“Urethral-Sparing” Robotic Radical Prostatectomy: Critical Appraisal of the Safety of the Technique Based on the Histologic Characteristics of the Prostatic Urethra
Abstract
1. Introduction
- (1)
- the plane failed to develop easily,
- (2)
- the urethra muscle became too thin (reaching mucosa or tearing),
- (3)
- or the dissection reached the transected bladder neck (i.e., complete urethral sparing).
2. Materials and Methods
- (1)
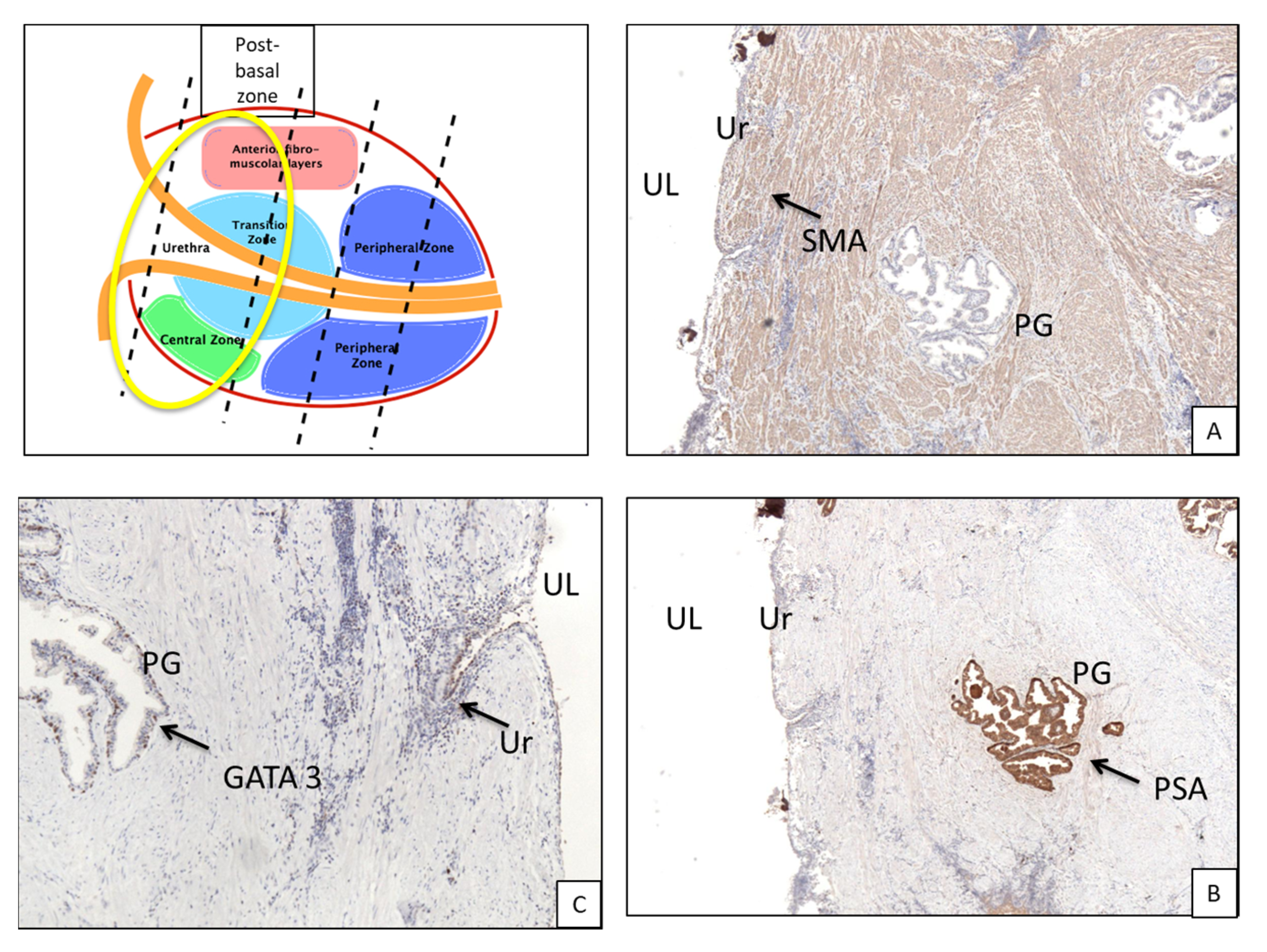
- Post-basal zone: between first post-basal section and verumontanum,
- (2)
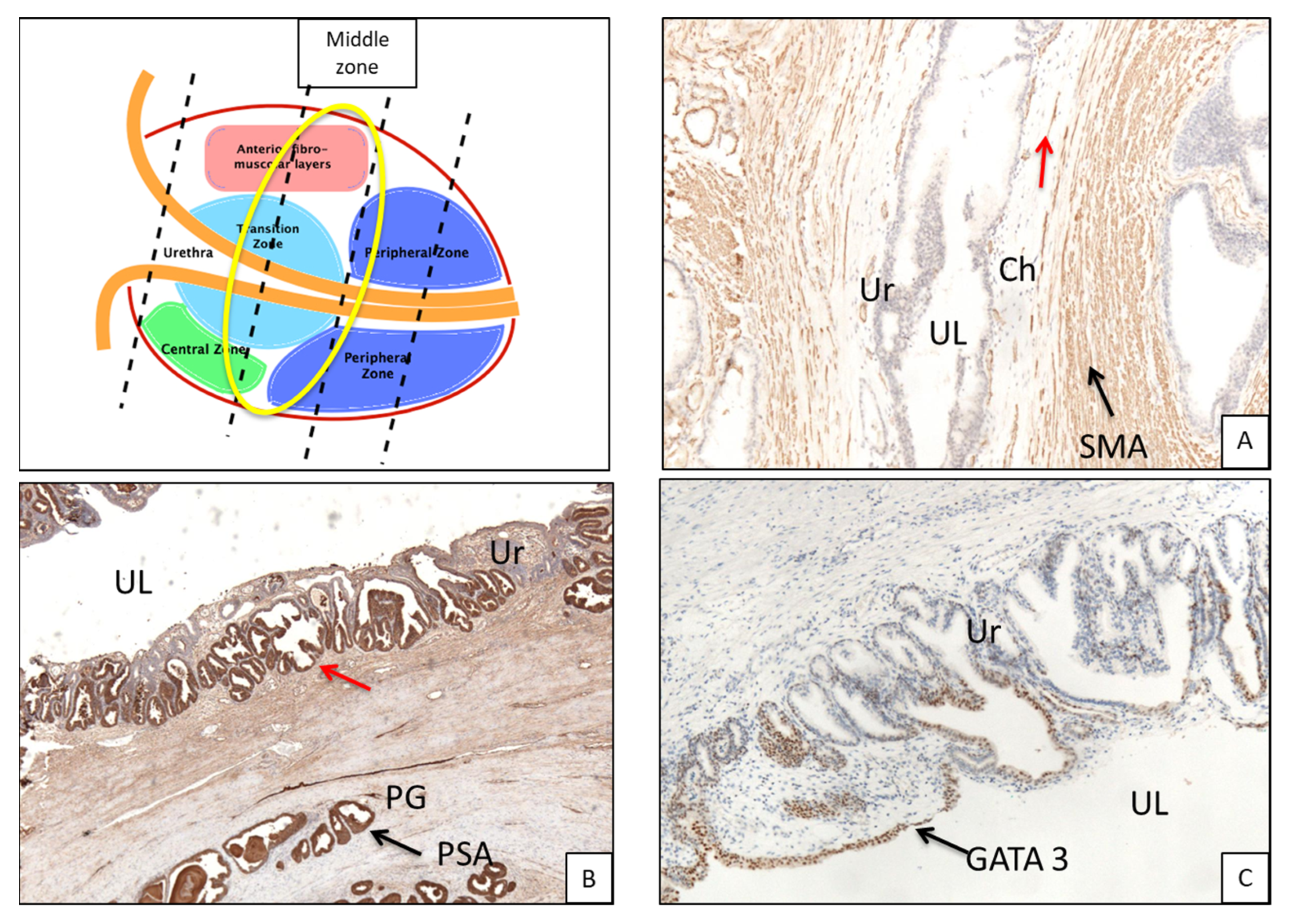
- Middle zone: between second half of verumontanum and pre-apical sections,
- (3)
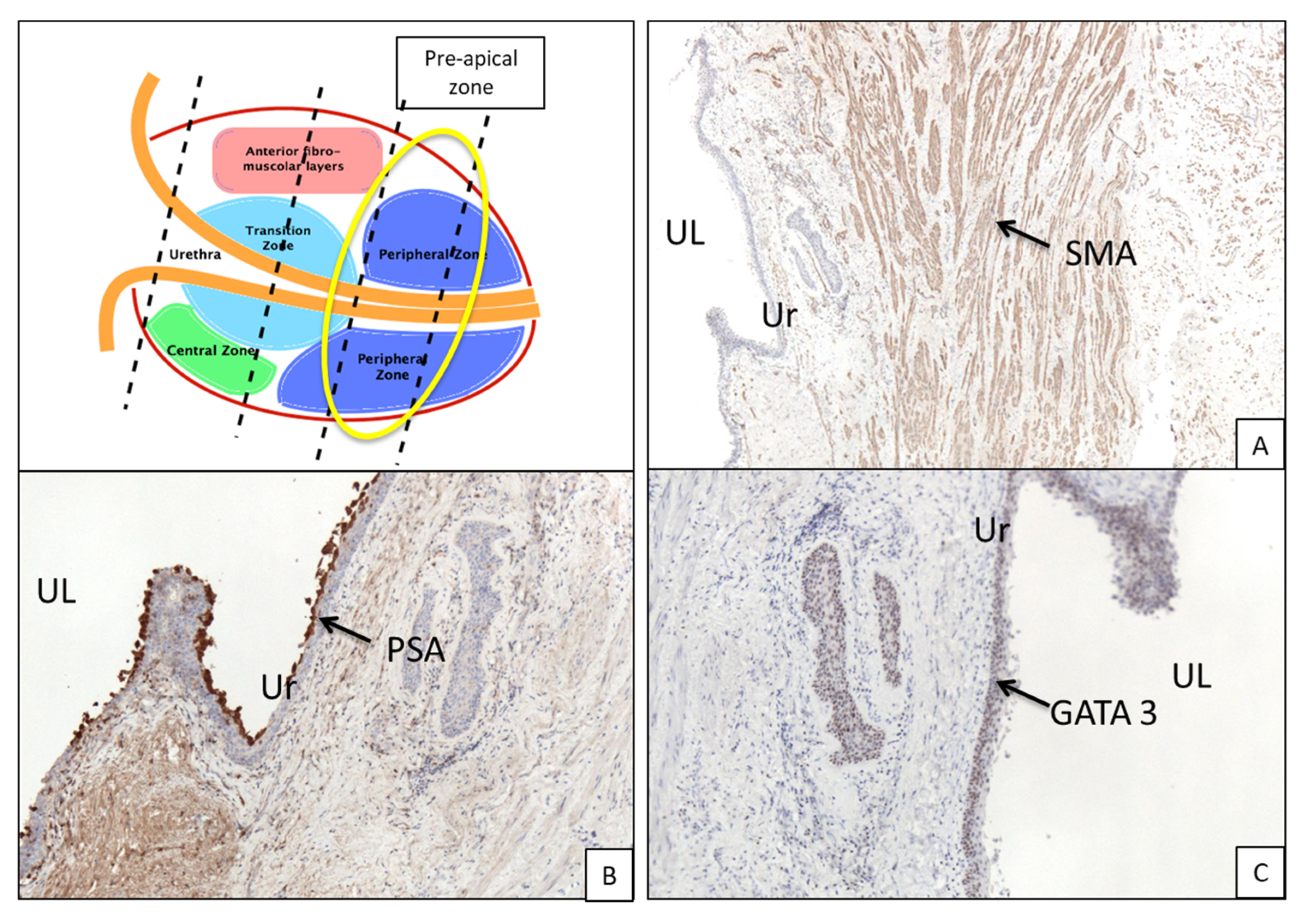
- Pre-apical zone: remaining pre-apical sections.
3. Results
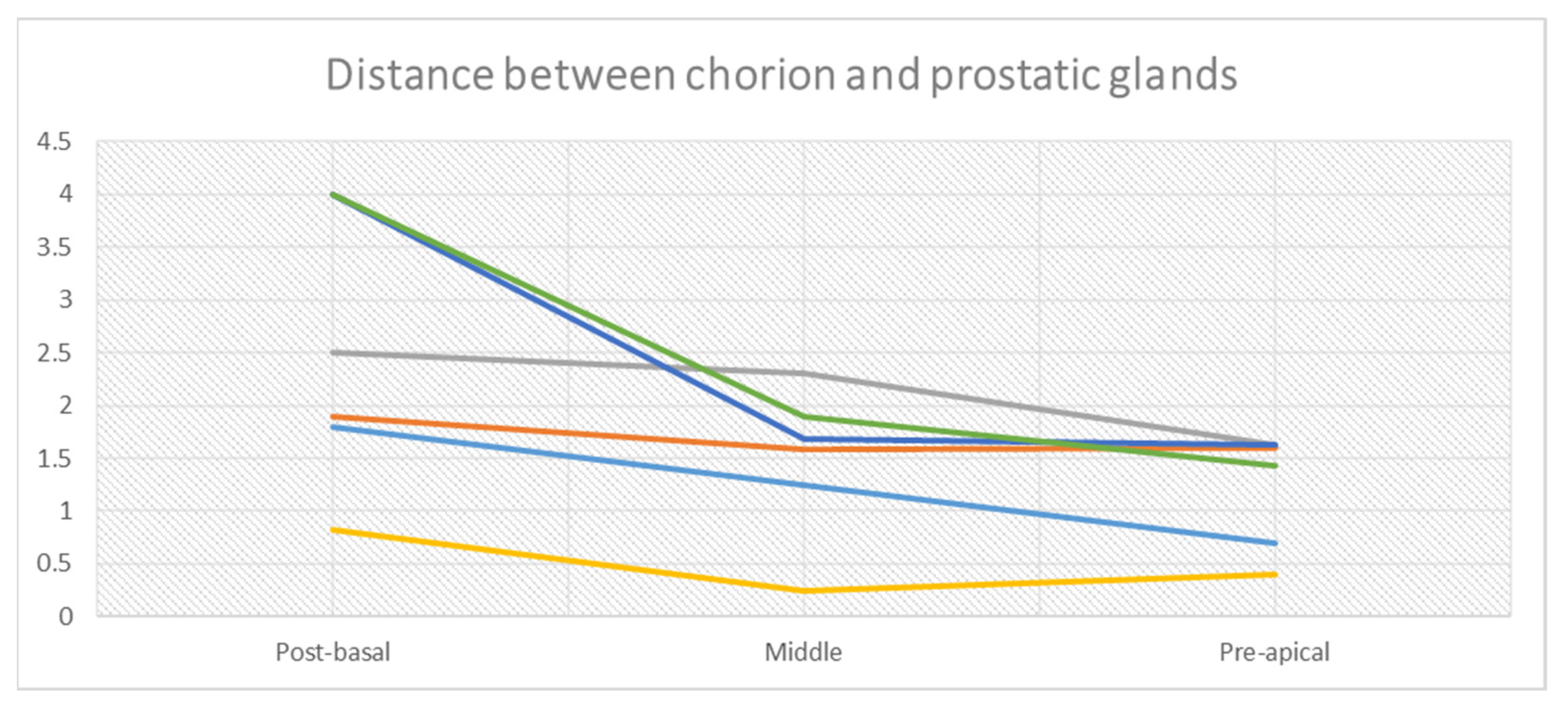
3.1. Histologic Features of the PU. Distance between Basal Membrane of the PU Epithelium and the Prostatic Glandular Tissue
- (1)
- urothelium with basement membrane,
- (2)
- subepithelial connective tissue (SCT) (chorion),
- (3)
- prostatic periurethral muscular tissue (PPUM).
3.2. Immunohistochemistry (Figure 3B,C and Figure 5B,C)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bill-Axelson, A.; Holmberg, L.; Ruutu, M.; Häggman, M.; Andersson, S.O.; Bratell, S.; Spångberg, A.; Busch, C.; Nordling, S.; Garmo, H.; et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N. Engl. J. Med. 2005, 352, 1977–1984. [Google Scholar] [CrossRef]
- Bauer, R.M.; Bastian, P.J.; Gozzi, C.; Stief, C.G. Postprostatectomy incontinence: All about diagnosis and management. Eur. Urol. 2009, 55, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Cambio, A.J.; Evans, C.P. Minimising postoperative incontinence following radical prostatectomy: Considerations and evidence. Eur. Urol. 2006, 50, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.P.; Weinberg, A.C.; Lei, Y.; Soukup, J.R.; Lipsitz, S.R.; Prasad, S.M.; Korkes, F.; Lin, T.; Hu, J.C. Anatomic bladder neck preservation during robotic-assisted laparoscopic radical prostatectomy: Description of technique and outcomes. Eur. Urol. 2009, 56, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Hamada, A.; Razdan, S.; Etafy, M.H.; Fagin, R.; Razdan, S. Early return of continence in patients undergoing robot-assisted laparoscopic prostatectomy using modified maximal urethral length preservation technique. J. Endourol. 2014, 28, 930–938. [Google Scholar] [CrossRef]
- Nunez Bragayrac, L.A.; Hussein, A.A.; Attwood, K.; Pop, E.; James, G.; Osei, J.; Murekeysoni, C.; Kauffman, E.C. Feasibility and continence outcomes of extended prostatic urethral preservation during robot-assisted radical prostatectomy. Prostate Cancer Prostatic Dis. 2020, 23, 286–294. [Google Scholar]
- Van der Kwast, T.H.; Amin, M.B.; Billis, A.; Epstein, J.I.; Griffiths, D.; Humphrey, P.A.; Montironi, R.; Wheeler, T.M.; Srigley, J.R.; Egevad, L.; et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 2: T2 substaging and prostate cancer volume. Mod. Pathol. 2011, 24, 16–25. [Google Scholar] [CrossRef]
- Coelho, R.F.; Chauhan, S.; Palmer, K.J.; Rocco, B.; Patel, M.B.; Patel, V.R. Robotic-assisted radical prostatectomy: A review of current outcomes. BJU Int. 2009, 104, 1428–1435. [Google Scholar] [CrossRef]
- Ficarra, V.; Novara, G.; Artibani, W.; Cestari, A.; Galfano, A.; Graefen, M.; Guazzoni, G.; Guillonneau, B.; Menon, M.; Montorsi, F. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: A systematic review and cumulative analysis of comparative studies. Eur. Urol. 2009, 55, 1037–1063. [Google Scholar] [CrossRef]
- Heesakkers, J.; Farag, F.; Bauer, R.M.; Sandhu, J.; De Ridder, D.; Stenzl, A. Pathophysiology and contributing factors in postprostatectomy incontinence: A review. Eur. Urol. 2017, 71, 936–944. [Google Scholar] [CrossRef]
- Sanda, M.G.; Dunn, R.L.; Michalski, J.; Sandler, H.M.; Northouse, L.; Hembroff, L.; Lin, X.; Greenfield, T.K.; Litwin, M.S.; Saigal, C.S.; et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N. Engl. J. Med. 2008, 358, 1250–1261. [Google Scholar] [CrossRef]
- Prabhu, V.; Sivarajan, G.; Taksler, G.B.; Laze, J.; Lepor, H. Long-term continence outcomes in men undergoing radical prostatectomy for clinically localized prostate cancer. Eur. Urol. 2014, 65, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.L.; Hamdy, F.C.; Lane, J.A.; Mason, M.; Metcalfe, C.; Walsh, E.; Blazeby, J.M.; Peters, T.J.; Holding, P.; Bonnington, S.; et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N. Engl. J. Med. 2016, 375, 1425–1437. [Google Scholar]
- Barry, M.J.; Gallagher, P.M.; Skinner, J.S.; Fowler, F.J., Jr. Adverse effects of robotic-assisted laparoscopic versus open retropubic radical prostatectomy among a nationwide random sample of medicare-age men. J. Clin. Oncol. 2012, 30, 513–518. [Google Scholar] [PubMed]
- Asimakopoulos, A.D.; Topazio, L.; De Angelis, M.; Agrò, E.F.; Pastore, A.L.; Fuschi, A.; Annino, F. Retzius-sparing versus standard robot-assisted radical prostatectomy: A prospective randomized comparison on immediate continence rates. Surg. Endosc. 2019, 33, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulos, A.D.; Annino, F.; D’Orazio, A.; Pereira, C.F.T.; Mugnier, C.; Hoepffner, J.L.; Piechaud, T.; Gaston, R. Complete periprostatic anatomy preservation during robot-assisted laparoscopic radical prostatectomy (RALP): The new pubovesical complex-sparing technique. Eur. Urol. 2010, 58, 407–417. [Google Scholar]
- Asimakopoulos, A.D.; Mugnier, C.; Hoepffner, J.L.; Piechaud, T.; Gaston, R. Bladder neck preservation during minimally invasive radical prostatectomy: A standardised technique using a lateral approach. BJU Int. 2012, 110, 1566–1571. [Google Scholar]
- von Bodman, C.; Matsushita, K.; Savage, C.; Matikainen, M.P.; Eastham, J.A.; Scardino, P.T.; Rabbani, F.; Akin, O.; Sandhu, J.S. Recovery of urinary function after radical prostatectomy: Predictors of urinary function on preoperative prostate magnetic resonance imaging. J. Urol. 2012, 187, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, A.A.; Faleck, D.M.; Agalliu, I.; Rozenblit, A.M.; Chernyak, V.; Ghavamian, R. Preoperative and intraoperative measurements of urethral length as predictors of continence after robot-assisted radical prostatectomy. J. Endourol. 2011, 25, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, Y.; Uehara, H.; Fujisue, Y.; Takagi, S.; Nishida, T.; Inamoto, T.; Ubai, T.; Nomi, H.; Katsuoka, Y.; Azuma, H. Urinary continence following laparoscopic radical prostatectomy: Association with postoperative membranous urethral length measured using real-time intraoperative transrectal ultrasonography. Oncol. Lett. 2012, 3, 181–184. [Google Scholar] [CrossRef]
- Hammerer, P.; Huland, H. Urodynamic Evaluation of Changes in Urinary Control After Radical Retropubic Prostatectomy. J. Urol. 1997, 157, 233–236. [Google Scholar] [CrossRef]
- Schlomm, T.; Heinzer, H.; Steuber, T.; Salomon, G.; Engel, O.; Michl, U.; Haese, A.; Graefen, M.; Huland, H. Full functional-length urethral sphincter preservation during radical prostatectomy. Eur. Urol. 2011, 60, 320–329. [Google Scholar] [PubMed]
- Van Randenborgh, H.; Paul, R.; Kubler, H.; Breul, J.; Hartung, R. Improved urinary continence after radical retropubic prostatectomy with preparation of a long, partially intraprostatic portion of the membraneous urethra: An analysis of 1013 consecutive cases. Prostate Cancer Prostatic Dis. 2004, 7, 253–257. [Google Scholar] [CrossRef]
- McNeal, J.E. The zonal anatomy of the prostate. Prostate 1981, 2, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Son, D.Y.; Ro, J.Y.; Kang, M.J.; Jang, W.Y.; Cho, Y.M. Histology and distribution of prostatic tissue on prostatic urethral margins: Evaluation of radical prostatectomy specimens and implications on frozen section analysis. Ann. Diagn. Pathol. 2012, 16, 79–84. [Google Scholar] [PubMed]
- Kohl, S.K.; Balaji, K.C.; Smith, L.M.; Wilson, N.P.; Johansson, S.L.; Sterrett, S.P.; Abrahams, N.A. Clinical significance of benign glands at surgical margins in robotic radical prostatectomy specimens. Urology 2007, 69, 1112–1116. [Google Scholar] [CrossRef]
- Ko, Y.H.; Kim, T.H.; Song, P.H.; Kim, B.H.; Kim, B.S.; Kim, K.H.; Cho, J.; KYUS Group. Structural Variations of the Prostatic Urethra Within the Prostate Predict the Severities of Obstructive Symptoms: A Prospective Multicenter Observational Study. Urology 2017, 104, 160–165. [Google Scholar] [CrossRef]
- Wilson, A.H. The Prostate Gland: A Review of its Anatomy, Pathology, and Treatment. JAMA 2014, 312, 562. [Google Scholar] [CrossRef]
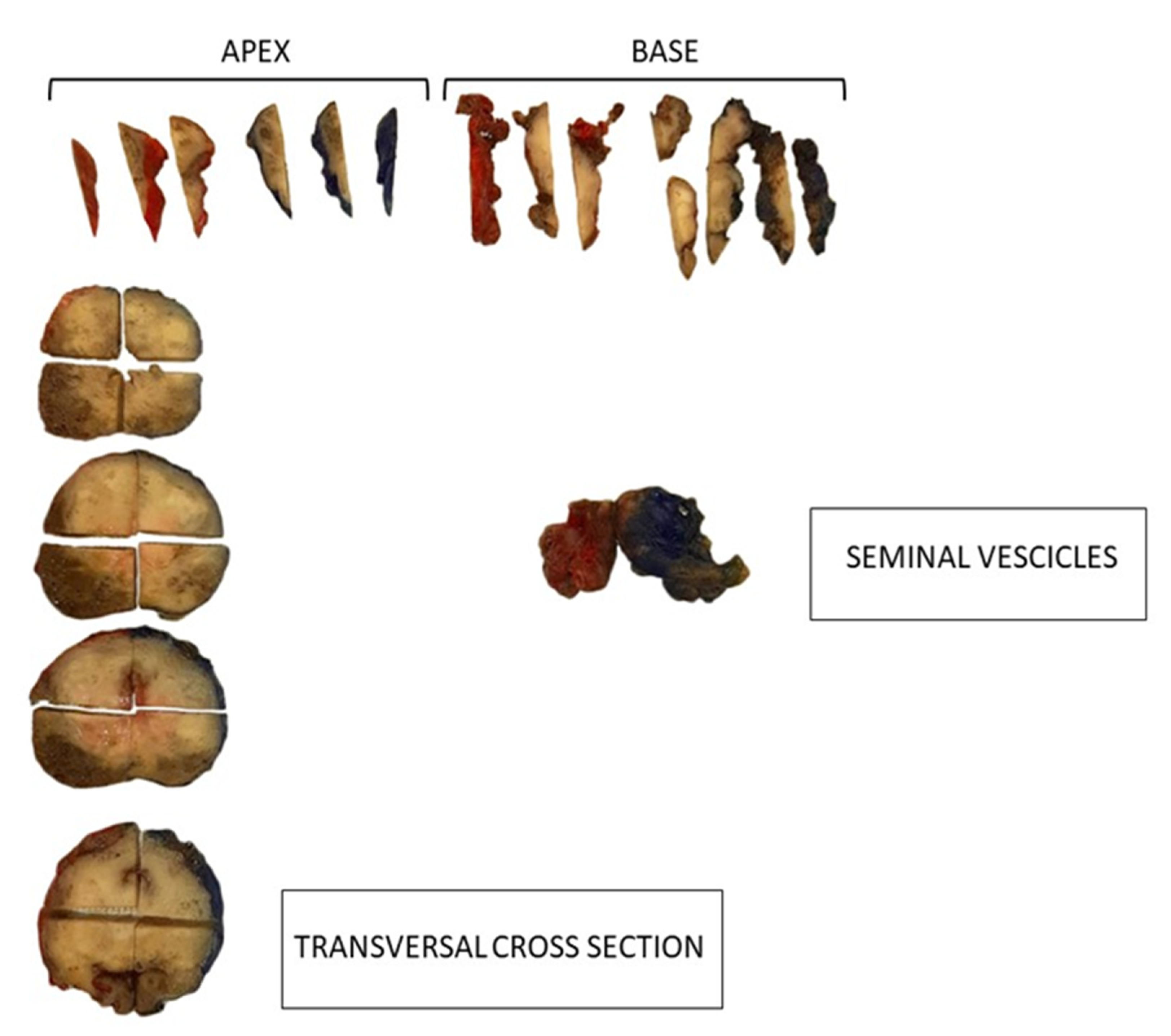


| Fixation | En bloc, 10% neutral buffered formalin |
| Coating (to delineate surgical margins) | India ink. |
| Specimen processing | Specimens were step-sectioned transversely at 3–4-mm intervals. |
| Apex and base | An apical and basal shaved section, 3–4-mm thick, was truncated perpendicular to the prostatic urethra and subsequently sectioned as slices parallel to the prostatic urethra. |
| Bladder neck section | Either by sampling portions of tissue at the junction of the prostatic capsule and bladder neck, or by sampling the most proximal portion of the submitted specimen corresponding to the anatomical bladder neck. |
| Staining | Hematoxylin and eosin. |
| Intraprostatic urethra evaluation | Three axial sections of proximal (post-basal), intermediate (middle), and distal (pre-apical) part; each section further divided in four subsections, left–right and anterior–posterior. |
| Evaluated parameters |
|
| Immunohistochemistry | Anti-GATA 3. Anti-PSA. |
| a | |||||||
|---|---|---|---|---|---|---|---|
| BASAL URETHRA Chorion Thickness | Anterior Right | Anterior Left | Posterior Right | Posterior Left | Average | Average Anterior | Average Posterior |
| n.1 Specimen | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| n.2 Specimen | 0.3 | 0.2 | 0.2 | 0.3 | 0.25 | 0.25 | 0.25 |
| n.3 Specimen | 0.4 | 0.2 | 02 | 0.2 | 0.25 | 0.3 | 0.2 |
| n.4 Specimen | 0.3 | 0.3 | 0.2 | 0.3 | 0.27 | 0.3 | 0.25 |
| n.5 Specimen | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| n.6 Specimen | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| b | |||||||
| BASAL URETHRA Distance Basal Membrane PU Epithelium-Prostate Glands | Anterior Right | Anterior Left | Posterior Right | Posterior Left | Average | Average Anterior | Average Posterior |
| n.1 Specimen | 2 | 2.5 | 0.2 | 2.5 | 1.8 | 2.25 | 1.35 |
| n.2 Specimen | 1.3 | 2.6 | 0.4 | 3.3 | 1.9 | 1.96 | 1.85 |
| n.3 Specimen | 4 | 4 | 0.3 | 1.7 | 2.5 | 4 | 1 |
| n.4 Specimen | 1 | 0.3 | 1 | 1 | 0.82 | 0.65 | 1 |
| n.5 Specimen | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| n.6 Specimen | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| a | |||||||
|---|---|---|---|---|---|---|---|
| MIDDLE URETHRA Chorion Thickness | Anterior Right | Anterior Left | Posterior Right | Posterior Left | Average | Average Anterior | Average Posterior |
| n.1 Specimen | 0.3 | 0.3 | 0.2 | 0.2 | 0.25 | 0.3 | 0.2 |
| n.2 Specimen | 0.3 | 0.2 | 0.2 | 0.2 | 0.22 | 0.25 | 0.2 |
| n.3 Specimen | 0.3 | 0.2 | 0.2 | 0.2 | 0.22 | 0.25 | 0.2 |
| n.4 Specimen | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| n.5 Specimen | 0.3 | 0,2 | 0,2 | 0.3 | 0.25 | 0.25 | 0.25 |
| n.6 Specimen | 0.3 | 0.2 | 0.3 | 0.2 | 0.25 | 0.25 | 0.25 |
| b | |||||||
| MIDDLE URETHRA Distance Basal Membrane PU Epithelium-Prostate Glands | Anterior Right | Anterior Left | Posterior Right | Posterior Left | Average | Average Anterior | Average Posterior |
| n.1 Specimen | 1.4 | 2 | 0.4 | 1.2 | 1.25 | 1.7 | 0.8 |
| n.2 Specimen | 1.2 | 2 | 1.1 | 2 | 1.58 | 1.6 | 1.55 |
| n.3 Specimen | 4 | 2.2 | 2 | 1 | 2.3 | 3.1 | 1.5 |
| n.4 Specimen | 0.4 | 0.1 | 0.4 | 0.1 | 0.25 | 0.25 | 0.25 |
| n.5 Specimen | 0.2 | 1.5 | 2 | 3 | 1.68 | 0.85 | 2.5 |
| n.6 Specimen | 2.3 | 3 | 1.3 | 1 | 1.9 | 2.65 | 1.15 |
| a | |||||||
|---|---|---|---|---|---|---|---|
| APICAL URETHRA Chorion Thickness | Anterior Right | Anterior Left | Posterior Right | Posterior Left | Average | Average Anterior | Average Posterior |
| n.1 Specimen | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| n.2 Specimen | 0.3 | 0.3 | 0.3 | 0.2 | 0.27 | 0.3 | 0.25 |
| n.3 Specimen | 0.3 | 0.3 | 0.3 | 0.2 | 0.27 | 0.3 | 0.25 |
| n.4 Specimen | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| n.5 Specimen | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| n.6 Specimen | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| b | |||||||
| APICAL URETHRA Distance Basal Membrane PU Epithelium-Prostate Glands | Anterior Right | Anterior Left | Posterior Right | Posterior Left | Average | Average Anterior | Average Posterior |
| n.1 Specimen | 0.7 | 0.3 | 1 | 0.8 | 0.7 | 0.5 | 0.9 |
| n.2 Specimen | 1.7 | 1.2 | 2 | 1.5 | 1,6 | 1.45 | 1.75 |
| n.3 Specimen | 2 | 1 | 1.5 | 2 | 1.63 | 1.5 | 1.75 |
| n.4 Specimen | 0.4 | 0.4 | 0.4 | 0.4 | 0,4 | 0.4 | 0.4 |
| n.5 Specimen | 2 | 1 | 1.5 | 2 | 1.63 | 1.5 | 1.75 |
| n.6 Specimen | 1.7 | 1 | 2 | 1 | 1.43 | 1.35 | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asimakopoulos, A.D.; Annino, F.; Colalillo, G.; Gaston, R.; Piechaud, T.; Mauriello, A.; Anceschi, U.; Borri, F. “Urethral-Sparing” Robotic Radical Prostatectomy: Critical Appraisal of the Safety of the Technique Based on the Histologic Characteristics of the Prostatic Urethra. Curr. Oncol. 2023, 30, 1065-1076. https://doi.org/10.3390/curroncol30010082
Asimakopoulos AD, Annino F, Colalillo G, Gaston R, Piechaud T, Mauriello A, Anceschi U, Borri F. “Urethral-Sparing” Robotic Radical Prostatectomy: Critical Appraisal of the Safety of the Technique Based on the Histologic Characteristics of the Prostatic Urethra. Current Oncology. 2023; 30(1):1065-1076. https://doi.org/10.3390/curroncol30010082
Chicago/Turabian StyleAsimakopoulos, Anastasios D., Filippo Annino, Gaia Colalillo, Richard Gaston, Thierry Piechaud, Alessandro Mauriello, Umberto Anceschi, and Filippo Borri. 2023. "“Urethral-Sparing” Robotic Radical Prostatectomy: Critical Appraisal of the Safety of the Technique Based on the Histologic Characteristics of the Prostatic Urethra" Current Oncology 30, no. 1: 1065-1076. https://doi.org/10.3390/curroncol30010082
APA StyleAsimakopoulos, A. D., Annino, F., Colalillo, G., Gaston, R., Piechaud, T., Mauriello, A., Anceschi, U., & Borri, F. (2023). “Urethral-Sparing” Robotic Radical Prostatectomy: Critical Appraisal of the Safety of the Technique Based on the Histologic Characteristics of the Prostatic Urethra. Current Oncology, 30(1), 1065-1076. https://doi.org/10.3390/curroncol30010082






