Should Laparoscopic Complete Mesocolic Excision Be Offered to Elderly Patients to Treat Right-Sided Colon Cancer?
Abstract
1. Introduction
2. Material and Methods
2.1. Study Overview
2.2. Study Endpoints
2.3. Variables and Definitions
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hohenberger, W.; Weber, K.; Matzel, K.; Papadopoulos, T.; Merkel, S. Standardized surgery for colonic cancer: Complete mesocolic excision and central ligation—Technical notes and outcome. Color. Dis. 2009, 11, 364–365. [Google Scholar] [CrossRef] [PubMed]
- Killeen, S.; Mannion, M.; Devaney, A.; Winter, D.C. Complete mesocolic resection and extended lymphadenectomy for colon cancer: A systematic review. Color. Dis. 2014, 16, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, C.A.; Neuenschwander, A.U.; Jansen, J.E.; Wilhelmsen, M.; Kirkegaard-Klitbo, A.; Tenma, J.R.; Bols, B.; Ingeholm, P.; Rasmussen, L.A.; Jepsen, L.V.; et al. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: A retrospective, population-based study. Lancet Oncol. 2015, 16, 161–168. [Google Scholar] [CrossRef]
- Merkel, S.; Weber, K.; Matzel, K.E.; Agaimy, A.; Göhl, J.; Hohenberger, W. Prognosis of patients with colonic carcinoma before, during and after implementation of complete mesocolic excision. Br. J. Surg. 2016, 103, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Kim, B.R.; Choi, E.H.; Kim, Y.W. Short-term and oncologic outcomes of laparoscopic and open complete mesocolic excision and central ligation. Int. J. Surg. 2016, 27, 151–157. [Google Scholar] [CrossRef]
- Li, J.; Yudong, L.; Chen, Y. Short- and long-term outcomes of laparoscopic complete mesocolic excision in elderly patients with right colon cancer. J. BUON 2018, 23, 1625–1632. [Google Scholar] [PubMed]
- Ceccarelli, G.; Andolfi, E.; Biancafarina, A.; Rocca, A.; Amato, M.; Milone, M.; Scricciolo, M.; Frezza, B.; Miranda, E.; De Prizio, M.; et al. Robot-assisted surgery in elderly and very elderly population: Our experience in oncologic and general surgery with literature review. Aging Clin. Exp. Res. 2017, 29, 55–63. [Google Scholar] [CrossRef]
- Hwang, D.Y.; Lee, G.R.; Kim, J.H.; Lee, Y.S. Laparoscopic complete mesocolic excision with D3 lymph node dissection for right colon cancer in elderly patients. Sci. Rep. 2020, 10, 126–133. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Engels, E.A.; Landgren, O.; Chiao, E.; Henderson, L.; Amaratunge, H.C.; Giordano, T.P. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology 2009, 49, 116–123. [Google Scholar] [CrossRef]
- Chéreau, E.; Ballester, M.; Selle, F.; Rouzier, R.; Daraï, E. Ovarian cancer in the elderly: Impact of surgery on morbidity and survival. Eur. J. Surg. Oncol. 2011, 37, 537–542. [Google Scholar] [CrossRef]
- Fornara, P.; Doehn, C.; Frese, R.; Jocham, D. Laparoscopic nephrectomy in young-old, old-old, and oldest-old adults. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, M.; Di Cristofaro, L.; Cortinovis, M.; Pinto, E.; Massa, M.; Alfieri, R.; Cagol, M.; Saadeh, L.; Costa, A.; Castoro, C.; et al. Minimally invasive surgery for colorectal cancer: Quality of life and satisfaction with care in elderly patients. Surg. Endosc. 2013, 27, 2911–2920. [Google Scholar] [CrossRef]
- Ripamonti, L.; De Carlis, R.; Lauterio, A.; Mangoni, I.; Frassoni, S.; Bagnardi, V.; Centonze, L.; Poli, C.; Buscemi, V.; Ferla, F.; et al. Major hepatectomy for perihilar cholangiocarcinoma in elderly patients: Is it reasonable? Updates Surg. 2022, 74, 203–211. [Google Scholar] [CrossRef]
- Famularo, S.; Di Sandro, S.; Giani, A.; Angrisani, M.; Lauterio, A.; Romano, F.; Gianotti, L.; De Carlis, L. The impact of age and ageing on hepatocarcinoma surgery: Short- and long-term outcomes in a multicentre propensity-matched cohort. Liver Int. 2019, 39, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Rocco, G.; Weder, W. Lung surgery in the elderly today. Lung Cancer 2013, 80, 115–119. [Google Scholar] [CrossRef]
- Magistro, C.; Bertoglio, C.L.; Giani, A.; Mazzola, M.; Rubicondo, C.; Maspero, M.; Carnevali, P.; Origi, M.; Ferrari, G. Laparoscopic complete mesocolic excision versus conventional resection for right-sided colon cancer: A propensity score matching analysis of short-term outcomes. Surg. Endosc. 2021, 36, 3049–3058. [Google Scholar] [CrossRef] [PubMed]
- Giusti, I.; Carnevali, P.; Bertoglio, C.L.; Giani, A.; Achilli, P.; Grimaldi, S.; Origi, M.; Mazzola, M.; Magistro, C.; Ferrari, G. Laparoscopic right hemicolectomy for hepatic flexure adenocarcinoma with complete mesocolic excision and 3D-CT vascular reconstruction. Tech. Coloproctol. 2022, 26, 1003–1004. [Google Scholar] [CrossRef]
- Giani, A.; Veronesi, V.; Bertoglio, C.L.; Mazzola, M.; Bernasconi, D.P.; Grimaldi, S.; Gualtierotti, M.; Magistro, C.; Ferrari, G. Multidimensional evaluation of the learning curve for laparoscopic complete mesocolic excision for right colon cancer: A risk-adjusted cumulative summation analysis. Color. Dis. 2022, 24, 577–586. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; Ronald MacKenzie, C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Weiser, M.R. AJCC 8th Edition: Colorectal Cancer. Ann. Surg. Oncol. 2018, 25, 1454–1455. [Google Scholar] [CrossRef] [PubMed]
- Kurniali, P.C.; Hrinczenko, B.; Al-Janadi, A. Management of locally advanced and metastatic colon cancer in elderly patients. World J. Gastroenterol. 2014, 20, 1910–1922. [Google Scholar] [CrossRef]
- Heald, R.J.; Husband, E.M.; Ryall, R.D.H. The mesorectum in rectal cancer surgery—The clue to pelvic recurrence? Br. J. Surg. 1982, 69, 613–616. [Google Scholar] [CrossRef] [PubMed]
- West, N.P.; Morris, E.J.A.; Rotimi, O.; Cairns, A.; Finan, P.J.; Quirke, P. Pathology grading of colon cancer surgical resection and its association with survival: A retrospective observational study. Lancet Oncol. 2008, 9, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B., 3rd; Venook, A.P.; Cederquist, L.; Chan, E.; Chen, Y.-J.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; Enzinger, P.C.; Fichera, A.; et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, P.; Francis, N.; Jayne, D.; Hohenberger, W.; Khan, J.; CME Project Working Group. Consensus statements on complete mesocolic excision for right-sided colon cancer-technical steps and training implications. Surg. Endosc. 2022, 36, 5595–5601. [Google Scholar] [CrossRef]
- Denet, C.; Fuks, D.; Cocco, F.; Chopinet, S.; Abbas, M.; Costea, C.; Levard, H.; Perniceni, T.; Gayet, B. Effects of age after laparoscopic right colectomy for cancer: Are there any specific outcomes? Dig. Liver Dis. 2017, 49, 562–567. [Google Scholar] [CrossRef]
- Amato, A.; Pescatori, M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst. Rev. 2006, 1, CD005033. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Z.; Shen, K.; Shen, Z.; Jiang, K.; Liang, B.; Yin, M.; Yang, X.; Wang, S.; Ye, Y. Safety, quality and effect of complete mesocolic excision vs non-complete mesocolic excision in patients with colon cancer: A systemic review and meta-analysis. Color. Dis. 2017, 19, 962–972. [Google Scholar] [CrossRef]
- Ouyang, M.; Luo, Z.; Wu, J.; Zhang, W.; Tang, S.; Lu, Y.; Hu, W.; Yao, X. Comparison of outcomes of complete mesocolic excision with conventional radical resection performed by laparoscopic approach for right colon cancer. Cancer Manag. Res. 2019, 25, 8647–8656. [Google Scholar] [CrossRef]
- An, M.S.; Baik, H.; Oh, S.H.; Park, Y.-H.; Seo, S.H.; Kim, K.H.; Hong, K.H.; Bae, K.B. Oncological outcomes of complete versus conventional mesocolic excision in laparoscopic right hemicolectomy. ANZ J. Surg. 2018, 88, E698–E702. [Google Scholar] [CrossRef] [PubMed]
- Vignali, A.; Elmore, U.; Guarneri, G.; De Ruvo, V.; Parise, P.; Rosati, R. Enhanced recovery after surgery in colon and rectal surgery: Identification of predictive variables of failure in a monocentric series including 733 patients. Updates Surg. 2021, 73, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, P.M.; Mazzola, M.; Giani, A.; Baleri, S.; Maspero, M.; De Martini, P.; Gualtierotti, M.; Ferrari, G. ERAS pathway for gastric cancer surgery: Adherence, outcomes and prognostic factors for compliance in a Western centre. Updates Surg. 2021, 73, 1857–1865. [Google Scholar] [CrossRef]
- Mazzola, M.; Bertoglio, C.; Boniardi, M.; Magistro, C.; De Martini, P.; Carnevali, P.; Morini, L.; Ferrari, G. Frailty in major oncologic surgery of upper gastrointestinal tract: How to improve postoperative outcomes. Eur. J. Surg. Oncol. 2017, 43, 1566–1571. [Google Scholar] [CrossRef]
- Baimas-George, M.; Watson, M.; Elhage, S.; Parala-Metz, A.; Vrochides, D.; Davis, B.R. Prehabilitation in Frail Surgical Patients: A Systematic Review. World J. Surg. 2020, 44, 3668–3678. [Google Scholar] [CrossRef] [PubMed]
- Achilli, P.; Mazzola, M.; Bertoglio, C.L.; Magistro, C.; Origi, M.; Carnevali, P.; Gervasi, F.; Mastellone, C.; Guanziroli, N.; Corradi, E.; et al. Preoperative immunonutrition in frail patients with colorectal cancer: An intervention to improve postoperative outcomes. Int. J. Color. Dis. 2020, 35, 19–27. [Google Scholar] [CrossRef]
- Giani, A.; Bertoglio, C.L.; Mazzola, M.; Giusti, I.; Achilli, P.; Carnevali, P.; Origi, M.; Magistro, C.; Ferrari, G. Mid-term oncological outcomes after complete versus conventional mesocolic excision for right-sided colon cancer: A propensity score matching analysis. Surg. Endosc. 2022, 36, 6489–6496. [Google Scholar] [CrossRef]
- Sundararajan, V.; Mitra, N.; Jacobson, J.S.; Grann, V.R.; Heitjan, D.F.; Neugut, A.I. Survival associated with 5-fluorouracil-based adjuvant chemotherapy among elderly patients with node-positive colon cancer. Ann. Intern. Med. 2002, 136, 349–357. [Google Scholar] [CrossRef]
- Schrag, D.; Cramer, L.D.; Bach, P.B.; Begg, C.B. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J. Natl. Cancer Inst. 2001, 93, 850–857. [Google Scholar] [CrossRef]
- Liska, D.; Stocchi, L.; Karagkounis, G.; Elagili, F.; Dietz, D.W.; Kalady, M.F.; Kessler, H.; Remzi, F.H.; Church, J. Incidence, Patterns, and Predictors of Locoregional Recurrence in Colon Cancer. Ann. Surg. Oncol. 2017, 24, 1093–1099. [Google Scholar] [CrossRef]
| Under-80 (n = 95) | Over-80 (n = 35) | p-Value | |
|---|---|---|---|
| Male | 50 (52) | 21 (60) | 0.454 |
| BMI | 26 (23–29) | 25 (23.8–28) | 0.545 |
| BMI > 30 | 75 (78.9) | 30 (85.7) | 0.385 |
| Charlson Comorbidity Index | 5 (4–6) | 6 (6–7) | <0.001 |
| Charlson Comorbidity Index > 6 | 80 (84.2) | 18 (51.4) | <0.001 |
| ASA | <0.001 | ||
| 1 | 19 (20) | 0 (0) | |
| 2 | 46 (48.4) | 18 (51.4) | |
| 3 | 30 (31.5) | 17 (48.5) | |
| ASA > 2 | 30 (31.6) | 17 (48.6) | 0.074 |
| CEA | 2.8 (1.3–6.9) | 4.45 (2.7–9.9) | 0.054 |
| Tumor Site | 0.209 | ||
| Cecum/Ascending colon | 75 (78.9) | 31 (88.6) | |
| Hepatic Flexure/Transverse colon | 20 (21.1) | 4 (11.4) | |
| Tumor Size | 4.1 (3–6) | 4 (2.6–5) | 0.478 |
| Grading | 0.932 | ||
| 1 | 16 (16.8) | 5 (14.3) | |
| 2 | 65 (68.4) | 25 (71.4) | |
| 3 | 14 (14.7) | 5 (14.3) | |
| T | 0.732 | ||
| 1 | 20 (21.1) | 9 (25.7) | |
| 2 | 21 (22.1) | 10 (28.6) | |
| 3 | 47 (49.5) | 14 (40) | |
| 4 | 7 (7.4) | 2 (5.7) | |
| N | 0.689 | ||
| X | 1 (1.1) | 0 (0) | |
| 0 | 68 (71.6) | 29 (82.8) | |
| 1 | 18 (18.9) | 3 (8.6) | |
| 2 | 8 (8.4) | 3 (8.6) | |
| Tumor Stage | 0.436 | ||
| 1 | 37 (38.9) | 17 (48.6) | |
| 2 | 32 (33.7) | 12 (34.3) | |
| 3 | 26 (27.4) | 6 (17.1) | |
| Tumor Stage > 2 | 26 (27.4) | 6 (17.1) | 0.229 |
| Under-80 (n = 95) | Over-80 (n = 35) | p-Value | |
|---|---|---|---|
| LOS, Median | 5 (4–7) | 8 (6–10) | <0.001 |
| Overall complications | 22 (23.2) | 10 (28.6) | 0.525 |
| Clavien–Dindo | 0.794 | ||
| 0 | 75 (78.9) | 27 (77.1) | |
| 1 | 2 (2.1) | 0 (0) | |
| 2 | 8 (8.4) | 5 (14.3) | |
| 3a | 3 (3.1) | 2 (5.7) | |
| 3b | 5 (5.3) | 1 (2.9) | |
| 4a | 1 (1.1) | 0 (0) | |
| 4b | 1 (1.1) | 0 (0) | |
| Severe Complication | 10 (10.5) | 3 (8.6) | 0.742 |
| Comprehensive Complication Index | 0 (0–0) | 0 (0–0) | 0.723 |
| 90-day mortality | 0 (0) | 0 (0) | 1 |
| Blood Transfusion | 10 (10.5) | 5 (14.3) | 0.552 |
| Readmission | 0 (0) | 0 (0) | 1 |
| Harvested Lymph nodes | 22.5 (16–29) | 18.5 (13.7–26) | 0.267 |
| R0 | 95 (100) | 35 (100) | 1 |
| Conversion rate | 2 (2) | 1 (3.1) | 0.8 |
| Under-80 | Over-80 | p-Value | |
|---|---|---|---|
| (n = 95) | (n = 35) | ||
| Adjuvant chemotherapy * | 25/26 (96.1) | 1/6 (16.7) | <0.001 |
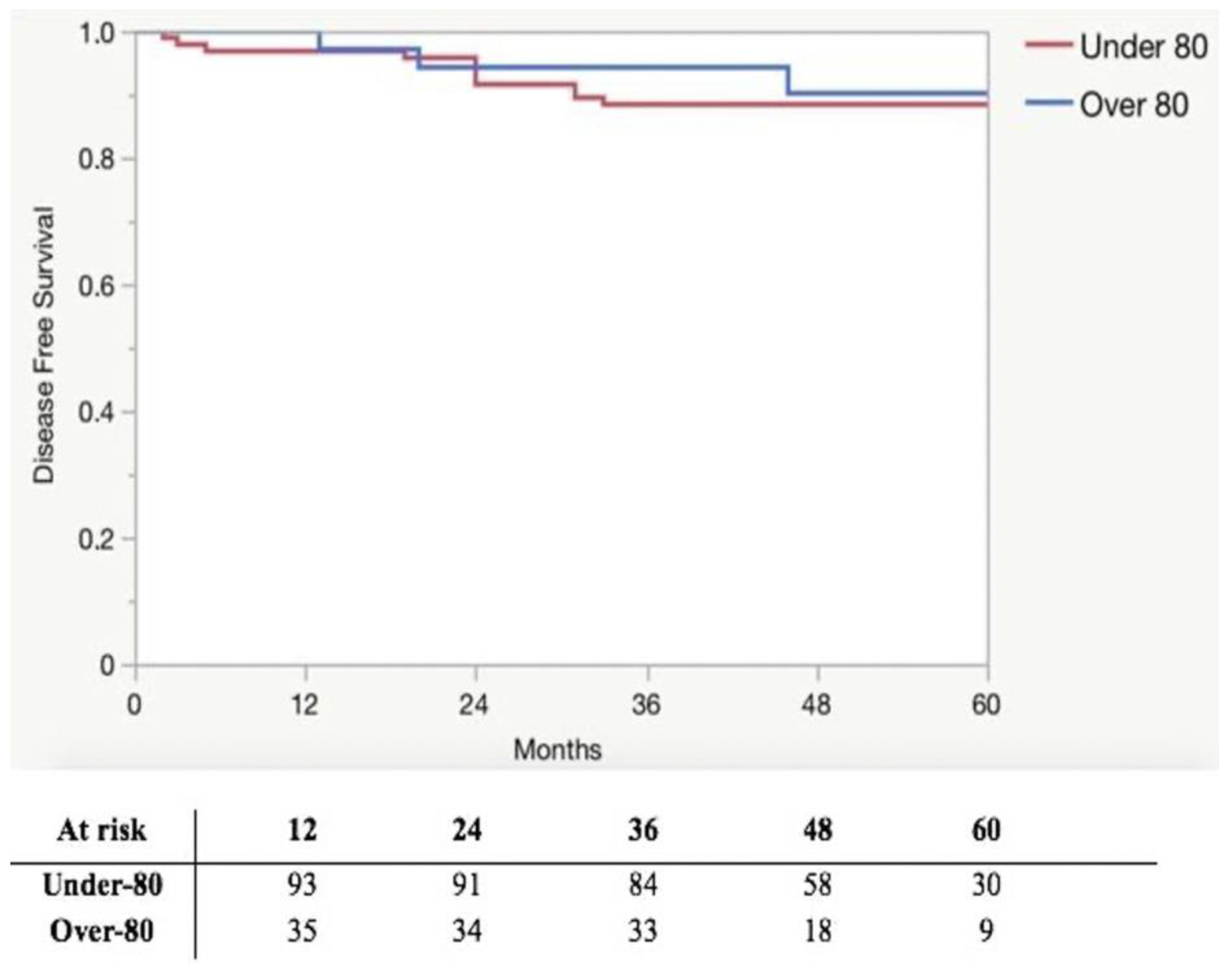
| Relapse | 12 (12.6) | 3 (8.6) | 0.509 |
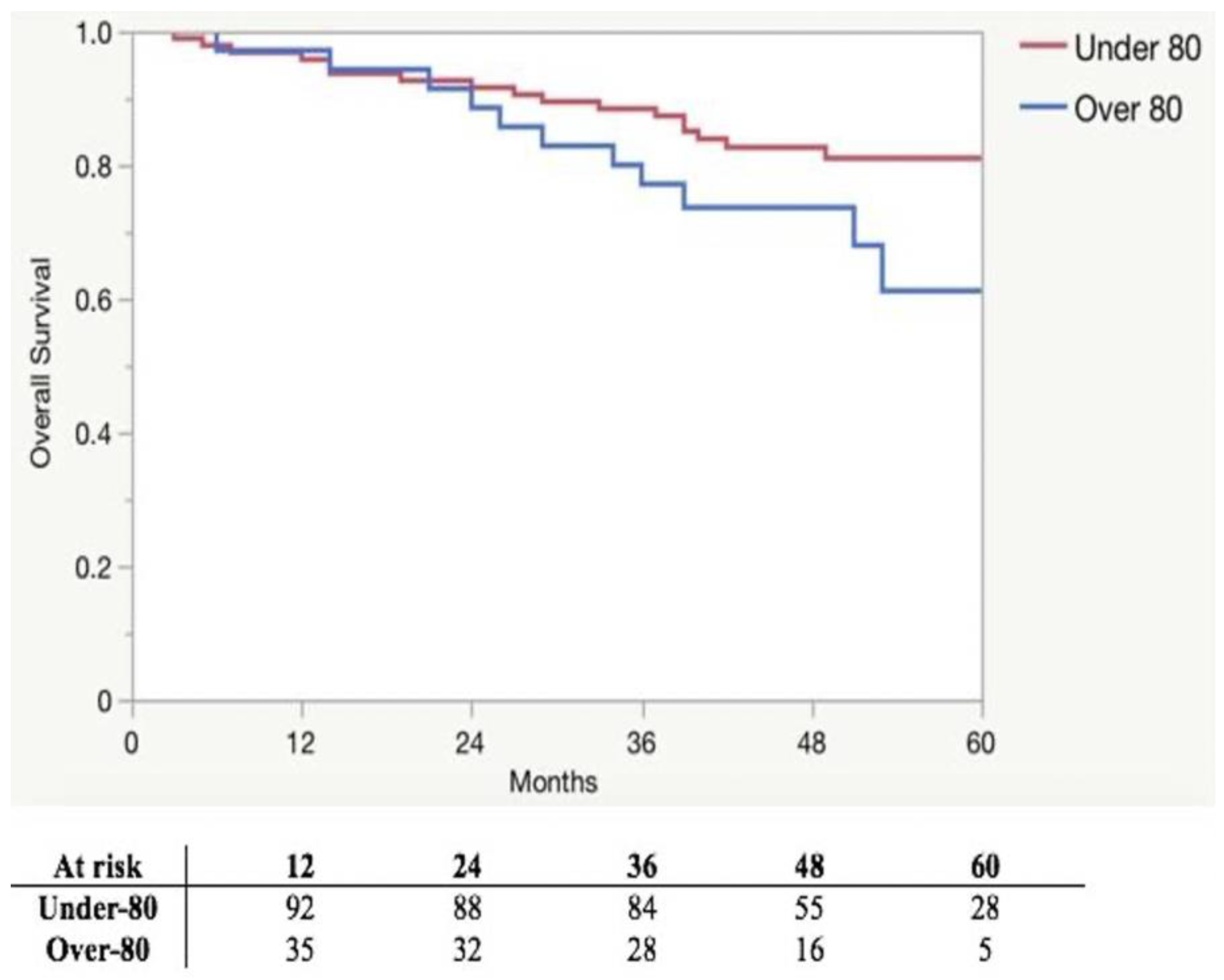
| Survival rate | 65 (79.3) | 23 (67.7) | 0.183 |
| Follow-up | 43 (32.5–54.5) | 32.5 (21.8–42.3) | 0.004 |
| Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age ≥ 80 | 1.327 (0.553–3.182) | 0.525 | 1.146 (0.432–2.893) | 0.777 |
| Sex (Male) | 1.082 (0.486–2.410) | 0.845 | 1.364 (0.577–3.269) | 0.478 |
| ASA > 2 | 3.059 (1.344–6.960) | 0.006 | 3.114 (1.314–7.630) | 0.009 |
| BMI > 30 | 1.244 (0.466–3.321) | 0.662 | 1.081 (0.354–2.500) | 0.885 |
| CCI > 6 | 5.125 (2.134–12.304) | 0 | ||
| Tumor Site (Hepatic Flexure/Transverse colon) | 0.769 (0.262–2.262) | 0.633 | 0.834 (0.241–2.500) | 0.755 |
| Tumor pStage > 2 | 1.570 (0.647–3.806) | 0.315 | 1.382 (0.518–3.519) | 0.507 |
| Conversion to open surgery | 1.55 (0.13–17.66) | 0.723 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzola, M.; Ripamonti, L.; Giani, A.; Carnevali, P.; Origi, M.; Alampi, B.; Giusti, I.; Achilli, P.; Bertoglio, C.L.; Magistro, C.; et al. Should Laparoscopic Complete Mesocolic Excision Be Offered to Elderly Patients to Treat Right-Sided Colon Cancer? Curr. Oncol. 2023, 30, 4979-4989. https://doi.org/10.3390/curroncol30050376
Mazzola M, Ripamonti L, Giani A, Carnevali P, Origi M, Alampi B, Giusti I, Achilli P, Bertoglio CL, Magistro C, et al. Should Laparoscopic Complete Mesocolic Excision Be Offered to Elderly Patients to Treat Right-Sided Colon Cancer? Current Oncology. 2023; 30(5):4979-4989. https://doi.org/10.3390/curroncol30050376
Chicago/Turabian StyleMazzola, Michele, Lorenzo Ripamonti, Alessandro Giani, Pietro Carnevali, Matteo Origi, BrunocDomenico Alampi, Irene Giusti, Pietro Achilli, Camillo Leonardo Bertoglio, Carmelo Magistro, and et al. 2023. "Should Laparoscopic Complete Mesocolic Excision Be Offered to Elderly Patients to Treat Right-Sided Colon Cancer?" Current Oncology 30, no. 5: 4979-4989. https://doi.org/10.3390/curroncol30050376
APA StyleMazzola, M., Ripamonti, L., Giani, A., Carnevali, P., Origi, M., Alampi, B., Giusti, I., Achilli, P., Bertoglio, C. L., Magistro, C., & Ferrari, G. (2023). Should Laparoscopic Complete Mesocolic Excision Be Offered to Elderly Patients to Treat Right-Sided Colon Cancer? Current Oncology, 30(5), 4979-4989. https://doi.org/10.3390/curroncol30050376





