3. Imaging
6. Challenges
7. Clinical Trials and Metanalysis
8. Future Directions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nguyen, N.C.; Beriwal, S.; Moon, C.H.; D’Ardenne, N.; Mountz, J.M.; Furlan, A.; Muthukrishnan, A.; Rangaswamy, B. Diagnostic Value of FDG PET/MRI in Females With Pelvic Malignancy-A Systematic Review of the Literature. Front. Oncol. 2020, 10, 519440. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Virarkar, M.; Viswanathan, C.; Iyer, R.; de Castro Faria, S.; Morani, A.; Carter, B.; Ganeshan, D.; Elsherif, S.; Bhosale, P.R. The Role of Positron Emission Tomography/Magnetic Resonance Imaging in Gynecological Malignancies. J. Comput. Assist. Tomogr. 2019, 43, 825–834. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef]
- Balcacer, P.; Shergill, A.; Litkouhi, B. MRI of cervical cancer with a surgical perspective: Staging, prognostic implications and pitfalls. Abdom. Radiol. 2019, 44, 2557–2571. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures. 2022. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html# (accessed on 1 October 2022).
- Saleh, M.; Virarkar, M.; Javadi, S.; Elsherif, S.B.; de Castro Faria, S.; Bhosale, P. Cervical Cancer: 2018 Revised International Federation of Gynecology and Obstetrics Staging System and the Role of Imaging. AJR Am. J. Roentgenol. 2020, 214, 1182–1195. [Google Scholar] [CrossRef]
- Corrigendum to “Revised FIGO staging for carcinoma of the cervix uteri” [Int J Gynecol Obstet 145(2019) 129–135]. Int. J. Gynaecol. Obstet. 2019, 147, 279–280. [CrossRef]
- Virarkar, M.; Vulasala, S.S.; Morani, A.C.; Waters, R.; Gopireddy, D.R.; Kumar, S.; Bhosale, P.; Lall, C. Neuroendocrine Neoplasms of the Gynecologic Tract. Cancers 2022, 14, 1835. [Google Scholar] [CrossRef]
- Ghani, M.A.; Liau, J.; Eskander, R.; Mell, L.; Yusufaly, T.; Obrzut, S. Imaging Biomarkers and Liquid Biopsy in Assessment of Cervical Cancer. J. Comput. Assist. Tomogr. 2022, 46, 707–715. [Google Scholar] [CrossRef]
- Tsuyoshi, H.; Tsujikawa, T.; Yamada, S.; Okazawa, H.; Yoshida, Y. Diagnostic Value of 18F-FDG PET/MRI for Revised 2018 FIGO Staging in Patients with Cervical Cancer. Diagnostics 2021, 11, 202. [Google Scholar] [CrossRef]
- Xiao, M.; Yan, B.; Li, Y.; Lu, J.; Qiang, J. Diagnostic performance of MR imaging in evaluating prognostic factors in patients with cervical cancer: A meta-analysis. Eur. Radiol. 2020, 30, 1405–1418. [Google Scholar] [CrossRef]
- Thomeer, M.G.; Gerestein, C.; Spronk, S.; van Doorn, H.C.; van der Ham, E.; Hunink, M.G. Clinical examination versus magnetic resonance imaging in the pretreatment staging of cervical carcinoma: Systematic review and meta-analysis. Eur. Radiol. 2013, 23, 2005–2018. [Google Scholar] [CrossRef]
- Nguyen, N.C.; Beriwal, S.; Moon, C.H.; Furlan, A.; Mountz, J.M.; Rangaswamy, B. F-FDG PET/MRI Primary Staging of Cervical Cancer: A Pilot Study with PET/CT Comparison. J. Nucl. Med. Technol. 2020, 48, 331–335. [Google Scholar] [CrossRef]
- Steiner, A.; Narva, S.; Rinta-Kiikka, I.; Hietanen, S.; Hynninen, J.; Virtanen, J. Diagnostic efficiency of whole-body 18F-FDG PET/MRI, MRI alone, and SUV and ADC values in staging of primary uterine cervical cancer. Cancer Imaging 2021, 21, 16. [Google Scholar] [CrossRef]
- Chen, X.L.; Chen, G.W.; Xu, G.H.; Ren, J.; Li, Z.L.; Pu, H.; Li, H. Tumor Size at Magnetic Resonance Imaging Association With Lymph Node Metastasis and Lymphovascular Space Invasion in Resectable Cervical Cancer: A Multicenter Evaluation of Surgical Specimens. Int. J. Gynecol. Cancer 2018, 28, 1545–1552. [Google Scholar] [CrossRef]
- Ayhan, A.; Aslan, K.; Öz, M.; Tohma, Y.A.; Kuşçu, E.; Meydanli, M.M. Para-aortic lymph node involvement revisited in the light of the revised 2018 FIGO staging system for cervical cancer. Arch. Gynecol. Obstet. 2019, 300, 675–682. [Google Scholar] [CrossRef]
- Sarabhai, T.; Schaarschmidt, B.M.; Wetter, A.; Kirchner, J.; Aktas, B.; Forsting, M.; Ruhlmann, V.; Herrmann, K.; Umutlu, L.; Grueneisen, J. Comparison of 18F-FDG PET/MRI and MRI for pre-therapeutic tumor staging of patients with primary cancer of the uterine cervix. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 67–76. [Google Scholar] [CrossRef]
- Wang, T.; Sun, H.; Han, F.; Sun, W.; Chen, Z. Evaluation of parametrial infiltration in cervical cancer with voxel-based segmentation of integrated 18F-FDG PET/MRI images: A preliminary study. Eur. J. Radiol. 2019, 118, 147–152. [Google Scholar] [CrossRef]
- Kitajima, K.; Suenaga, Y.; Ueno, Y.; Kanda, T.; Maeda, T.; Deguchi, M.; Ebina, Y.; Yamada, H.; Takahashi, S.; Sugimura, K. Fusion of PET and MRI for staging of uterine cervical cancer: Comparison with contrast-enhanced (18)F-FDG PET/CT and pelvic MRI. Clin. Imaging 2014, 38, 464–469. [Google Scholar] [CrossRef]
- Wiebe, E.; Denny, L.; Thomas, G. Cancer of the cervix uteri. Int. J. Gynaecol. Obstet. 2012, 119 (Suppl. 2), S100–S109. [Google Scholar] [CrossRef]
- Wang, M.; Ma, M.; Yang, L.; Liang, C. Development and validation of a nomogram for predicting pelvic lymph node metastasis and prognosis in patients with cervical cancer. Front. Oncol. 2022, 12, 952347. [Google Scholar] [CrossRef]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. 2), 22–36. [Google Scholar] [CrossRef]
- Zigras, T.; Lennox, G.; Willows, K.; Covens, A. Early Cervical Cancer: Current Dilemmas of Staging and Surgery. Curr. Oncol. Rep. 2017, 19, 51. [Google Scholar] [CrossRef]
- Anner, P.; Mayerhöfer, M.; Wadsak, W.; Geleff, S.; Dudczak, R.; Haug, A.; Hacker, M.; Karanikas, G. [18F]FDG-PET/CT and MRI for initial pelvic lymph node staging in patients with cervical carcinoma: The potential usefulness of [18F]FDG-PET/MRI. Oncol. Lett. 2018, 15, 3951–3956. [Google Scholar] [CrossRef]
- Lv, K.; Guo, H.M.; Lu, Y.J.; Wu, Z.X.; Zhang, K.; Han, J.K. Role of 18F-FDG PET/CT in detecting pelvic lymph-node metastases in patients with early-stage uterine cervical cancer: Comparison with MRI findings. Nucl. Med. Commun. 2014, 35, 1204–1211. [Google Scholar] [CrossRef]
- Sironi, S.; Buda, A.; Picchio, M.; Perego, P.; Moreni, R.; Pellegrino, A.; Colombo, M.; Mangioni, C.; Messa, C.; Fazio, F. Lymph node metastasis in patients with clinical early-stage cervical cancer: Detection with integrated FDG PET/CT. Radiology 2006, 238, 272–279. [Google Scholar] [CrossRef]
- Beiderwellen, K.; Grueneisen, J.; Ruhlmann, V.; Buderath, P.; Aktas, B.; Heusch, P.; Kraff, O.; Forsting, M.; Lauenstein, T.C.; Umutlu, L. [(18)F]FDG PET/MRI vs. PET/CT for whole-body staging in patients with recurrent malignancies of the female pelvis: Initial results. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 56–65. [Google Scholar] [CrossRef]
- Narva, S.I.; Seppänen, M.P.; Raiko, J.R.H.; Forsback, S.J.; Orte, K.J.; Virtanen, J.M.; Hynninen, J.; Hietanen, S. Imaging of Tumor Hypoxia With 18F-EF5 PET/MRI in Cervical Cancer. Clin. Nucl. Med. 2021, 46, 952–957. [Google Scholar] [CrossRef]
- Kidd, E.A.; Grigsby, P.W. Intratumoral metabolic heterogeneity of cervical cancer. Clin. Cancer Res. 2008, 14, 5236–5241. [Google Scholar] [CrossRef]
- Sapienza, L.G.; Salcedo, M.P.; Ning, M.S.; Jhingran, A.; Klopp, A.H.; Calsavara, V.F.; Schmeler, K.M.; Leite Gomes, M.J.; de Freitas Carvalho, E.; Baiocchi, G. Pelvic Insufficiency Fractures After External Beam Radiation Therapy for Gynecologic Cancers: A Meta-analysis and Meta-regression of 3929 Patients. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 475–484. [Google Scholar] [CrossRef]
- Azumi, M.; Matsumoto, M.; Suzuki, K.; Sasaki, R.; Ueno, Y.; Nogami, M.; Terai, Y. PET/MRI is useful for early detection of pelvic insufficiency fractures after radiotherapy for cervical cancer. Oncol. Lett. 2021, 22, 776. [Google Scholar] [CrossRef]
- Kidd, E.A.; El Naqa, I.; Siegel, B.A.; Dehdashti, F.; Grigsby, P.W. FDG-PET-based prognostic nomograms for locally advanced cervical cancer. Gynecol. Oncol. 2012, 127, 136–140. [Google Scholar] [CrossRef]
- Kidd, E.A.; Siegel, B.A.; Dehdashti, F.; Grigsby, P.W. The standardized uptake value for F-18 fluorodeoxyglucose is a sensitive predictive biomarker for cervical cancer treatment response and survival. Cancer 2007, 110, 1738–1744. [Google Scholar] [CrossRef]
- Zhao, Q.; Feng, Y.; Mao, X.; Qie, M. Prognostic value of fluorine-18-fluorodeoxyglucose positron emission tomography or PET-computed tomography in cervical cancer: A meta-analysis. Int. J. Gynecol. Cancer 2013, 23, 1184–1190. [Google Scholar] [CrossRef]
- Ho, J.C.; Allen, P.K.; Bhosale, P.R.; Rauch, G.M.; Fuller, C.D.; Mohamed, A.S.; Frumovitz, M.; Jhingran, A.; Klopp, A.H. Diffusion-Weighted Magnetic Resonance Imaging as a Predictor of Outcome in Cervical Cancer After Chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 546–553. [Google Scholar] [CrossRef]
- Das, S.; Chandramohan, A.; Reddy, J.K.; Mukhopadhyay, S.; Kumar, R.M.; Isiah, R.; John, S.; Oommen, R.; Jeyaseelan, V. Role of conventional and diffusion weighted MRI in predicting treatment response after low dose radiation and chemotherapy in locally advanced carcinoma cervix. Radiother. Oncol. 2015, 117, 288–293. [Google Scholar] [CrossRef]
- Park, J.J.; Kim, C.K.; Park, B.K. Prediction of disease progression following concurrent chemoradiotherapy for uterine cervical cancer: Value of post-treatment diffusion-weighted imaging. Eur. Radiol. 2016, 26, 3272–3279. [Google Scholar] [CrossRef]
- Park, J.J.; Kim, C.K.; Park, S.Y.; Park, B.K.; Kim, B. Value of diffusion-weighted imaging in predicting parametrial invasion in stage IA2-IIA cervical cancer. Eur. Radiol. 2014, 24, 1081–1088. [Google Scholar] [CrossRef]
- Olsen, J.R.; Esthappan, J.; DeWees, T.; Narra, V.R.; Dehdashti, F.; Siegel, B.A.; Schwarz, J.K.; Grigsby, P.W. Tumor volume and subvolume concordance between FDG-PET/CT and diffusion-weighted MRI for squamous cell carcinoma of the cervix. J. Magn. Reson. Imaging 2013, 37, 431–434. [Google Scholar] [CrossRef]
- Kidd, E.A.; Spencer, C.R.; Huettner, P.C.; Siegel, B.A.; Dehdashti, F.; Rader, J.S.; Grigsby, P.W. Cervical cancer histology and tumor differentiation affect 18F-fluorodeoxyglucose uptake. Cancer 2009, 115, 3548–3554. [Google Scholar] [CrossRef]
- Mendez, L.E.; Manci, N.; Cantuaria, G.; Gomez-Marin, O.; Penalver, M.; Braunschweiger, P.; Nadji, M. Expression of glucose transporter-1 in cervical cancer and its precursors. Gynecol. Oncol. 2002, 86, 138–143. [Google Scholar] [CrossRef]
- Yen, T.C.; See, L.C.; Lai, C.H.; Yah-Huei, C.W.; Ng, K.K.; Ma, S.Y.; Lin, W.J.; Chen, J.T.; Chen, W.J.; Lai, C.R.; et al. 18F-FDG uptake in squamous cell carcinoma of the cervix is correlated with glucose transporter 1 expression. J. Nucl. Med. 2004, 45, 22–29. [Google Scholar]
- Brandmaier, P.; Purz, S.; Bremicker, K.; Höckel, M.; Barthel, H.; Kluge, R.; Kahn, T.; Sabri, O.; Stumpp, P. Simultaneous [18F]FDG-PET/MRI: Correlation of Apparent Diffusion Coefficient (ADC) and Standardized Uptake Value (SUV) in Primary and Recurrent Cervical Cancer. PLoS ONE 2015, 10, e0141684. [Google Scholar] [CrossRef]
- Grueneisen, J.; Schaarschmidt, B.M.; Heubner, M.; Aktas, B.; Kinner, S.; Forsting, M.; Lauenstein, T.; Ruhlmann, V.; Umutlu, L. Integrated PET/MRI for whole-body staging of patients with primary cervical cancer: Preliminary results. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1814–1824. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.C.; Lin, G.; Wang, J.J.; Lai, C.H.; Chang, C.J.; Yen, T.C. Correlation of apparent diffusion coefficients measured by 3T diffusion-weighted MRI and SUV from FDG PET/CT in primary cervical cancer. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 200–208. [Google Scholar] [CrossRef]
- Surov, A.; Meyer, H.J.; Schob, S.; Höhn, A.K.; Bremicker, K.; Exner, M.; Stumpp, P.; Purz, S. Parameters of simultaneous 18F-FDG-PET/MRI predict tumor stage and several histopathological features in uterine cervical cancer. Oncotarget 2017, 8, 28285–28296. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yu, S.; Xin, J.; Guo, Q. Relationship of 18F-FDG PET/CT metabolic, clinical and pathological characteristics of primary squamous cell carcinoma of the cervix. J. Investig. Med. 2016, 64, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Sun, H.; Gao, S.; Xin, J.; Lu, Z.; Chen, Z.; Pan, S.; Guo, Q. Relationship between 18F-FDG PET metabolic parameters and MRI intravoxel incoherent motion (IVIM) histogram parameters and their correlations with clinicopathological features of cervical cancer: Evidence from integrated PET/MRI. Clin. Radiol. 2019, 74, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Floberg, J.M.; Fowler, K.J.; Fuser, D.; DeWees, T.A.; Dehdashti, F.; Siegel, B.A.; Wahl, R.L.; Schwarz, J.K.; Grigsby, P.W. Spatial relationship of 2-deoxy-2-[18F]-fluoro-D-glucose positron emission tomography and magnetic resonance diffusion imaging metrics in cervical cancer. EJNMMI Res. 2018, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Umutlu, L.; Nensa, F.; Demircioglu, A.; Antoch, G.; Herrmann, K.; Forsting, M.; Grueneisen, J.S. Radiomics Analysis of Multiparametric PET/MRI for N- and M-Staging in Patients with Primary Cervical Cancer. Rofo 2020, 192, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.J.; Purz, S.; Sabri, O.; Surov, A. Cervical Cancer: Associations between Metabolic Parameters and Whole Lesion Histogram Analysis Derived from Simultaneous 18F-FDG-PET/MRI. Contrast Media Mol. Imaging 2018, 2018, 5063285. [Google Scholar] [CrossRef] [PubMed]
- Vojtíšek, R.; Baxa, J.; Kovářová, P.; Almortaza, A.; Hošek, P.; Sukovská, E.; Tupý, R.; Ferda, J.; Fínek, J. Prediction of treatment response in patients with locally advanced cervical cancer using midtreatment PET/MRI during concurrent chemoradiotherapy. Strahlenther. Onkol. 2021, 197, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Ahangari, S.; Hansen, N.L.; Olin, A.B.; Nøttrup, T.J.; Ryssel, H.; Berthelsen, A.K.; Löfgren, J.; Loft, A.; Vogelius, I.R.; Schnack, T.; et al. Toward PET/MRI as one-stop shop for radiotherapy planning in cervical cancer patients. Acta Oncol. 2021, 60, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Choi, H.J.; Park, S.Y.; Lee, H.Y.; Seo, S.S.; Yoo, C.W.; Jung, D.C.; Kang, S.; Cho, K.S. Additional value of MR/PET fusion compared with PET/CT in the detection of lymph node metastases in cervical cancer patients. Eur. J. Cancer 2009, 45, 2103–2109. [Google Scholar] [CrossRef]
- Ahangari, S.; Littrup Andersen, F.; Liv Hansen, N.; Jakobi Nøttrup, T.; Berthelsen, A.K.; Folsted Kallehauge, J.; Richter Vogelius, I.; Kjaer, A.; Espe Hansen, A.; Fischer, B.M. Multi-parametric PET/MRI for enhanced tumor characterization of patients with cervical cancer. Eur. J. Hybrid Imaging 2022, 6, 7. [Google Scholar] [CrossRef]
- Gong, J.; Liu, H.; Bao, Z.; Bian, L.; Li, X.; Meng, Y. Relative clinical utility of simultaneous 18F-fluorodeoxyglucose PET/MRI and PET/CT for preoperative cervical cancer diagnosis. J. Int. Med. Res. 2021, 49, 3000605211019190. [Google Scholar] [CrossRef]
- Xin, J.; Ma, Q.; Guo, Q.; Sun, H.; Zhang, S.; Liu, C.; Zhai, W. PET/MRI with diagnostic MR sequences vs PET/CT in the detection of abdominal and pelvic cancer. Eur. J. Radiol. 2016, 85, 751–759. [Google Scholar] [CrossRef]
- Queiroz, M.A.; Kubik-Huch, R.A.; Hauser, N.; Freiwald-Chilla, B.; von Schulthess, G.; Froehlich, J.M.; Veit-Haibach, P. PET/MRI and PET/CT in advanced gynaecological tumours: Initial experience and comparison. Eur. Radiol. 2015, 25, 2222–2230. [Google Scholar] [CrossRef]
- Spick, C.; Herrmann, K.; Czernin, J. 18F-FDG PET/CT and PET/MRI Perform Equally Well in Cancer: Evidence from Studies on More Than 2,300 Patients. J. Nucl. Med. 2016, 57, 420–430. [Google Scholar] [CrossRef]
- Grueneisen, J.; Schaarschmidt, B.M.; Beiderwellen, K.; Schulze-Hagen, A.; Heubner, M.; Kinner, S.; Forsting, M.; Lauenstein, T.; Ruhlmann, V.; Umutlu, L. Diagnostic value of diffusion-weighted imaging in simultaneous 18F-FDG PET/MR imaging for whole-body staging of women with pelvic malignancies. J. Nucl. Med. 2014, 55, 1930–1935. [Google Scholar] [CrossRef]
- Schwartz, M.; Gavane, S.C.; Bou-Ayache, J.; Kolev, V.; Zakashansky, K.; Prasad-Hayes, M.; Taouli, B.; Chuang, L.; Kostakoglu, L. Feasibility and diagnostic performance of hybrid PET/MRI compared with PET/CT for gynecological malignancies: A prospective pilot study. Abdom. Radiol. 2018, 43, 3462–3467. [Google Scholar] [CrossRef]
- Nakajo, K.; Tatsumi, M.; Inoue, A.; Isohashi, K.; Higuchi, I.; Kato, H.; Imaizumi, M.; Enomoto, T.; Shimosegawa, E.; Kimura, T.; et al. Diagnostic performance of fluorodeoxyglucose positron emission tomography/magnetic resonance imaging fusion images of gynecological malignant tumors: Comparison with positron emission tomography/computed tomography. Jpn. J. Radiol. 2010, 28, 95–100. [Google Scholar] [CrossRef]
- Passarello, K.; Kurian, S.; Villanueva, V. Endometrial Cancer: An Overview of Pathophysiology, Management, and Care. Semin. Oncol. Nurs. 2019, 35, 157–165. [Google Scholar] [CrossRef]
- National Cancer Institute. Endometrial Cancer Treatment (PDQ®): Health Professional Version. Available online: https://www.cancer.gov/types/uterine/hp/endometrial-treatment-pdq (accessed on 1 October 2022).
- Ironi, G.; Mapelli, P.; Bergamini, A.; Fallanca, F.; Candotti, G.; Gnasso, C.; Taccagni, G.L.; Sant’Angelo, M.; Scifo, P.; Bezzi, C.; et al. Hybrid PET/MRI in Staging Endometrial Cancer: Diagnostic and Predictive Value in a Prospective Cohort. Clin. Nucl. Med. 2022, 47, e221–e229. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef]
- Tsuyoshi, H.; Tsujikawa, T.; Yamada, S.; Chino, Y.; Shinagawa, A.; Kurokawa, T.; Okazawa, H.; Yoshida, Y. FDG-PET/MRI with high-resolution DWI characterises the distinct phenotypes of endometrial cancer. Clin. Radiol. 2020, 75, 209–215. [Google Scholar] [CrossRef]
- Shih, I.L.; Yen, R.F.; Chen, C.A.; Chen, B.B.; Wei, S.Y.; Chang, W.C.; Sheu, B.C.; Cheng, W.F.; Tseng, Y.H.; Chen, X.J.; et al. Standardized uptake value and apparent diffusion coefficient of endometrial cancer evaluated with integrated whole-body PET/MR: Correlation with pathological prognostic factors. J. Magn. Reson. Imaging 2015, 42, 1723–1732. [Google Scholar] [CrossRef]
- Saleh, M.; Virarkar, M.; Bhosale, P.; El Sherif, S.; Javadi, S.; Faria, S.C. Endometrial Cancer, the Current International Federation of Gynecology and Obstetrics Staging System, and the Role of Imaging. J. Comput. Assist. Tomogr. 2020, 44, 714–729. [Google Scholar] [CrossRef]
- Ota, T.; Hori, M.; Onishi, H.; Sakane, M.; Tsuboyama, T.; Tatsumi, M.; Nakamoto, A.; Kimura, T.; Narumi, Y.; Tomiyama, N. Preoperative staging of endometrial cancer using reduced field-of-view diffusion-weighted imaging: A preliminary study. Eur. Radiol. 2017, 27, 5225–5235. [Google Scholar] [CrossRef]
- Jónsdóttir, B.; Ripoll, M.A.; Bergman, A.; Silins, I.; Poromaa, I.S.; Ahlström, H.; Stålberg, K. Validation of 18F-FDG PET/MRI and diffusion-weighted MRI for estimating the extent of peritoneal carcinomatosis in ovarian and endometrial cancer—A pilot study. Cancer Imaging 2021, 21, 34. [Google Scholar] [CrossRef]
- Tsuyoshi, H.; Tsujikawa, T.; Yamada, S.; Okazawa, H.; Yoshida, Y. Diagnostic value of 18F-FDG PET/MRI for staging in patients with endometrial cancer. Cancer Imaging 2020, 20, 75. [Google Scholar] [CrossRef]
- Bian, L.H.; Wang, M.; Gong, J.; Liu, H.H.; Wang, N.; Wen, N.; Fan, W.S.; Xu, B.X.; Wang, M.Y.; Ye, M.X.; et al. Comparison of integrated PET/MRI with PET/CT in evaluation of endometrial cancer: A retrospective analysis of 81 cases. PeerJ 2019, 7, e7081. [Google Scholar] [CrossRef]
- Bezzi, C.; Zambella, E.; Ghezzo, S.; Fallanca, F.; Samanes Gajate, A.M.; Franchini, A.; Ironi, G.; Bergamini, A.; Monaco, L.; Evangelista, L.; et al. 18F-FDG PET/MRI in endometrial cancer: Systematic review and meta-analysis. Clin. Transl. Imaging 2021, 10, 45–58. [Google Scholar] [CrossRef]
- Stecco, A.; Buemi, F.; Cassarà, A.; Matheoud, R.; Sacchetti, G.M.; Arnulfo, A.; Brambilla, M.; Carriero, A. Comparison of retrospective PET and MRI-DWI (PET/MRI-DWI) image fusion with PET/CT and MRI-DWI in detection of cervical and endometrial cancer lymph node metastases. Radiol. Med. 2016, 121, 537–545. [Google Scholar] [CrossRef]
- American Cancer Society. Key Statistics for Ovarian Cancer. Available online: https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html (accessed on 2 October 2022).
- National Cancer Institute. Cancer Stat Facts: Ovarian Cancer. Available online: https://seer.cancer.gov/statfacts/html/ovary.html (accessed on 2 October 2022).
- Javadi, S.; Ganeshan, D.M.; Qayyum, A.; Iyer, R.B.; Bhosale, P. Ovarian Cancer, the Revised FIGO Staging System, and the Role of Imaging. AJR Am. J. Roentgenol. 2016, 206, 1351–1360. [Google Scholar] [CrossRef]
- Virarkar, M.; Ganeshan, D.; Gulati, A.T.; Palmquist, S.; Iyer, R.; Bhosale, P. Diagnostic performance of PET/CT and PET/MR in the management of ovarian carcinoma-a literature review. Abdom. Radiol. 2021, 46, 2323–2349. [Google Scholar] [CrossRef]
- Forstner, R.; Meissnitzer, M.; Cunha, T.M. Update on Imaging of Ovarian Cancer. Curr. Radiol. Rep. 2016, 4, 31. [Google Scholar] [CrossRef]
- Elsherif, S.; Javadi, S.; Viswanathan, C.; Faria, S.; Bhosale, P. Low-grade epithelial ovarian cancer: What a radiologist should know. Br. J. Radiol. 2019, 92, 20180571. [Google Scholar] [CrossRef] [PubMed]
- Sassone, A.M.; Timor-Tritsch, I.E.; Artner, A.; Westhoff, C.; Warren, W.B. Transvaginal sonographic characterization of ovarian disease: Evaluation of a new scoring system to predict ovarian malignancy. Obstet. Gynecol. 1991, 78, 70–76. [Google Scholar]
- Van Calster, B.; Van Hoorde, K.; Valentin, L.; Testa, A.C.; Fischerova, D.; Van Holsbeke, C.; Savelli, L.; Franchi, D.; Epstein, E.; Kaijser, J.; et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: Prospective multicentre diagnostic study. BMJ 2014, 349, g5920. [Google Scholar] [CrossRef] [PubMed]
- Khiewvan, B.; Torigian, D.A.; Emamzadehfard, S.; Paydary, K.; Salavati, A.; Houshmand, S.; Werner, T.J.; Alavi, A. An update on the role of PET/CT and PET/MRI in ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Forstner, R.; Thomassin-Naggara, I.; Cunha, T.M.; Kinkel, K.; Masselli, G.; Kubik-Huch, R.; Spencer, J.A.; Rockall, A. ESUR recommendations for MR imaging of the sonographically indeterminate adnexal mass: An update. Eur. Radiol. 2017, 27, 2248–2257. [Google Scholar] [CrossRef] [PubMed]
- Rieber, A.; Nüssle, K.; Stöhr, I.; Grab, D.; Fenchel, S.; Kreienberg, R.; Reske, S.N.; Brambs, H.J. Preoperative diagnosis of ovarian tumors with MR imaging: Comparison with transvaginal sonography, positron emission tomography, and histologic findings. AJR Am. J. Roentgenol. 2001, 177, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.N.; Sarian, L.O.; Yoshida, A.; Araújo, K.G.; Barros, R.H.O.; Baião, A.C.; Parente, D.B.; Derchain, S. Accuracy of the ADNEX MR scoring system based on a simplified MRI protocol for the assessment of adnexal masses. Diagn. Interv. Radiol. 2018, 24, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Suzuki, K.; Senda, M.; Kita, M.; Nakamoto, Y.; Onishi, Y.; Maeda, T.; Yoshikawa, T.; Ohno, Y.; Sugimura, K. FDG-PET/CT for diagnosis of primary ovarian cancer. Nucl. Med. Commun. 2011, 32, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Tsuyoshi, H.; Yoshida, Y. Diagnostic imaging using positron emission tomography for gynecological malignancy. J. Obstet. Gynaecol. Res. 2017, 43, 1687–1699. [Google Scholar] [CrossRef]
- Fiaschetti, V.; Calabria, F.; Crusco, S.; Meschini, A.; Nucera, F.; Schillaci, O.; Simonetti, G. MR-PET fusion imaging in evaluating adnexal lesions: A preliminary study. Radiol. Med. 2011, 116, 1288–1302. [Google Scholar] [CrossRef]
- Tsuyoshi, H.; Tsujikawa, T.; Yamada, S.; Okazawa, H.; Yoshida, Y. Diagnostic value of [18F]FDG PET/MRI for staging in patients with ovarian cancer. EJNMMI Res. 2020, 10, 117. [Google Scholar] [CrossRef]
- Pelissier, A.; Bonneau, C.; Chéreau, E.; de La Motte Rouge, T.; Fourchotte, V.; Daraï, E.; Rouzier, R. CA125 kinetic parameters predict optimal cytoreduction in patients with advanced epithelial ovarian cancer treated with neoadjuvant chemotherapy. Gynecol. Oncol. 2014, 135, 542–546. [Google Scholar] [CrossRef]
- Vallius, T.; Peter, A.; Auranen, A.; Carpén, O.; Kemppainen, J.; Matomäki, J.; Oksa, S.; Roering, P.; Seppänen, M.; Grénman, S.; et al. 18F-FDG-PET/CT can identify histopathological non-responders to platinum-based neoadjuvant chemotherapy in advanced epithelial ovarian cancer. Gynecol. Oncol. 2016, 140, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, L.M.; Kirchner, J.; Grueneisen, J.; Ruhlmann, V.; Aktas, B.; Schaarschmidt, B.M.; Forsting, M.; Herrmann, K.; Antoch, G.; Umutlu, L. Comparison of 18F–FDG PET/MRI and MRI alone for whole-body staging and potential impact on therapeutic management of women with suspected recurrent pelvic cancer: A follow-up study. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Grueneisen, J.; Beiderwellen, K.; Heusch, P.; Gratz, M.; Schulze-Hagen, A.; Heubner, M.; Kinner, S.; Forsting, M.; Lauenstein, T.; Ruhlmann, V.; et al. Simultaneous positron emission tomography/magnetic resonance imaging for whole-body staging in patients with recurrent gynecological malignancies of the pelvis: A comparison to whole-body magnetic resonance imaging alone. Investig. Radiol. 2014, 49, 808–815. [Google Scholar] [CrossRef]
- Kirchner, J.; Sawicki, L.M.; Suntharalingam, S.; Grueneisen, J.; Ruhlmann, V.; Aktas, B.; Deuschl, C.; Herrmann, K.; Antoch, G.; Forsting, M.; et al. Whole-body staging of female patients with recurrent pelvic malignancies: Ultra-fast 18F-FDG PET/MRI compared to 18F-FDG PET/CT and CT. PLoS ONE 2017, 12, e0172553. [Google Scholar] [CrossRef]
- Grueneisen, J.; Schaarschmidt, B.M.; Heubner, M.; Suntharalingam, S.; Milk, I.; Kinner, S.; Heubner, A.; Forsting, M.; Lauenstein, T.; Ruhlmann, V.; et al. Implementation of FAST-PET/MRI for whole-body staging of female patients with recurrent pelvic malignancies: A comparison to PET/CT. Eur. J. Radiol. 2015, 84, 2097–2102. [Google Scholar] [CrossRef]
- Kitajima, K.; Suenaga, Y.; Ueno, Y.; Kanda, T.; Maeda, T.; Makihara, N.; Ebina, Y.; Yamada, H.; Takahashi, S.; Sugimura, K. Value of fusion of PET and MRI in the detection of intra-pelvic recurrence of gynecological tumor: Comparison with 18F-FDG contrast-enhanced PET/CT and pelvic MRI. Ann. Nucl. Med. 2014, 28, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Chehade, H.; Tedja, R.; Ramos, H.; Bawa, T.S.; Adzibolosu, N.; Gogoi, R.; Mor, G.; Alvero, A.B. Regulatory Role of the Adipose Microenvironment on Ovarian Cancer Progression. Cancers 2022, 14, 2267. [Google Scholar] [CrossRef] [PubMed]
- Spiliotis, J.D.; Iavazzo, C.; Kopanakis, N.D.; Christopoulou, A. Secondary debulking for ovarian carcinoma relapse: The R-R dilemma—Is the prognosis different for residual or recurrent disease? J. Turk. Ger. Gynecol. Assoc. 2019, 20, 213–217. [Google Scholar] [CrossRef]
- Zheng, M.; Xie, D.; Pan, C.; Xu, Y.; Yu, W. Diagnostic value of 18F-FDG PET/MRI in recurrent pelvis malignancies of female patients: A systematic review and meta-analysis. Nucl. Med. Commun. 2018, 39, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Chandarana, H.; Heacock, L.; Rakheja, R.; DeMello, L.R.; Bonavita, J.; Block, T.K.; Geppert, C.; Babb, J.S.; Friedman, K.P. Pulmonary nodules in patients with primary malignancy: Comparison of hybrid PET/MR and PET/CT imaging. Radiology 2013, 268, 874–881. [Google Scholar] [CrossRef]
- Virarkar, M.; Devine, C.; Bassett, R.; Javadi, S.; Faria, S.C.; Bhosale, P. Update on Diagnostic Performance of PET/MRI in Gynecological Malignancies: A Systematic Review and Meta-Analysis. J. Belg. Soc. Radiol. 2020, 104, 4. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Zhang, J.; Gao, J.; Guo, L.; Zhou, H.; Hu, Y.; Zhu, C.; Li, Q.; Ma, X. Diagnostic role of 18F-FDG PET/MRI in patients with gynecological malignancies of the pelvis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0175401. [Google Scholar] [CrossRef] [PubMed]
- Virarkar, M.; Ganeshan, D.; Devine, C.; Bassett, R.; Kuchana, V.; Bhosale, P. Diagnostic value of PET/CT versus PET/MRI in gynecological malignancies of the pelvis: A meta-analysis. Clin. Imaging 2020, 60, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Luo, Y.; Jiang, H.; Meng, N.; Huang, Z.; Feng, P.; Fang, T.; Fu, F.; Li, X.; Bai, Y.; et al. The value of diffusion kurtosis imaging, diffusion weighted imaging and 18F-FDG PET for differentiating benign and malignant solitary pulmonary lesions and predicting pathological grading. Front. Oncol. 2022, 12, 873669. [Google Scholar] [CrossRef]
- Yang, L.; Rao, S.; Wang, W.; Chen, C.; Ding, Y.; Yang, C.; Grimm, R.; Yan, X.; Fu, C.; Zeng, M. Staging liver fibrosis with DWI: Is there an added value for diffusion kurtosis imaging? Eur. Radiol. 2018, 28, 3041–3049. [Google Scholar] [CrossRef]
- Wang, P.; Thapa, D.; Wu, G.; Sun, Q.; Cai, H.; Tuo, F. A study on diffusion and kurtosis features of cervical cancer based on non-Gaussian diffusion weighted model. Magn. Reson. Imaging 2018, 47, 60–66. [Google Scholar] [CrossRef]
- Chen, T.; Li, Y.; Lu, S.S.; Zhang, Y.D.; Wang, X.N.; Luo, C.Y.; Shi, H.B. Quantitative evaluation of diffusion-kurtosis imaging for grading endometrial carcinoma: A comparative study with diffusion-weighted imaging. Clin. Radiol. 2017, 72, 995.e11–995.e20. [Google Scholar] [CrossRef]
- Yue, W.; Meng, N.; Wang, J.; Liu, W.; Wang, X.; Yan, M.; Han, D.; Cheng, J. Comparative analysis of the value of diffusion kurtosis imaging and diffusion-weighted imaging in evaluating the histological features of endometrial cancer. Cancer Imaging 2019, 19, 9. [Google Scholar] [CrossRef]
- Li, H.M.; Zhao, S.H.; Qiang, J.W.; Zhang, G.F.; Feng, F.; Ma, F.H.; Li, Y.A.; Gu, W.Y. Diffusion kurtosis imaging for differentiating borderline from malignant epithelial ovarian tumors: A correlation with Ki-67 expression. J. Magn. Reson. Imaging 2017, 46, 1499–1506. [Google Scholar] [CrossRef]
- Lawrenson, K.; Fonseca, M.A.S.; Liu, A.Y.; Segato Dezem, F.; Lee, J.M.; Lin, X.; Corona, R.I.; Abbasi, F.; Vavra, K.C.; Dinh, H.Q.; et al. A Study of High-Grade Serous Ovarian Cancer Origins Implicates the SOX18 Transcription Factor in Tumor Development. Cell Rep. 2019, 29, 3726–3735.e3724. [Google Scholar] [CrossRef]
- Wallbillich, J.J.; Tran, P.M.; Bai, S.; Tran, L.K.; Sharma, A.K.; Ghamande, S.A.; She, J.X. Identification of a transcriptomic signature with excellent survival prediction for squamous cell carcinoma of the cervix. Am. J. Cancer Res. 2020, 10, 1534–1547. [Google Scholar]
- Mysona, D.P.; Tran, L.K.H.; Tran, P.M.H.; Gehrig, P.A.; Van Le, L.; Ghamande, S.; Rungruang, B.J.; Java, J.; Mann, A.K.; Liao, J.; et al. Clinical calculator predictive of chemotherapy benefit in stage 1A uterine papillary serous cancers. Gynecol. Oncol. 2020, 156, 77–84. [Google Scholar] [CrossRef]
- Ladbury, C.; Li, R.; Shiao, J.; Liu, J.; Cristea, M.; Han, E.; Dellinger, T.; Lee, S.; Wang, E.; Fisher, C.; et al. Characterizing impact of positive lymph node number in endometrial cancer using machine-learning: A better prognostic indicator than FIGO staging? Gynecol. Oncol. 2022, 164, 39–45. [Google Scholar] [CrossRef]

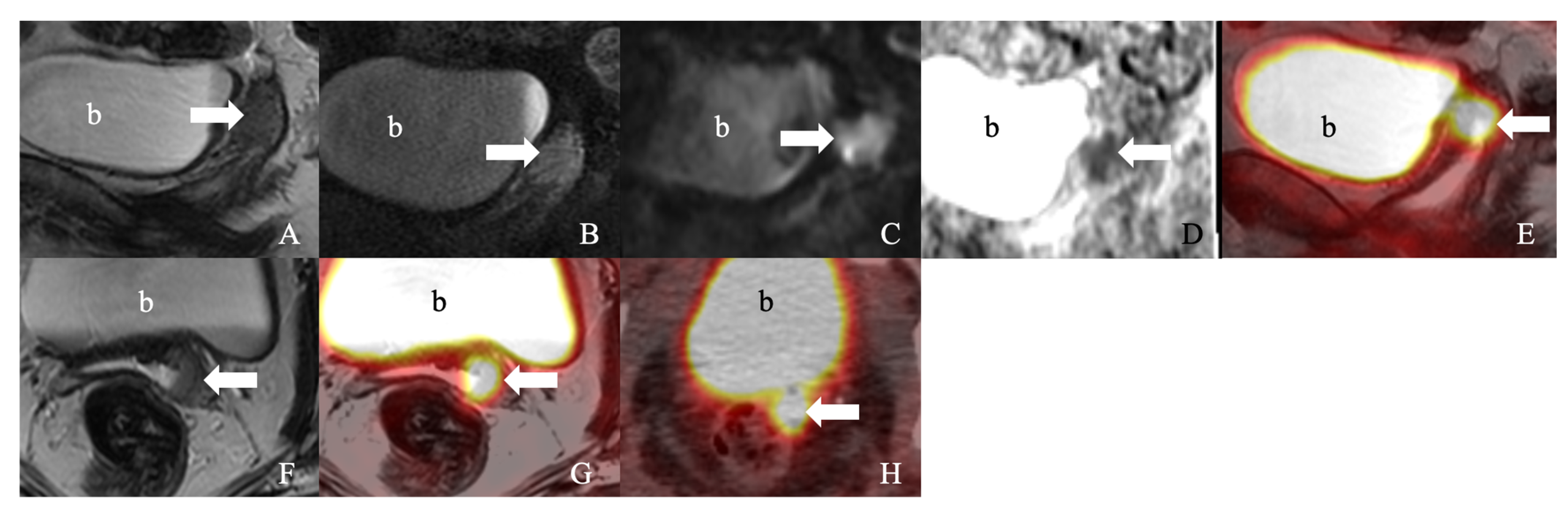
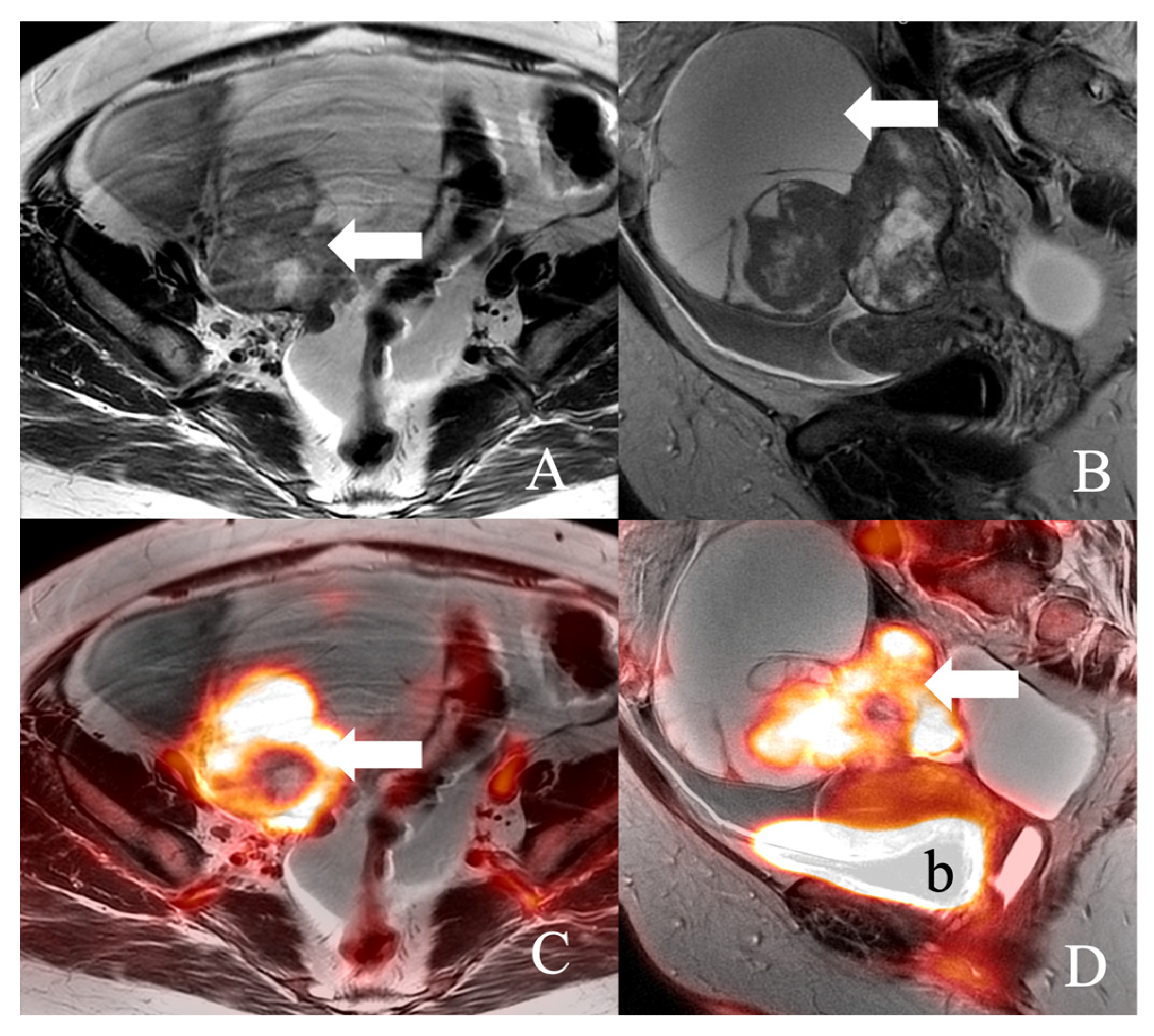
| Sr. No. | Series Description | Field of Vision | Slice Thickness | Spacing | Frequency Encoding | Frequency × Phase |
|---|---|---|---|---|---|---|
| 1 | Coronal T2WI | 420 | 5 | 0 | Superior/Inferior | 288 × 192 |
| 2 | Sagittal T2WI | 240 | 5 | 0 | Anterior/Posterior | 320 × 224 |
| 3 | FOV Sagittal b = 50, 600 | 240 | 5 | 0 | Superior/Inferior | 96 × 80 |
| 4 | Axial T2WI | 240 | 5 | 0 | Left/Right | 320 × 224 |
| 5 | Axial T1WI | 240 | 5 | 0 | Left/Right | 320 × 224 |
| 6 | Axial Diffusion b = 50, 400, 800 Sagittal Diffusion pelvis 0, 600 | 380 | 5 | 0 | Left/Right | 96 × 160 |
| 7 | Axial 3D pre-contrast T1WI | 240 | 5 | −2.5 | Left/Right | 320 × 224 |
| 8 | Dynamic | 240 | 5 | −2.5 | Superior/Inferior | 256 × 224 |
| 9 | Axial 3D post-contrast T1WI immediate delay | 240 | 5 | −2.5 | Left/Right | 320 × 224 |
| PET/MRI Sequences |
|---|
| Focused Pelvis (performed first). |
| Fused axial 3D T1WI LAVA. |
| Fused sagittal T2WI FSE. |
| Fused axial T2WI FSE. |
| Fused post-contrast axial T1WI. |
| Whole body (performed post-contrast after focused pelvis) |
| Auto-bind axial 3D T1WI LAVA water images. |
| Fused axial 3D T1WI LAVA. |
| Fused sagittal 3D T1WI LAVA (reformat). |
| Fused coronal 3D T1WI LAVA (reformat). |
| MIP PET MAC |
| Serial Number | Study | Year of Publication | Type of Study | Total Patient Number | Objective of Study | PET MRI Machine Details | Result | Limitations |
|---|---|---|---|---|---|---|---|---|
| 1 | Floberg et al. [51] | 2018 | Retrospective | 17 | To describe the relation between ADC and SUV values on MRI and PET imaging, respectively. | nMR-integrated PET/MRI | SUVmean and ADCmean (p = 0.007) and SUVmean and ADCT/M (p = 0.008) are inversely correlated. Such inverse correlation was not statistically significant when the tumors were divided into Adenocarcinomas and SCC. | Retrospective study with small sample size; Heterogeneous patient cohort including patients treated with surgery or chemoradiation and cancers of varied sizes, grades, histology, and stages. |
| 2 | Nguyen et al. [15] | 2020 | Prospective | 6 | To compare the diagnostic performance of FDG PET/MRI vs. PET/CT. | Discovery 710 PET/CT and Biograph mMR 3T scanner | There is a strong correlation between the tumor SUVs on PET/CT and PET/MRI (p < 0.001). PET/MRI has superior diagnostic interpretation and identified 4 of the 6 tumors not identified on PET/CT. | Small sample size; Lack of histological confirmation and correlation; Confounding bias as a result of the time gap between the two imaging methods |
| 3 | Surov et al. [48] | 2017 | Prospective | 21 | To study the relation between ADC and SUV values, and their importance in estimating tumor proliferation (KI 67). | Biograph mMR PET/MRI | SUVmax (p = 0.005), SUVmean (p = 0.04), ADCmin (p = 0.03), SUVmax/ADCmin (p = 0.001), and SUVmax/ADCmean (p = 0.001) are significantly correlated with KI-67 | Small sample size |
| 4 | Anner et al. [26] | 2016 | Retrospective | 27 | To study the quality of MRI, PET/CT, and PET/MRI in the lymph nodal staging of cervical carcinoma. Authors compared the diagnostic efficacy of imaging compared to histological analyses. | 64-row multidetector PET/CT, Magnetom trio 3-T MRI; PET/MRI images were reconstructed virtually from individual MRI and PET/CT images | PET/MRI has similar sensitivity (64%) and moderate specificity (77% vs. 69%), PPV (75% vs. 69%), and NPV (67% vs. 64%) compared to PET/CT images. Hence, the study concluded that PET/MRI is not superior to PET/CT in the lymph nodal staging of cervical cancer patients. | Small population; Retrospective study design; Discrepancy between the imaging and histological analyses; Virtually reconstructed PET/MRI images rather than originally obtained scanner images. |
| 5 | Wang et al. [20] | 2019 | Retrospective | 79 | To study the diagnostic efficacy of integrated PET/MRI in identifying the parametrial involvement and the importance of gray value while interpreting PET/MRI. | Signa PET/MRI (Integrated scanner) | The accuracy, sensitivity, and NPV of PET/MRI are higher than conventional MRI; however, it was not significant (p = 1.0). The accuracy, sensitivity, and NPV of combined PET/MRI+ gray values are significantly superior to conventional MRI (p < 0.05). | Retrospective analysis resulting in selection bias; Small sample size; No evaluation between multiple observers. |
| 6 | Narva et al. [30] | 2021 | Prospective | 9 | To evaluate the correlation between PET/MRI imaging (18F-EF5) and endogenous hypoxia (such as HIF1, CAIX, and GLUT1) tracers. | Ingenuity TF PET/MRI | 18F-EF5 max T/M ratio (p = 0.036) and HSV (p = 0.040) correlated with advanced-stage tumors and HSV correlated with tumor size (p = 0.02). | Small sample size; the chemistry of EF5 is complex, which may limit its broad application. |
| 7 | Brandmaier et al. [45] | 2015 | Prospective | 31 | To study the correlation between ADC and SUV values on simultaneous PET/MRI and their importance in primary and recurrent cervical cancer. | Magnetom Biograph mMR PET/MRI scanner | There was a significant inverse correlation between ADCmin and SUVmax (p = 0.05) and SUVmean and ADCmin (p = 0.03) in patients with primary tumors, primary metastases, and recurrent tumors (p = 0.002); No significant correlation among patients with recurrent metastases (p > 0.05). | Histopathological correlation was not performed; Included are the visible lesions on both imaging modalities; Average uptake time for FDG on PET/MRI is approximately 30 min, which could affect the SUV measurements. |
| 8 | Umutlu et al. [52] | 2020 | Prospective | 30 | To evaluate if PET/MRI can identify N- and M-staging of primary cervical cancers and, based on the results, if it can be a platform for radiomics analysis and artificial intelligence algorithms. | Biograph mMR PET/MRI scanner | PET/MRI is superior in determining the M-stage than the N-stage, with a sensitivity and specificity of 91% and 92%, respectively. AUC was 0.97 for the M-staging and 0.82 for the N-staging. | Small patient cohort; Heterogeneous histopathology and tumor sizes. |
| 9 | Meyer et al. [53] | 2018 | Prospective | 18 | To study the correlation between the parameters of cervical cancer’s histopathology and PET/MRI imaging. | Biograph mMR PET/MRI scanner | Authors identified no significant correlation between SUVmax, SUVmean, and ADC histogram parameters; Total lesion glycolysis was correlated inversely with p25, p75, p90, ADCmedian, and ADCmode. MTV also significantly corelated with ADCmean, p10, p25, p75, p90, ADCmedian, and ADCmode. | Retrospective study; Small sample size; Only squamous cell carcinomas were evaluated. |
| 10 | Sarabhai et al. [19] | 2017 | Prospective | 53 | To compare the efficacy of PET/MRI and MRI alone for evaluating primary and metastatic cervical tumors. | Biograph mMR whole-body PET/MRI scanner | T-staging: PET/MRI vs. MRI alone classified 85% vs. 87% of tumors (p > 0.1); N-staging: Sensitivity, specificity, and accuracy of PET/MRI were 83%, 90%, and 87%, respectively, and that of MRI alone were 71%, 83%, and 77%, respectively (p > 0.05); M-staging: Sensitivity, specificity, and accuracy of PET/MRI were 87%, 92%, and 91%, respectively, while that of MRI alone were 67%, 90%, and 83%, respectively (p > 0.05). | Small patient cohort and statistical power; Authors used restricted reference standards for all suspicious lesions. |
| 11 | Steiner et al. [16] | 2021 | Retrospective | 33 | To compare the efficiency of PET/MRI and MRI alone; Role of ADC and SUV values in primary cervical cancer. | Hybrid 3T Ingenuity TF PET/MRI scanner on a phased-array SENSE XL | PET/MRI has higher AUC compared to MRI alone in detecting deep stromal invasion (0.96 vs. 0.74), parametrial invasion (0.89 vs. 0.73), and vaginal invasion (0.85 vs. 0.74); PET/MRI is more sensitive than MRI alone in ruling out residual tumors after radical cone biopsy or hysterectomy (89% vs. 44%); PET/MRI has equal AUC to MRI alone in pelvic nodal staging (0.73 vs. 0.73) but not distant metastases (0.80 vs. 0.67). | Retrospective study; Small cohort; ADC values were obtained from ROI-based mean, rather than whole tumor volume. |
| 12 | Vojtisek et al. [54] | 2021 | Retrospective | 66 | To identify the role of PET/MRI in predicting tumor treatment response to chemoradiotherapy. | Biograph mMR PET/MRI scanner | The PET/MRI parameters, including mid-MTV, mid-TLG, mid-TLG-S, mid-MTV-s, mid-tumor size, and change in % SUVmax, were significantly different between the responders and non-responders. Of all the parameters, mid-MTV-s showed moderate discrimination ability to identify non-responders. | Small cohort; Shorter follow-up interval. |
| 13 | Ahangari et al. [55] | 2021 | Retrospective | 18 | To evaluate the workflow with PET/MRI in cervical cancer patients undergoing radiotherapy. | Biograph mMR PET/MRI scanner | PET images reconstructed with sCT and CT had no significant difference in quantification for all patients. | Residual error due to alignment issues between CT and MRI; As the weight is the limiting factor, one of the patients in the current study did not fit the PET/MRI coil holder. |
| 14 | Kim et al. [56] | 2009 | Retrospective | 79 | To study the efficacy of fusion PET/MRI in detecting of metastatic lymph nodes in cervical cancer. | Signa 1.5T MRI; Biograph LSO or Discovery LS PET/CT scanner; Images are fused using advantage windows workstation. | PET/MRI has higher diagnostic performance than PET/CT in identifying the metastatic lymph nodes (p = 0.0259); In addition, it has sensitivity and specificity of 54% and 93%, respectively, and that of PET/CT are 44% and 94%, respectively. | Verification bias as the surgeons are guided by pre-operative MRI and PET/CT; Node-by-node comparison was not performed; instead, the notable lymph node identified grossly or on imaging was considered. |
| 15 | Ahangari et al. [57] | 2022 | Prospective | 10 | To study the role of simultaneous PET/MRI in the characterization of tumor heterogeneity before chemoradiotherapy. | Biograph Vision 600 PET/CT scanner; Biograph mMR whole-body PET/MRI scanner | There was a strong correlation between the SUV and ADC values in patients with cervical cancer (r = −0.7). | Small patient population. |
| 16 | Azumi et al. [33] | 2021 | Retrospective | 149 | To study the risk factors associated with pelvic insufficiency fractures in cervical cancer and the role of PET/MRI in PIF diagnosis. | The pelvic insufficiency fractures were detected earlier on PET/MRI compared to PET/CT (p < 0.05). | Retrospective study; Measured SUV values on PET/CT and PET/MRI may differ due to the difference in detectors and reconstruction methods. | |
| 17 | Gong et al. [58] | 2021 | Retrospective | 114 | To study the role of PET/MRI as diagnostic imaging in cervical cancer. | Biograph Truepoint 64-row multidetector PET/CT and Magnetom Biograph mMR PET/MRI | PET/MRI is more sensitive (90–100% vs. 62–67%) and specific (96% vs. 93%) than PET/CT in detecting primary tumors and bladder invasion. The SUVmax and SUVmean values obtained on PET/MRI were higher than PET/CT in patients with primary tumors, bladder involvement, and para-aortic lymph nodal invasion (p < 0.001). The difference is insignificant in patients with vaginal (p = 0.3) or pelvic lymph node involvement (p = 0.4). | Different FDG uptake periods between the PET/CT and PET/MRI were considered; the diagnostic value of lymph nodal size was not analyzed |
| Serial Number | Study | Year of Publication | Type of Study | Total Patients in Study | Objective | PET MR Machine Details | Result | Limitations |
|---|---|---|---|---|---|---|---|---|
| 1 | Xin et al. [59] | 2016 | Prospective | 45 | To evaluate the diagnostic performance of PET/MRI in abdominal and pelvic tumors compared to PET/CT. | Discovery 690 PET/CT; Ingenuity TF PET/MRI scanner | There was no significant difference in tumor identification on PET/CT and PET/MRI (p = 0.18); However, PET/MRI images had better quality than PET/CT; There was an excellent correlation of SUV value to the focal lesions (R = 0.948). | PET/MRI was obtained 105 min after PET/CT, which might have led to physical decay and tracer biokinetics; The position of arms varied between the PET/CT and PET/MRI, which could be the reason for the difference in image quality. |
| 2 | Queiroz et al. [60] | 2015 | Prospective | 26 | To study the role of PET/CT and PET/MRI in staging and re-staging of advanced gynecological cancers. | Discovery PET/CT 690; the fusion was performed on the Advantage workstation | PET/MRI is superior to PET/CT for primary tumor identification (p < 0.001). No difference was found in the evaluation of lymph nodes and abdominal metastases. | Small patient population; PET/MRI was not obtained from whole-body imaging. |
| 3 | Spick et al. [61] | 2016 | Retrospective | 69 | To study whether PET/MRI has improved diagnostic performance in cancer assessment. | PET/MRI has similar diagnostic accuracy as PET/CT in the detection of primary and recurrent pelvic cancers; However, the diagnostic confidence of PET/MRI is higher than PET/CT in benign (p < 0.05) and malignant (p < 0.01) lesions. In addition, lesion conspicuity was better on PET/MRI compared to PET/CT. | ||
| 4 | Grueneisen et al. [62] | 2014 | Prospective | 48 | To study the role of DWI in PET/MRI imaging for primary and recurrent tumor evaluation. | Biograph mMR 3-T PET/MRI scanner | There was no significant effect of DWI on the diagnostic performance of PET/MRI (p > 0.05); In fact, higher diagnostic confidence was noted with PET than with the DWI (p < 0.05). | Included 48 patients; however, further studies are required to validate the results. Image and histopathological correlation were performed using restricted reference standards. |
| 5 | Schwartz et al. [63] | 2018 | Prospective | 18 | To compare the diagnostic ability of PET/MRI to PET/CT for patients with gynecologic malignancy. | Biograph mCT PET/CT scanner; Biograph mMR 3-T PET/MRI scanner | PET/CT and PET/MRI have similar diagnostic potential in visualizing the regional lymph nodes and abdominal metastases, whereas PET/MRI is more sensitive than PET/CT in demonstrating the soft-tissue involvement. | Small cohort; Heterogeneous sample; PET/MRI was limited to only abdominopelvic cavity. |
| 6 | Nakajo et al. [64] | 2010 | Retrospective | 31 | To compare the diagnostic accuracy of FDG PET/CT vs. PET/MRI in gynecological malignancies. | Fusion of PET/CT and MRI images was conducted using Osirix imaging software | PET/T2W MRI images localized the lesion better than the PET/T1W or PET/CT images during the first (p < 0.01), second (p < 0.01), and third (p < 0.01) evaluation. | Misregistration secondary to motion artifact between PET and MRI; Pelvis MR images, instead of whole-body MR images, were used for fusion images; Only two readers scored the images. |
| Serial Number | Study | Year of Publication | Type of Study | Total Patients in Study | Objective | PET MR Machine Details | Result | Limitations |
|---|---|---|---|---|---|---|---|---|
| 1 | Ironi et al. [67] | 2021 | Retrospective | 35 | To study the pre-operative diagnostic role of PET/MRI in assessing myometrial and lymph nodal involvement of endometrial cancer. | Signa Hybrid PET/MRI scanner | Lymph node involvement: PET/MRI demonstrated a sensitivity, specificity, accuracy, NPV, and PPV of 85%, 92%, 91%, 96%, and 75%, respectively; Myometrial invasion: PET/MRI demonstrated a sensitivity, specificity, accuracy, NPV, and PPV of 72%, 84%, 77%, 64%, and 88%. The MRI- and PET-derived tumor volume, volume index, tumor–volume ratio, and total-lesion glycolysis were significant predictors of lymph nodal space invasion (p = 0.002, 0.006, 0.002, 0.013, respectively). | Small study population. |
| 2 | Jonsdottir et al. [73] | 2021 | Prospective | 34 | To investigate the efficacy of PET/MRI, in comparison to DWI–MRI, for evaluating peritoneal carcinomatosis in gynecological tumors. | Signa 3T PET/MRI scanner | The PCI of PET/MRI (p-0.6) is closer to surgical PCI than DWI–MRI (p = 0.007). In addition, PET/MRI is a useful tool that aids in deciding the operability of cancer. | Small cohort of patients; Included the patients who received neoadjuvant chemotherapy and those who were primarily operated on. |
| 3 | Tsuyoshi et al. [74] | 2020 | Prospective | 36 | To study the role of PET/MRI in pre-operative staging of endometrial cancer. | Signa PET/MRI scanner | PET/MRI is equivalent to contrast-enhanced in assessing nodal and distant metastatic staging (p > 0.05); hence, it can be considered an alternative imaging modality during the pre-operative staging of endometrial cancer. | Retrospective study; A few MRI images were not performed at the author’s institution, however, were re-read by the radiologists at the author’s institution; Small population study; Histological correlation was not performed in 36% of patients, as they did not undergo lymphadenectomy. |
| 4 | Bian et al. [75] | 2019 | Retrospective | 81 | To evaluate the diagnostic performance of PET/MRI and PET/CT for regional lymph node metastases and myometrial involvement in endometrial cancer. | Biograph 64 PET/CT; Biograph mMR PET/MRI scanner | Regional lymph node metastases: PET/MRI has superior sensitivity (p = 0.015) and specificity (p < 0.001) than PET/CT; Myometrial involvement: Accuracy of PET/MRI is higher than PET/CT (82% vs. 46%). | Patients did not undergo simultaneous PET/CT and PET/MRI; Retrospective study; Small study population. |
| 5 | Tsuyoshi et al. [69] | 2019 | Retrospective | 31 | To evaluate the role of PET/MRI in determining the phenotypes of endometrial cancer. | Signa 3-T PET/MRI scanne | Lower ADC values (p < 0.05) and higher SUV-to-ADC ratio (p < 0.005) are associated with high-risk cancers more than low-risk cancers. In turn, the SUV-to-ADC ratio demonstrated higher diagnostic accuracy and higher AUC values (p < 0.05); The SUV-to-ADC ratio cut-off value of 16.9 × 109 has a sensitivity, specificity, and accuracy of 73%, 81%, and 77%, respectively, in predicting high-risk cancer groups. | Small sample size; Mean SUV and ADC values at the center of the lesion were considered. |
| 6 | Bezzi et al. [76] | 2021 | Systematic review and meta-analysis | To study the role of PET/MRI in staging and re-staging of endometrial cancer. | PET/MRI has the highest diagnostic accuracy in detecting the soft-tissue involvement and metastases. SUV to ADC ratio seems to be a more reliable index to describe the aggressiveness of endometrial cancer; PET/MRI detects the post-therapeutic changes of the lesion and even small recurrent lymph node lesions. | Small cohort study; Heterogeneous patient population. | ||
| 7 | Stecco et al. [77] | 2016 | Retrospective | 27 | To evaluate the clinical utility of retrospective fusion PET/MRI–DWI obtained through individual PET/CT and MRI–DWI. | Biograph 16 HI-REZ PET/CT; Achieva Intera 1.5T MRI scanner | Although on a per-patient basis PET/MRI has similar sensitivity, specificity, and accuracy to PET/CT, PET/MRI is superior in terms of per-node basis. The sensitivity (89% vs. 70%), specificity (92% vs. 91%), accuracy (91% vs. 87%), PPV (69% vs. 60%), and NPV (98% vs. 94%) are better for PET/MRI than PET/CT. There was no significant difference between PET/CT and PET/MRI–DWI in the detection of metastatic lymph nodes. | Small sample size; Pelvic MR images are considered in the study instead of whole-body MRI; Scores were subjective, and only two interpreters were involved in the study. |
| 8 | Shih et al. [70] | 2015 | Prospective | 36 | To study the correlation between SUVmax and ADCmin, and their roles in determining the prognosis. | Biograph mMR PET/MRI scanner | SUVmax and ADCmin are inversely correlated with each other (p = 0.001); Higher SUVmax and lower ADCmin are associated with advanced tumor stages (p < 0.05); Study also found that a higher ratio of SUVmax to ADCmin is indicative of advanced tumor (p < 0.05). | Small sample size; Diagnostic accuracy of PET/MRI during the pre-operative period of endometrial cancer was not evaluated; Correlation of SUVmax and ADCmin to the parameters, including disease-free and overall survival, was not studied. |
| Serial Number | Study | Year of Publication | Type of Study | Total Number of Patients in the Study | Objective of the Study | PET MR Machine Details | Results | Limitations |
|---|---|---|---|---|---|---|---|---|
| 1 | Sawicki et al. [96] | 2017 | Retrospective | 71 | To evaluate the impact of PET/MRI findings in the management of recurrent pelvic cancer, compared with MRI alone. | Biograph mMR PET/MRI scanner | PET/MRI is significantly superior in categorizing malignant lesions compared to MRI (99.2% vs. 79.3%; p < 0.001). It identified 100% of cancer recurrence compared to MRI, which identified only 83.6% of the recurrent tumors (p < 0.01). | Histopathological sampling was not performed; Heterogeneous patient population in terms of cancer types. |
| 2 | Grueneisen et al. [97] | 2014 | Prospective | 34 | To compare the diagnostic value of whole-body PET/MRI to whole-body MRI in the assessment of recurrent gynecological pelvic malignancies. | Biograph mMR PET/MRI scanner | In addition to superior lesion contrast and diagnostic confidence (p <0.001), PET/MRI identified 99% of malignant lesions compared to MRI alone, which discovered 89% of the malignant lesions. | Small sample size; Absence of standard criteria for the histological confirmation of the suspected malignant lesions. |
| 3 | Kirchner et al. [98] | 2017 | Retrospective | 43 | To study the diagnostic performance of ultrafast PET/MRI sequences (T2W, contrast-enhanced T1W, and SUV) compared to PET/CT and CT for the staging of recurrent pelvic cancers. | Biograph mMR PET/MRI scanner, Biograph mCT PET/CT | PET/MRI identified tumor recurrence equivalent to PET/CT. In addition, PET/MRI and PET/CT have equivalent diagnostic accuracies (94% vs. 92%), compared to the lower value of CT (53%). | Small patient cohort. |
| 4 | Grueneisen et al. [99] | 2015 | Retrospective | 24 | To compare the efficacy of fast PET/MRI and PET/CT in whole-body staging for recurrent pelvic malignancies. | Biograph mMR PET/MRI scanner | Although fast PET/MRI and PET/CT demonstrated equivalent diagnostic performance (86% vs. 84%), PET/MRI enables high-quality tumor restaging, though with a slightly prolonged scan duration. | Retrospective study; fast PET/MRI protocol is based on pre-obtained prolonged examination protocols; There is a time delay between the PET/MRI and PET/CT acquisitions that might have provided a gap for alteration in tumor metabolic activity. |
| 5 | Beirderwellen et al. [29] | 2014 | Retrospective | 19 | To study the diagnostic performance of PET/MRI and PET/CT in the evaluation of recurrent ovarian and cervical cancers. | Biograph mMR PET/MRI scanner; Biograph mCT 128 PET/CT scanner | Although the lesions were equivalently identified on PET/MRI and PET/CT (p > 0.05), the PET/MRI demonstrated higher diagnostic confidence in malignant (p < 0.01) and benign (p < 0.05) lesions. | Limited patient cohort; Modified reference standard was applied for the histological tumor classification. |
| 6 | Kitajima et al. [100] | 2013 | Retrospective | 30 | To study the accuracy of retrospectively fused PET and MRI images in the assessment of locoregional and nodal staging of endometrial cancer. | Discovery PET/CT 690, Signa Echo speed plus Excite MRI; Fused PET/MR images on advantage windows workstation | Fused PET/MRI is equivalent to MRI in regards to T-staging and PET/CT in regards to N-staging. | Retrospective study; PET/CT was not performed in all cases; Small sample size; Histopathological correlation was not possible for 53% of cases, as they did not undergo lymphadenectomy; Pelvic MR images instead of whole-body images were used for PET/MRI fusion; hence, distant metastases could not be studied. |
| Serial Number | Study | Year of Publication | Type of Study | Total Number of Patients in Study | Objective of Study | PET MR Machine Details | Results | Limitations |
|---|---|---|---|---|---|---|---|---|
| 1 | Tsuyoshi et al. [93] | 2020 | Retrospective | 103 | To evaluate the diagnostic value of PET/MRI in ovarian cancer. | Whole-body PET/MRI scanner | PET/MRI has superior sensitivity and specificity than ceCT and ceMRI on M-staging and has equivalent diagnostic potential to ceCt/ceMRI on T- and N-staging. | Retrospective study; Small sample size; Heterogeneous population; Histopathological correlation could not be performed in a few patients. |
| Serial Number | ClinicalTrials.gov Identifier | Recruitment Status | Location (Sponsor/Collaborator/Country) | Study Design (Study Type/Actual Enrollment) | Official Title | Outcome Measure (Primary Outcome) | Outcome Measure (Secondary Outcome) |
|---|---|---|---|---|---|---|---|
| 1 | NCT03965481 | Recruiting | M.D. Anderson Cancer Center, Houston/National Cancer Institute (NCI)/United States | Interventional/60 Participants | Comparing Accuracy of PET/MRI vs. ceCT in Assessment of Peritoneal Disease for resectability in Patients with Ovarian Cancer or Highly Suspected Ovarian Cancer | The accuracy of lesion detection will be summarized by modality using frequencies and percentages. The McNemar test will be utilized to compare the accuracies of PET/MRI and ceCT. Other diagnostic metrics (sensitivity, specificity, positive predictive value, and negative predictive value), and 95% confidence intervals will be estimated. The effect of patient and tumor characteristics on diagnostic precision will be evaluated using a logistic regression model. | Diagnostic accuracy by location will be analyzed using linear regression or generalized linear regression models where applicable. Where applicable, response status will be analyzed using linear regression or generalized linear regression models. Imaging and genomic data analysis correlation between imaging and genomic data will be analyzed using linear regression or generalized linear regression models where applicable. |
| 2 | NCT04454450 | Active, not recruiting | Memorial Sloan Kettering Cancer Center, New York/Not provided/United States | Interventional/10 participants | Integration of Radiomic Analysis into the Multi-Modal Profiling of High-Grade Serous Ovarian Cancer | Radiologist-defined tumor volumes will be used to train and validate a machine-learning algorithm for generating segmentations. The data will be split into 70–30% training and test sets. The comparisons will be performed on a slice-by-slice and lesion-by-lesion basis, which should ensure sufficient examples for testing. | Not Provided |
| 3 | NCT03302156 | Recruiting | University of Wisconsin, Madison/University of Wisconsin, Madison/United States | Interventional/52 participants | PSMA PET/MRI in Gynecological Cancers | Estimate the frequency with which PSMA PET and MR imaging and final IHC staining disagree in their classifications of the presence of disease. | Record the normal biodistribution of PSMA as detected in normal female controls by the resulting PET imaging. The radiodosimetry of PSMA-based 18F-DCFPyL will be measured in normal female controls via the resulting PET images. Record the distribution of PSMA in cancer tissue. |
| 4 | NCT04212910 | Recruiting | IRCCS San Raffaele/IRCCS San Raffaele/Italy | Observational/101 participants | Stratifying Endometrial Cancer Patients Using a PET/MRI Prognostic Model | MRI evaluation with tumor volume measurement and correlation to surgical specimen, as well as changes in ADC normal versus tumor myometrium. Myometrial invasion depth. DCE-MRI perfusion parameters will distinguish between normal and tumor myometrium perfusion. Positive lymph node evaluation. Evaluation of PET imaging with PET-positive lymph nodes and SUV values for tumor/positive lymph node/metastases. | Measure tumor angiogenesis with angiogenesis marker CD1. Correlate PET- and MRI-derived functional and morphological parameters with histology using imaging-derived parameters correlated with histopathology. |
| 5 | NCT05483023 | Not yet recruiting | University of North Carolina Lineberger Comprehensive Cancer Center/Radiological Society of North America/United States | Interventional/8 participants | 18-FFNP PET/MRI as a Potential Biomarker of Response to Progesterone Therapy in CAH and Grade 1 Endometrial Cancer | Sensitivity of 18F-FFNP PET/MRI for predicting response to progestin therapy in CAH/EC patients and specificity of 18F-FFNP PET/MRI for predicting response to progestin therapy in CAH/Endometrial cancer patients. | First, to correlate FFNP Mean Standardized Uptake Value (SUVmean) at baseline and on repeat examination with estrogen and progesterone receptor expression in the CAH/Endometrial cancer tissues at baseline and after six months of treatment. Second, to correlate FFNP SUVmax at baseline and on repeat examination with estrogen and progesterone receptor expression in the CAH/Endometrial cancer tissues at baseline and after six months of treatment. |
| 6 | NCT02285192 | Active, not recruiting | Memorial Sloan Kettering Cancer Center/Memorial Sloan Kettering Cancer Center/United States | Interventional/42 participants | Positron Lymphography Via Intracervical 18F-FDG Injection for Pre-surgical Lymphatic Mapping in Stage IB1 Cervical Cancer and High-grade Endometrial Cancer | The diagnostic accuracy of the Positron Lymphograph will be defined in terms of sensitivity and will consist of a pathology review of labeled, excised specimens compared with lymph node imaging data acquired preoperatively. | To evaluate several standard uptake values (SUV) (18F-FDG avidity), they will assess the ability of SUV to predict malignant disease. The continuous variable of SUV assigned to a given lymph node during Positron Lymphography will be compared with each labeled lymph node’s pathologic assessment (benign vs. malignant). The SUV assigned to a given lymph node is performed using imaging software and is not up to the radiologist’s discretion. |
| 7 | NCT05390021 | Not yet recruiting | Massachusetts General Hospital/Massachusetts General Hospital/United States | Interventional/33 participants | Diagnostic Performance of PET/MRI Versus Standard of Care Imaging (PET/CT and/or CT and/or PET) in Preoperative Women With Presumed Early-stage High-Grade Endometrial Carcinoma | Percentage of patients with a metastatic lesion noted on PET/MRI that have a true malignancy noted on surgical pathology of that lesion (Sensitivity of PET/MRI to detect metastatic lesions). Percentage of patients without any metastatic lesions noted on PET/MRI that do not have malignancy noted on surgical pathology (Specificity of PET/MRI to detect an absence of metastatic lesions). | Not Provided |
| 8 | NCT05480995 | Not yet recruiting | University of North Carolina, Chapel Hill/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)/United States | Interventional/24 participants | Evaluation of Endometriosis With 18F-FFNPe PET/MRI | Sensitivity and Specificity of 18F-FFNP PET/MRI for evaluating endometriosis. | Diagnostic accuracy of PET/MRI. Correlation of uptake values (SUV-max) with EHP-30scale controlling for covariates. Correlation of uptake values (SUV-max) with pain level using a controlling for covariates. |
| 9 | NCT04347135 | Enrolling by invitation | Annie (Annie) T. Packard, Mayo Clinic/Annie (Annie) T. Packard/United States | Interventional/10 participants | Pilot Study Evaluating Endometriosis With 16α-(18)F-fluoro-17β-estradiol ([F-18] FES) PET/MRI | Detection of Endometriosis: Comparisons will be made descriptively between conventional MRI imaging, FES PET/MRI imaging, and surgical/pathological findings. Outcome data include a number of detected lesions, differences in the accuracy of detection of active disease versus inactive fibrosis, and confidence score. | Not Provided |
| 10 | NCT04219904 | Recruiting | M.D. Anderson Cancer Center/National Cancer Institute (NCI)/United States | Interventional/25 participants | Evaluation of Resectable Cervical Carcinoma With PET/MRI | Accuracy of diagnosing the depth of invasion on positron emission tomography/magnetic resonance imaging (PET/MRI). | Determine the relationship with pathology while assessing the lymph node involvement by PET/MRI. Inter-observer variability of PET/MRI. Quantitative imaging parameters of the tumor to correlate volumetric size, BOLD, ADC, IVIM analysis, DTI, DCE, MTV, TLG, standardized uptake value (SUV), and GMR with LVSI and tumor grade on surgical pathology which serves as the gold standard. |
| 11 | NCT01899404 | Active, not recruiting | University Health Network, Toronto/Princess Margaret Hospital, Canada/Canada | Interventional/25 participants | A Pilot Prospective Study of the Utility of DCE-MRI, Diffusion Weighted MRI (DWI), and Positron Emission Tomography (PET) Imaging With 18F-Fluorodeoxyglucose (18FDG) in Brachytherapy for Cervix Cancer | Target delineation in brachytherapy using standard T2-weighted MRI versus DCE-MRI, DWI, and FDG-PET/CT imaging: gross tumor volume and high-risk clinical target volume in patients with cervical cancer. Target delineation using standard MRI acquired during the last week of EBRT fused to planning CT versus full MRI-guided brachytherapy. | Follow-up imaging (MRI and 18FDG PET) versus the imaging done at the time of brachytherapy. Imaging techniques for visualizing the brachytherapy applicator. |
| 12 | NCT05355558 | Recruiting | National Cancer Center, Singapore/National Cancer Center, Singapore/Singapore | Interventional/15 participants | Novel Functional Imaging Technique With FLT-PET/MRI For Staging, Response Assessment, and Radiation Treatment Planning in Cervix Cancer | To determine the feasibility of [18F]FLT-PET/MRI imaging for early prediction of treatment by comparison of changes in baseline SUV uptake at week 4–5 of External Beam Radiotherapy. | Compare SUV uptake of FLT with FDG PET at diagnosis. Compare SUV uptake of FLT before and after chemoradiotherapy. To compare differences in tumor and regional staging between PET/MRI, PET/CT, and MRI scans, determined from the tumor size and extent of local involvement. Assess the feasibility of PET/MRI in the radiation treatment planning workflow with respect to the adequacy of image quality and image fusion of PET/MRI data with the treatment planning CT for marrow-sparing RT plan. Compare changes in stimulated radiation treatment volume derived from PET/MRI vs. PET/CT vs. MRI. Compare VMAT versus IMRT versus proton versus tomotherapy for the best marrow-sparing plan. To determine the correlation of [18F]FLT parameters as a baseline during treatment and change in [18F] FLT parameters with clinical outcome and response. |
| Serial Number | Study | Year of Publication | Type of Study | Total Patients in Study | Objective | Result | Limitation |
|---|---|---|---|---|---|---|---|
| 1 | Virarkar et al. [105] | 2020 | Systematic review and meta-analysis | Meta-analysis of studies performed on PET/MRI in gynecologic cancers. | Patient-based analysis: Sensitivity, specificity, diagnostic odds ratio, and AUC of PET/MRI were 74%, 90%, 26, 0.834, respectively; While the respective values were 88%, 88%, 50, and 0.922, respectively, on lesion-based analyses. | The studies that were included were heterogeneous; Publication bias could not be ruled out entirely; Lack of standard guidelines, scanning protocols, and timing of PET/MRI among the included studies. | |
| 2 | Nie et al. [106] | 2017 | Systematic review and meta-analysis | Meta-analysis of studies performed on PET/MRI in gynecologic cancers. | Patient-based analysis: Sensitivity and specificity of PET/MRI were 95% and 95%, respectively, while the values for lesion-based analyses were 89% and 87%, respectively. | Heterogeneity of the studies; Publication-biased tests were not performed; Lower number of studies were included. | |
| 3 | Nguyen et al. [1] | 2020 | Systematic review and meta-analysis | Systematic review of studies on the role of PET/MRI in gynecological malignancies. | PET/MRI has comparable efficacy to PET/CT in the staging and restaging of gynecologic tumors; Mild-to-moderate inverse correlation was observed between SUV and ADC values which may predict the risk stratification and grading. | ||
| 4 | Virarkar et al. [107] | 2020 | Systemic review and meta-analysis | Meta-analyses of the studies to compare the diagnostic performance of PET/CT and PET/MRI in gynecological malignancies. | Patient-based analysis: Sensitivity, specificity of PET/CT were 63% and 92%, while that of PET/MRI were 73% and 92%, respectively. Lesion-based analysis: Sensitivity and specificity of PET/CT were 82% and 87%, while that of PET/MRI were 85% and 89%, respectively. | Small cohort of studies; High heterogeneity; Publication biases could not be excluded completely; Spectrum bias due to non-standardized protocols in the included studies. | |
| 5 | Zheng et al. [103] | 2018 | Systemic review and meta-analysis | Meta-analyses of studies to describe the role of PET/MRI in recurrent female pelvic malignancies; malignancies of female patients using a meta-analysis. | Patient-based analysis: Sensitivity and specificity of PET/MRI were 96% and 95%, respectively, while the respective values based on lesion analysis were 99% and 94%, respectively. | Small cohort of studies; Unpublished studies were not included; 57% of the included studies were retrospective. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virarkar, M.; Vulasala, S.S.; Calimano-Ramirez, L.; Singh, A.; Lall, C.; Bhosale, P. Current Update on PET/MRI in Gynecological Malignancies—A Review of the Literature. Curr. Oncol. 2023, 30, 1077-1105. https://doi.org/10.3390/curroncol30010083
Virarkar M, Vulasala SS, Calimano-Ramirez L, Singh A, Lall C, Bhosale P. Current Update on PET/MRI in Gynecological Malignancies—A Review of the Literature. Current Oncology. 2023; 30(1):1077-1105. https://doi.org/10.3390/curroncol30010083
Chicago/Turabian StyleVirarkar, Mayur, Sai Swarupa Vulasala, Luis Calimano-Ramirez, Anmol Singh, Chandana Lall, and Priya Bhosale. 2023. "Current Update on PET/MRI in Gynecological Malignancies—A Review of the Literature" Current Oncology 30, no. 1: 1077-1105. https://doi.org/10.3390/curroncol30010083
APA StyleVirarkar, M., Vulasala, S. S., Calimano-Ramirez, L., Singh, A., Lall, C., & Bhosale, P. (2023). Current Update on PET/MRI in Gynecological Malignancies—A Review of the Literature. Current Oncology, 30(1), 1077-1105. https://doi.org/10.3390/curroncol30010083





