Adherence to Oral Chemotherapy in Acute Lymphoblastic Leukemia during Maintenance Therapy in Children, Adolescents, and Young Adults: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Article Retrieval
2.2. Article Selection
2.3. Data Extraction and Synthesis
3. Results
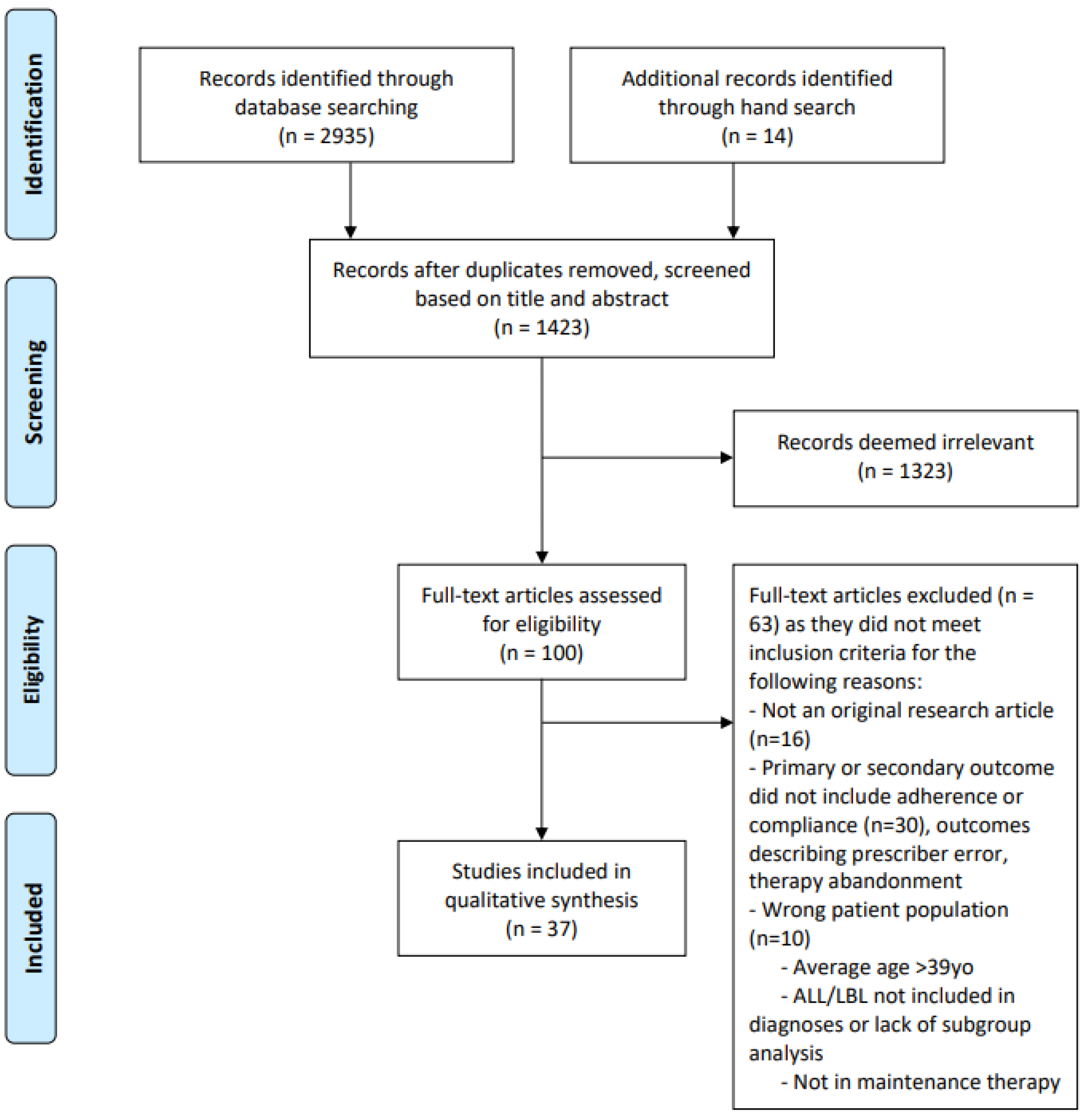
3.1. Literature Search
3.2. Study Characteristics
4. Assessment of Adherence and Prevalence of Nonadherence
5. Pharmacologic Adherence
5.1. Thiopurines (6-mercaptopurine)
5.2. Methotrexate
5.3. Steroids
6. Behavioral Adherence
6.1. Medication Event Monitoring System (MEMS)
6.2. Tablet Counting
6.3. Prescription Review
6.4. Medical Chart Review
7. Subjective Adherence
7.1. Self-Report
7.2. Text Messaging
7.3. Provider Survey
8. Comparison of Objective and Subjective Measures
9. Correlates of Nonadherence
9.1. Age
9.2. Sex
9.3. Race/Ethnicity
9.4. Family Structure and Parental Characteristics
9.5. Socioeconomic Status
9.6. Therapy Related Factors
9.7. Reasons for Nonadherence
10. Survival and Outcomes
11. Interventions
12. Discussion
Strengths and Limitations
13. Conclusions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Ward, E.M.; Johnson, C.J.; Cronin, K.A.; Ma, J.; Ryerson, B.; Mariotto, A.; Lake, A.J.; Wilson, R.; Sherman, R.L.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J. Natl. Cancer Inst. 2017, 109, djx030. [Google Scholar] [CrossRef] [PubMed]
- Hunger, S.P.; Lu, X.; Devidas, M.; Camitta, B.M.; Gaynon, P.S.; Winick, N.J.; Reaman, G.H.; Carroll, W.L. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children’s oncology group. J. Clin. Oncol. 2012, 30, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Teachey, D.T.; Hunger, S.P.; Loh, M.L. Optimizing therapy in the modern age: Differences in length of maintenance therapy in acute lymphoblastic leukemia. Blood 2021, 137, 168–177. [Google Scholar] [CrossRef]
- Group, C.A.C. Duration and intensity of maintenance chemotherapy in acute lymphoblastic leukaemia: Overview of 42 trials involving 12 000 randomised children. Lancet 1996, 347, 1783–1788. [Google Scholar] [CrossRef]
- Simchowitz, B.; Shiman, L.; Spencer, J.; Brouillard, D.; Gross, A.; Connor, M.; Weingart, S.N. Perceptions and experiences of patients receiving oral chemotherapy. Clin. J. Oncol. Nurs. 2010, 14, 447–453. [Google Scholar] [CrossRef]
- Ruddy, K.; Mayer, E.; Partridge, A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J. Clin. 2009, 59, 56–66. [Google Scholar] [CrossRef]
- Weingart, S.N.; Brown, E.; Bach, P.B.; Eng, K.; Johnson, S.A.; Kuzel, T.M.; Langbaum, T.S.; Leedy, R.D.; Muller, R.J.; Newcomer, L.N.; et al. NCCN Task Force Report: Oral chemotherapy. J. Natl. Compr. Cancer Netw. 2008, 6 (Suppl. 3), S1–S14. [Google Scholar] [CrossRef]
- Osterberg, L.; Blaschke, T. Adherence to medication. N. Engl. J. Med. 2005, 353, 487–497. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Alsous, M.; Abu Farha, R.; Alefishat, E.; Al Omar, S.; Momani, D.; Gharabli, A.; McElnay, J.; Horne, R.; Rihani, R. Adherence to 6-Mercaptopurine in children and adolescents with Acute Lymphoblastic Leukemia. PLoS ONE 2017, 12, e0183119. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Landier, W.; Shangguan, M.; Hageman, L.; Schaible, A.N.; Carter, A.R.; Hanby, C.L.; Leisenring, W.; Yasui, Y.; Kornegay, N.M.; et al. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: A report from the children’s oncology group. J. Clin. Oncol. 2012, 30, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Landier, W.; Hageman, L.; Kim, H.; Chen, Y.; Crews, K.R.; Evans, W.E.; Bostrom, B.; Casillas, J.; Dickens, D.S.; et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: A Children’s Oncology Group study. Blood 2014, 124, 2345–2353. [Google Scholar] [CrossRef]
- Bhatia, S.; Landier, W.; Hageman, L.; Chen, Y.; Kim, H.; Sun, C.L.; Kornegay, N.; Evans, W.E.; Angiolillo, A.L.; Bostrom, B.; et al. Systemic Exposure to Thiopurines and Risk of Relapse in Children With Acute Lymphoblastic Leukemia: A Children’s Oncology Group Study. JAMA Oncol. 2015, 1, 287–295. [Google Scholar] [CrossRef]
- Bhatia, S.; Hageman, L.; Chen, Y.; Wong, F.L.; McQuaid, E.L.; Duncan, C.; Mascarenhas, L.; Freyer, D.; Mba, N.; Aristizabal, P.; et al. Effect of a Daily Text Messaging and Directly Supervised Therapy Intervention on Oral Mercaptopurine Adherence in Children With Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA Netw. Open. 2020, 3, e2014205. [Google Scholar] [CrossRef]
- Davies, H.A.; Lennard, L.; Lilleyman, J.S. Variable mercaptopurine metabolism in children with leukaemia: A problem of non-compliance? BMJ 1993, 306, 1239–1240. [Google Scholar] [CrossRef][Green Version]
- de Oliveira, B.M.; Viana, M.B.; Zani, C.L.; Romanha, A.J. Clinical and laboratory evaluation of compliance in acute lymphoblastic leukaemia. Arch. Dis. Child. 2004, 89, 785–788. [Google Scholar] [CrossRef][Green Version]
- de Oliveira, B.M.; Viana, M.B.; de Mattos Arruda, L.; Ybarra, M.I.; Romanha, A.J. Evaluation of compliance through specific interviews: A prospective study of 73 children with acute lymphoblastic leukemia. J. Pediatr. (Rio J.) 2005, 81, 245–250. [Google Scholar] [CrossRef][Green Version]
- Farberman, D.; Valente, P.; Malpiedi, L.; Morosi, M.; Luisella, L.; Colaboradores. Adherence to oral antineoplastic agents in pediatric oncology. A multicenter study. Arch. Argent Pediatr. 2021, 119, 44–50. [Google Scholar] [CrossRef]
- Hawwa, A.F.; Millership, J.S.; Collier, P.S.; McCarthy, A.; Dempsey, S.; Cairns, C.; McElnay, J.C. The development of an objective methodology to measure medication adherence to oral thiopurines in paediatric patients with acute lymphoblastic leukaemia—An exploratory study. Eur. J. Clin. Pharmacol. 2009, 65, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, M.B.; Hussain, T.; Barrera, L.; Cai, S.W.; Haugen, M.; Duff, A.; Shoop, J.; Morgan, E.; Rossoff, J.; Weinstein, J.; et al. Applying the COM-B model to patient-reported barriers to medication adherence in pediatric acute lymphoblastic leukemia. Pediatr. Blood Cancer 2020, 67, e28216. [Google Scholar] [CrossRef] [PubMed]
- Hoppmann, A.L.; Chen, Y.; Landier, W.; Hageman, L.; Evans, W.E.; Wong, F.L.; Relling, M.V.; Bhatia, S. Individual prediction of nonadherence to oral mercaptopurine in children with acute lymphoblastic leukemia: Results from COG AALL03N1. Cancer 2021, 127, 3832–3839. [Google Scholar] [CrossRef] [PubMed]
- Isaac, E.I.; Sivagnanalingam, U.; Meisman, A.R.; Wetherington Donewar, C.; Ewing, L.J.; Katz, E.R.; Muriel, A.C.; Rohan, J.M. Longitudinal Patterns of Social Problem-Solving Skills in an Ethnically Diverse Sample of Pediatric Patients with Cancer and their Caregivers. Int. J. Environ. Res. Public Health 2020, 17, 1581. [Google Scholar] [CrossRef]
- Jaime-Pérez, J.C.; Gómez-Almaguer, D.; Sandoval-González, A.; Chapa-Rodríguez, A.; Gonzàlez-Llano, O. Random serum methotrexate determinations for assessing compliance with maintenance therapy for childhood acute lymphoblastic leukemia. Leuk Lymphoma 2009, 50, 1843–1847. [Google Scholar] [CrossRef]
- Kato, P.M.; Cole, S.W.; Bradlyn, A.S.; Pollock, B.H. A video game improves behavioral outcomes in adolescents and young adults with cancer: A randomized trial. Pediatrics 2008, 122, e305–e317. [Google Scholar] [CrossRef]
- Khalek, E.R.; Sherif, L.M.; Kamal, N.M.; Gharib, A.F.; Shawky, H.M. Acute lymphoblastic leukemia: Are Egyptian children adherent to maintenance therapy? J. Cancer Res. Ther. 2015, 11, 54–58. [Google Scholar] [CrossRef]
- Kremeike, K.; Juergens, C.; Alz, H.; Reinhardt, D. Patients’ Adherence in the Maintenance Therapy of Children and Adolescents with Acute Lymphoblastic Leukemia. Klin. Padiatr. 2015, 227, 329–334. [Google Scholar] [CrossRef]
- Kristjansdottir, E.R.; Toksvang, L.N.; Schmiegelow, K.; Rank, C.U. Prevalence of non-adherence and non-compliance during maintenance therapy in adults with acute lymphoblastic leukemia and their associations with survival. Eur. J. Haematol. 2021, 108, 109–117. [Google Scholar] [CrossRef]
- Lancaster, D.; Lennard, L.; Lilleyman, J.S. Profile of non-compliance in lymphoblastic leukaemia. Arch. Dis. Child 1997, 76, 365–366. [Google Scholar] [CrossRef]
- Landier, W.; Chen, Y.; Hageman, L.; Kim, H.; Bostrom, B.C.; Casillas, J.N.; Dickens, D.S.; Evans, W.E.; Maloney, K.W.; Mascarenhas, L.; et al. Comparison of self-report and electronic monitoring of 6MP intake in childhood ALL: A Children’s Oncology Group study. Blood 2017, 129, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Landier, W.; Hageman, L.; Chen, Y.; Kornegay, N.; Evans, W.E.; Bostrom, B.C.; Casillas, J.; Dickens, D.S.; Angiolillo, A.L.; Lew, G.; et al. Mercaptopurine Ingestion Habits, Red Cell Thioguanine Nucleotide Levels, and Relapse Risk in Children With Acute Lymphoblastic Leukemia: A Report From the Children’s Oncology Group Study AALL03N1. J. Clin. Oncol. 2017, 35, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- Lansky, S.B.; Smith, S.D.; Cairns, N.U.; Cairns, G.F., Jr. Psychological correlates of compliance. Am. J. Pediatr. Hematol. Oncol. 1983, 5, 87–92. [Google Scholar] [PubMed]
- Lau, R.C.; Matsui, D.; Greenberg, M.; Koren, G. Electronic measurement of compliance with mercaptopurine in pediatric patients with acute lymphoblastic leukemia. Med. Pediatr. Oncol. 1998, 30, 85–90. [Google Scholar] [CrossRef]
- Lennard, L.; Welch, J.; Lilleyman, J.S. Intracellular metabolites of mercaptopurine in children with lymphoblastic leukaemia: A possible indicator of non-compliance? Br. J. Cancer 1995, 72, 1004–1006. [Google Scholar] [CrossRef]
- Lennard, L.; Cartwright, C.S.; Wade, R.; Richards, S.M.; Vora, A. Thiopurine methyltransferase genotype-phenotype discordance and thiopurine active metabolite formation in childhood acute lymphoblastic leukaemia. Br. J. Clin. Pharmacol. 2013, 76, 125–136. [Google Scholar] [CrossRef]
- Lennard, L.; Cartwright, C.S.; Wade, R.; Vora, A. Thiopurine dose intensity and treatment outcome in childhood lymphoblastic leukaemia: The influence of thiopurine methyltransferase pharmacogenetics. Br. J. Haematol. 2015, 169, 228–240. [Google Scholar] [CrossRef]
- Macdougall, L.G.; McElligott, S.E.; Ross, E.; Greeff, M.C.; Poole, J.E. Pattern of 6-mercaptopurine urinary excretion in children with acute lymphoblastic leukemia: Urinary assays as a measure of drug compliance. Ther. Drug Monit. 1992, 14, 371–375. [Google Scholar] [CrossRef]
- Mancini, J.; Simeoni, M.C.; Parola, N.; Clement, A.; Vey, N.; Sirvent, N.; Michel, G.; Auquier, P. Adherence to leukemia maintenance therapy: A comparative study among children, adolescents, and adults. Pediatr. Hematol. Oncol. 2012, 29, 428–439. [Google Scholar] [CrossRef]
- Pai, A.L.; Drotar, D.; Kodish, E. Correspondence between objective and subjective reports of adherence among adolescents with acute lymphoblastic leukemia. Children’s Health Care 2008, 37, 225–235. [Google Scholar] [CrossRef]
- Phillips, B.; Richards, M.; Boys, R.; Hodgkin, M.; Kinsey, S. A home-based maintenance therapy program for acute lymphoblastic leukemia-practical and safe? J. Pediatr. Hematol. Oncol. 2011, 33, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Psihogios, A.M.; Li, Y.; Ahmed, A.; Huang, J.; Kersun, L.S.; Schwartz, L.A.; Barakat, L.P. Daily text message assessments of 6-mercaptopurine adherence and its proximal contexts in adolescents and young adults with leukemia: A pilot study. Pediatr. Blood Cancer 2021, 68, e28767. [Google Scholar] [CrossRef] [PubMed]
- Rohan, J.M.; Drotar, D.; Alderfer, M.; Donewar, C.W.; Ewing, L.; Katz, E.R.; Muriel, A. Electronic monitoring of medication adherence in early maintenance phase treatment for pediatric leukemia and lymphoma: Identifying patterns of nonadherence. J. Pediatr. Psychol. 2015, 40, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Rohan, J.M.; Fukuda, T.; Alderfer, M.A.; Wetherington Donewar, C.; Ewing, L.; Katz, E.R.; Muriel, A.C.; Vinks, A.A.; Drotar, D. Measuring Medication Adherence in Pediatric Cancer: An Approach to Validation. J. Pediatr. Psychol. 2017, 42, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Schroder, H.; Clausen, N.; Ostergaard, E.; Pressler, T. Pharmacokinetics of erythrocyte methotrexate in children with acute lymphoblastic leukemia during maintenance treatment. Cancer Chemother. Pharmacol. 1986, 16, 190–193. [Google Scholar] [CrossRef]
- Schroder, H. Methotrexate in neutrophils in children with acute lymphoblastic leukemia. Cancer Chemother. Pharmacol. 1987, 19, 339–342. [Google Scholar] [CrossRef]
- Smith, S.D.; Rosen, D.; Trueworthy, R.C.; Lowman, J.T. A reliable method for evaluating drug compliance in children with cancer. Cancer 1979, 43, 169–173. [Google Scholar] [CrossRef]
- Wu, Y.P.; Stenehjem, D.D.; Linder, L.A.; Yu, B.; Parsons, B.G.; Mooney, R.; Fluchel, M.N. Adherence to Oral Medications During Maintenance Therapy Among Children and Adolescents With Acute Lymphoblastic Leukemia: A Medication Refill Analysis. J. Pediatr. Oncol. Nurs. 2018, 35, 86–93. [Google Scholar] [CrossRef]
- Lam, W.Y.; Fresco, P. Medication Adherence Measures: An Overview. Biomed Res. Int. 2015, 2015, 217047. [Google Scholar] [CrossRef]
- Butow, P.; Palmer, S.; Pai, A.; Goodenough, B.; Luckett, T.; King, M. Review of adherence-related issues in adolescents and young adults with cancer. .J Clin. Oncol. 2010, 28, 4800–4809. [Google Scholar] [CrossRef]
- Taddeo, D.; Egedy, M.; Frappier, J.Y. Adherence to treatment in adolescents. Paediatr. Child Health 2008, 13, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Tebbi, C.K. Treatment compliance in childhood and adolescence. Cancer 1993, 71, 3441–3449. [Google Scholar] [CrossRef] [PubMed]
- Tebbi, C.K.; Cummings, K.M.; Zevon, M.A.; Smith, L.; Richards, M.; Mallon, J. Compliance of pediatric and adolescent cancer patients. Cancer 1986, 58, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Psihogios, A.M.; Fellmeth, H.; Schwartz, L.A.; Barakat, L.P. Family Functioning and Medical Adherence Across Children and Adolescents With Chronic Health Conditions: A Meta-Analysis. J. Pediatr. Psychol. 2019, 44, 84–97. [Google Scholar] [CrossRef]
- Levin, J.B.; Sams, J.; Tatsuoka, C.; Cassidy, K.A.; Sajatovic, M. Use of automated medication adherence monitoring in bipolar disorder research: Pitfalls, pragmatics, and possibilities. Ther. Adv. Psychopharmacol. 2015, 5, 76–87. [Google Scholar] [CrossRef]
- Garfield, S.; Clifford, S.; Eliasson, L.; Barber, N.; Willson, A. Suitability of measures of self-reported medication adherence for routine clinical use: A systematic review. BMC Med. Res. Methodol. 2011, 11, 149. [Google Scholar] [CrossRef]
- Stirratt, M.J.; Dunbar-Jacob, J.; Crane, H.M.; Simoni, J.M.; Czajkowski, S.; Hilliard, M.E.; Aikens, J.E.; Hunter, C.M.; Velligan, D.I.; Huntley, K.; et al. Self-report measures of medication adherence behavior: Recommendations on optimal use. Transl. Behav. Med. 2015, 5, 470–482. [Google Scholar] [CrossRef]
- Doro, P.; Benko, R.; Czako, A.; Matuz, M.; Thurzo, F.; Soos, G. Optimal recall period in assessing the adherence to antihypertensive therapy: A pilot study. Int. J. Clin. Pharm. 2011, 33, 690–695. [Google Scholar] [CrossRef]
- Lu, M.; Safren, S.A.; Skolnik, P.R.; Rogers, W.H.; Coady, W.; Hardy, H.; Wilson, I.B. Optimal recall period and response task for self-reported HIV medication adherence. AIDS Behav. 2008, 12, 86–94. [Google Scholar] [CrossRef]
- Ramsey, W.A.; Heidelberg, R.E.; Gilbert, A.M.; Heneghan, M.B.; Badawy, S.M.; Alberts, N.M. eHealth and mHealth interventions in pediatric cancer: A systematic review of interventions across the cancer continuum. Psychooncology 2020, 29, 17–37. [Google Scholar] [CrossRef]
- Badawy, S.M.; Barrera, L.; Sinno, M.G.; Kaviany, S.; O’Dwyer, L.C.; Kuhns, L.M. Text Messaging and Mobile Phone Apps as Interventions to Improve Adherence in Adolescents With Chronic Health Conditions: A Systematic Review. JMIR Mhealth Uhealth 2017, 5, e66. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.; Kurup, R.; Laba, T.L.; Santo, K.; Thiagalingam, A.; Rodgers, A.; Woodward, M.; Redfern, J.; Chow, C.K. Mobile Telephone Text Messaging for Medication Adherence in Chronic Disease: A Meta-analysis. JAMA Intern. Med. 2016, 176, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, M.B.; Hussain, T.; Barrera, L.; Cai, S.W.; Haugen, M.; Morgan, E.; Rossoff, J.; Weinstein, J.; Hijiya, N.; Cella, D.; et al. Access to Technology and Preferences for an mHealth Intervention to Promote Medication Adherence in Pediatric Acute Lymphoblastic Leukemia: Approach Leveraging Behavior Change Techniques. J. Med. Internet. Res. 2021, 23, e24893. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.M.; Shah, R.; Beg, U.; Heneghan, M.B. Habit Strength, Medication Adherence, and Habit-Based Mobile Health Interventions Across Chronic Medical Conditions: Systematic Review. J. Med. Internet. Res. 2020, 22, e17883. [Google Scholar] [CrossRef] [PubMed]
| Source, Year (Country) | STUDY DESIGN | Study Aim | Medication | Sample Size N (Subgroup n) | Age at Study Entry, Mean or Median (SD or Range) in Years | Study Length Follow Up Duration | Grade |
|---|---|---|---|---|---|---|---|
| Alsous et al., 2017 [12] (Jordan) | Cross-sectional; single center ≤19 year old, ALL | Assess adherence to 6MP; identify factors influencing adherence | 6MP | 52 | 8.9 (4.4) | Single time point | VL |
| Bhatia et al., 2012 [13] (USA/Canada) | Prospective observational; multicenter ≤21 year old, ALL a | Assess adherence to 6MP, impact on relapse, relation to ethnicity (Hispanic vs. non-Hispanic white) | 6MP | 327 | 4 (1–19) | Data collected over 6 months Median (range): 3.7 (0.4–8.8) years | M |
| Bhatia et al., 2014 [14] (USA/Canada) | Prospective observational; multicenter ≤21 year old, ALL a | Assess adherence to 6MP, impact on relapse, relation to ethnicity (African Americans/Black, Asian, non-Hispanic white) | 6MP | 298 | 6 (2–20) | Data collected over 5 months Median (range): 5 (0.07–9.1) years | M |
| Bhatia et al., 2015 [15] (USA/Canada) | Prospective observational; multicenter ≤21 year old, ALL a | Evaluate intra-individual variability of 6MP on relapse risk | 6MP | 742 (Adherence data n = 470) | 6 (2–21) | Data collected over 6 months. Median (range): 5.2 (0.07–9.4) years | M |
| Bhatia et al., 2020 [16] (USA/Canada) | Randomized control trial with intervention; multicenter ≤21 year old, ALL | Determine if multicomponent intervention (text + direct supervision + education) will increase adherence to 6MP compared to education alone | 6MP | 444 (Intervention n = 230; Education alone n = 214) | 8.1 (IQR 5.3–14.3) | Data collected over 28 days for baseline, intervention period of 16 weeks | H |
| Davies et al., 1993 [17] (UK) | Prospective observational; single center “Children”, ALL | Assess adherence to 6MP | 6MP | 35 | NR (NR) | Data collected at least 2 time points, unknown duration | VL |
| De Oliveira et al., 2004 [18] (Brazil) | Prospective observational; single center <18 year old, ALL b | Assess adherence to 6MP | 6MP | 39 | 4.8 (1.5–16.3) | Data collected over entirety of maintenance Median (range): 5.25 (1.38–6.9) years | L |
| De Oliveira et al., 2005 [19] (Brazil) | Prospective observational; single center <18 year old, ALL b | Assess adherence to 6MP | 6MP | 73 | 4.0 (1.2–16.3) | Data collected over entirety of maintenance Median (range): 4.75 (1.33–8.5) years | L |
| Farberman et al., 2021 [20] (Argentina) | Cross-sectional; multicenter 0–17 year old, ALL/LBL | Assesses adherence to oral chemotherapy and beliefs | Oral maintenance | 203 (ALL n = 163) | NR (NR) 0–2 (2.5%); 2–6 (34.5%); 6–11 (36.6%); 11–18 (27.1%) | Single time point | L |
| Hawwa et al., 2009 [21] (Northern Ireland) | Prospective observational; single center “Children”, ALL | Develop method to prospectively assess adherence to 6MP | 6MP | 19 | 10 (3–17) | Data collected over 6 months | L |
| Heneghan et al., 2020 [22] (USA) | Cross-sectional; single center 1–27 year old (parent of 1–18 year old, patient 12–24 year old), ALL | Assess parent and patient reported adherence and barriers to adherence | 6MP | 57 families (Patient n = 16; Parent n = 49) | Patient participants: 17 (IQR 16–19) Patient reported on by parents: 6 (IQR 5–10) | Single time point | L |
| Hoppmann et al., 2021 [23] (USA/Canada) | Prospective observational; multicenter ≤21 year old, ALL a | Develop risk prediction model for 6MP nonadherence | 6MP | 407 | 7.7 (4.4) | Data collected over 6 months | M |
| Isaac et al., 2020 [24] (USA) | Prospective observational; multicenter 7–19 year old, ALL/LBL c | Assess ethnic differences in parent and child social problem-solving abilities and impact on 6MP adherence | 6MP | 139 | 12.3 (3.4) | Data collected over 15 months | M |
| Jaime-Perez et al., 2009 [25] (Mexico) | Prospective observational; single center ≤15 year old, ALL | Assess adherence to MTX | MTX | 49 | 8 (5–15) | Data collected over 6–7 months | L |
| Kato et al., 2008 [26] (USA/Canada/Australia) | Randomized control trial; multicenter 13–29 year old, multiple malignancies (subgroup ALL/LBL on 6MP) | Determine if video-game intervention will increase adherence and alter other behavioral outcomes in adolescents with malignancies | 6MP | 375 (ALL n = 152; 6MP n = 54) | NR (13–29) Adolescent [13,14,15,16] (66%); Young adult [17,18,19,20,21,22,23,24,25,26,27,28,29] (34%) | Data collected over 3 months | M |
| Khalek et al., 2015 [27] (Egypt) | Prospective observational; single center “Children”, ALL | Assess adherence to 6MP | 6MP | 129 | 6 (1.6–16.1) | Data collected over 15 months | L |
| Kremeike et al., 2015 [28] (Germany) | Prospective observational; single center ≤18 year old, ALL | Assess factors influencing adherence during maintenance therapy | 6MP, MTX | 33 | 8.2 (1–16) | 1–3 time points, unknown time-period | VL |
| Kristjansdottir et al., 2021 [29] (Denmark) | Retrospective; multicenter 18–45 year old, ALL | Assess adherence to 6MP and association with survival in young adults | 6MP | 62 (Adherence data n = 51) | 24.2 (IQR 19.4–33.5) | Data extracted over 11 year period Median (range): 4.1 (0.6–10.7) | M |
| Lancaster et al., 1997 [30] (UK) | Cross-sectional cohort; multicenter “Children”, ALL | Assess interpatient variability at standardized dose of 6MP | 6MP | 496 | NR | Single time point | VL |
| Landier et al., 2017 [31] (USA/Canada) | Prospective observational; multicenter ≤21 year old, ALL a | Comparison of self-reported adherence to electronic monitoring; identify predictors of overreporting | 6MP | 416 | 7 (2–20) | Data collected over 4 months | M |
| Landier et al., 2017 [32] (USA/Canada) | Prospective observational; multicenter ≤21 year old, ALL a | Assess 6MP ingestion habits and impact on adherence and relapse | 6MP | 441 | 6 (2–20) | Data collected over 6 months Median (range): 6.1 (0.8–11) years | M |
| Lansky et al., 1983 [33] (USA) | Cross-sectional; single center “Children”, ALL | Correlate urinary assay of prednisone with demographic and psychological testing | Prednisone | 31 | 7.2 (2–14) | Single time point | VL |
| Lau et al., 1998 [34] (Canada) | Prospective observational with subgroup randomization; single center “Children”, ALL | Assess adherence to 6MP; subgroup randomized to AM followed by PM medication administration to determine if timing affects adherence | 6MP | 24 (Randomized n = 8) | 7.3 (4.6) | Mean (SD, range): 44 (20.2, 15–94) days | L |
| Lennard et al., 1995 [35] (UK) | Cross-sectional; multicenter “Children”, ALL | Assess use of intracellular thioguanine metabolites as indicator of nonadherence | 6MP | 327 | 5 (1–15) | Single time point | VL |
| Lennard et al., 2013 [36] (UK/Ireland) | Prospective observational; multicenter 1–18 year old, ALL d | Assess TPMT phenotype-genotype concordance; influence of TPMT on thiopurine metabolite formation; use of metabolites as marker of nonadherence | 6MP | 1194 (6TG n = 450; 6MP n = 744) | NR (1–18) | Data collected over 2 years | M |
| Lennard et al., 2015 [37] (UK/Ireland) | Prospective observational; multicenter 1–18 year old, ALL d | Assess TPMT polymorphism on thiopurine dose intensity, myelosuppression and treatment outcomes; use of metabolites as marker of nonadherence | 6MP | 1082 | NR (1–18) <2 (8%); 2–9 (77%); 10–18 (15%) | Data collected over 2 years | M |
| MacDougall et al., 1992 [38] (South Africa) | Cross-sectional; single center 3–14 year old, ALL | Assess use of urine 6MP assay as indicator for adherence | 6MP | 21 | NR (3–14) | Single time point | VL |
| Mancini et al., 2012 [39] (France) | Cross-sectional; multicenter all ages, multiple malignancies | Assess concordance between self-reported and physician reported adherence to oral chemotherapy, and factors associated with nonadherence | 6MP, MTX | 52 (ALL n = 49) | 8 (3–77) Children [<11] (60%); Adolescent [11,12,13,14,15,16,17] (23%); Adult [>17] (17%) | Data collected over 7 months | L |
| Pai et al., 2008 [40] (USA) | Prospective observational; multicenter 12–19 year old, ALL | Assess concordance between self-reported adherence to 6MP and intracellular metabolites among adolescents | 6MP | 51 | 15 (12–19) | Data collected over 4 months | L |
| Phillips et al., 2011 [41] (UK) | Prospective single arm pilot study; multicenter “Children”, ALL | Assess safety and parental satisfaction of home-based maintenance intervention to improve adherence to oral chemotherapy | 6MP, MTX | 50 | 8 (3–19) | Data collected over 2 years | M |
| Psihogios et al., 2021 [42] (USA) | Prospective observational; single center 15–25 year old, ALL | Assess feasibly and acceptability of text-based assessment of adherence to 6MP | 6MP | 18 | 17.94 (2.31) | Data collected over 28 days | L |
| Rohan et al., 2015 [43] (USA) | Prospective observational; multicenter 7–19 year old, ALL/LBL c | Assess adherence to 6MP and relationship to patient demographics | 6MP | 139 | 12.3 (3.4) | Data collected over 30 days | M |
| Rohan et al., 2017 [44] (USA) | Prospective observational; multicenter 7–19 year old, ALL/LBL c | Assess concordance of pharmacological (intracellular metabolites) and behavioral (MEMS) measures of 6MP adherence | 6MP | 139 | 12.3 (3.4) | Data collected over 15 months | M |
| Schroder et al., 1986 [45] (Denmark) | Cross-sectional; multicenter “Children”, ALL | Describe pharmacokinetics of MTX in erythrocytes during maintenance therapy; assess use as marker of nonadherence | MTX | 47 | NR | Single time point | VL |
| Schroder et al., 1987 [46] (Denmark) | Cross-sectional; multicenter “Children”, ALL | Describe pharmacokinetics of MTX in neutrophils during maintenance therapy; assess use as marker of nonadherence | MTX | 16 | NR | Single time point | VL |
| Smith et al., 1979 [47] (USA) | Prospective observational; single center “Children”, multiple malignancies (subgroup ALL/LBL) | Assess prednisone adherence in pediatric malignancies | Prednisone | 52 (ALL n = 43) | NR (0.67–17) | Data collected over 16 months | L |
| Wu et al., 2008 [48] (USA) | Cross-sectional; national database ≤21 year old, ALL | Assess adherence to 6MP and MTX using prescription refills recorded in national claims database Medical Outcomes Research for Effectiveness and Economics (MORE) Registry | 6MP, MTX | 900 | 12.7 (4.2) Children [<12] (42%); Adolescents [12,13,14,15,16,17] (42%); Young adult [18,19,20,21] (16%) | Single time point | L |
| Author, Year | Adherence Assessment [S] Subjective [O] Objective | Adherence Rate | Definition and Prevalence of Nonadherence | Clinical Outcomes Related to Nonadherence |
|---|---|---|---|---|
| Mercaptopurine | ||||
| Alsous et al., 2017 [12] | [S] Survey—MARS a for parents and adolescents [O) Metabolite—TGN, MMP b | NR | [S] MARS score <90%: parents 5.8% (n = 3/52), adolescents 0% (n = 0/15) [O] TGN and MMP <20%ile: 15.4% (n = 8/52) Overall detected by at least 1 method: 19.2% (n = 10/52) | NA |
| Bhatia et al., 2012 [13] | [O] Electronic—MEMS c | 94.7% month 1 to 90.2% month 6 | [O] MEMS adherence <95%: 44% (n = 142/327) | Increased incidence and risk of relapse with nonadherence Cumulative incidence of relapse at 4 years: 11% (nonadherent 17% vs. adherent 4.9%, p = 0.0001); Relapse OR2.5 (p = 0.002); Adjusted risk of relapse attributed to nonadherence 58.8% |
| Bhatia et al., 2014 [14] | [O] Electronic—MEMS | 95% month 1 to 91.8% month 5 | [O] MEMS adherence <90%: Overall 20.5% (n = 61/298); non-Hispanic white 13% (n = 20/159), Asian 15% (n = 11/71), African American/black 44% (n = 30/68) | Increased risk of relapse with nonadherence Relapse: 6.4% (n = 19/298); Relapse risk from nonadherence HR3.9 (p = 0.01); Adjusted risk of relapse attributed to nonadherence 33% |
| Bhatia et al., 2015 [15] | [O] Electronic—MEMS | NR | [O] MEMS adherence <95%: 42% (n = 198/470) | Increased incidence and risk of relapse with nonadherence Cumulative incidence of relapse at 6 years: 9% (nonadherent 13.9% vs. adherent 4.7% p = 0.001); Relapse risk from nonadherence HR2.7 (p = 0.01) Varying metabolite (TGN) levels not predictive of relapse overall, but among adheres, highly variable TGN levels can predict relapse (HR4.4, p = 0.02) |
| Bhatia et al., 2020 [16] | [O] Electronic—MEMS * Intervention: Education + daily text reminders prompting supervised therapy | Intervention group: Baseline 92.2%; post 94% Education only group: Baseline 93.5%; post 92.5% | [O] MEMS adherence <95%: Baseline 31% (n = 138/444) Intervention group: Baseline 32% (n = 74/230); post 35% (n = 81/230) Education only group: Baseline 29.5% (n = 64/214); post 41% (n = 88/214) | Intervention did not improve overall prevalence of nonadherence (p = 0.08), but intervention increased mean adherence rate in patients ≥12 years old (93% vs. 90%, p = 0.04) and ≥12 year old with baseline adherence <90% (83.4% vs. 74.6%, p = 0.008) |
| Davies et al., 1993 [17] | [S] Interview of parents [O] Metabolite—TGN | NR | [S] Admitted nonadherence: 9% (n = 2/22); Equivocal history of adherence: 27% (n = 6/22) [O] Wide fluctuation of TGN levelb: 27% (n = 6/22) | NA |
| De Oliveira et al., 2004 [18] | [S] Interview of parents [O1] Review of medical chart [O2] Metabolite—TGN, MMP | NR | [S] Report 2+ missed doses: 33% (n = 13/39) [O1] Record of interruption or irregular dose administration: 30.7% (n = 12/39) [O2] Significant TGN and MMP decrease without decrease in prescribed dose: 16.6% (n = 6/36) Overall detected by at least 1 method: 53.8% (n = 21/39); by at least 2 methods: 20.5% (n = 8/39) | Increased relapse prevalence in nonadherent group Relapse: 26% (n = 10/39); nonadherent 33% (n = 7/21) vs. adherent 17% (n = 3/18) |
| De Oliveira et al., 2005 [19] | [S] Interview of parents [O] Review of medical chart | NR | [S] Report 2+ missed doses: 27% (n = 20/73) [O] Record of interruption or irregular dose administration: 30% (n = 22/73) | No difference in EFS and relapse with nonadherence Overall 8.5 year EFS 72.4% (nonadherent 72% vs. adherent 72.8%, p = 0.88); Relapse: 25% (n = 18/73); nonadherent 25% (n = 5/20) vs. adherent 25% (n = 13/25) |
| Hawwa et al., 2009 [21] | [S] Survey—MAS-4 d for parents [O] Metabolite—TGN, MMP | NR | [S] MAS ≥2: 15.8% (n = 3/19) [O] Low TGN and MMP cluster: 21.1% (n = 4/19) [O] Wide fluctuation of TGN level: 5.3% (n = 1/19) Overall detected by at least 1 method: 26.3% (n = 5/19) | |
| Heneghan et al., 2020 [22] | [S1] Survey—MMAS-8 e for parents and adolescents [S2] Survey—VAS f for parents and adolescents | NR | [S1] MMAS <8: Parents 43% (n = 21/49); adolescents 73% (n = 12/16) [S2] VAS <95%: Parents 10% (n = 5/49); adolescents 12% (n = 2/16) | |
| Hoppmann et al., 2021 [23] | [O] Electronic—MEMS | NR | [O] MEMS <95%: 36% (n = 148/407); MEMS <90%: 28% (n = 115/407) Month 3 data for MEMS <90% used to develop prediction model; predicated probability of 0.3 used as cut off for binary risk classifier of high or low risk of nonadherence with sensitivity 71%, specificity 76% | Risk of relapse higher with higher probability of nonadherence Cumulative incidence of relapse in 5 years: 11.9% for at high-risk nonadherence vs. 4.5% for at low risk (p = 0.006) Relapse risk at high risk nonadherence HR2.2 (p = 0.07) |
| Isaac et al., 2020 [24] | [O] Electronic—MEMS | Mean (SD): Non-minority 82.5% (3.3%), minority 82.3% (1.5%), p >0.05 | NR | Relapse: 8.6% (n = 12/139) |
| Kato et al., 2008 [26] | [S1] Survey—MAS-4 d (n = 375) [S2] Survey—CDCI g (n = 375) [O] Metabolite—MMP (n = 54) * Intervention: Cancer based videogame | Mean MAS-4 score (SD): Intervention 2.9 (1.1), control 3.0 (1.1) (p = ns) Mean CDCI score (SD): Intervention 81 (8.7), control 78.4 (7.5) (p = ns) | [O] MMP level <1000 pmol/8 × 108 erythrocytes: NR, lower nonadherence with intervention than control, p < 0.001 | Videogame intervention significantly improved prevalence of nonadherence |
| Khalek et al., 2015 [27] | [S] Survey for parents [O] Drug level—serum 6MP | NR | [S] Reported 2+ missed doses: 55% (n = 71/129) [O] Serum 6MP <50%ile (<9.3 ng): 50% (n = 65/129) | |
| Kremeike et al., 2015 [28] | [S] Survey for parents [O] Metabolite—TGN, MMP | NR | [S] Reported non-exact medication intake: 12% (n = 4/33) [O] TGN and MMP below therapeutic range: TGN 58% (n = 23/40), MMP 67% (n = 27/40)M | |
| Kristjansdottir et al., 2021 [29] | [O] Metabolite—TGN, MMP | NR | [O] Undetectable MMP in TPMT WT: 9.8% (n = 5/51); TGN <100 nmol/mmol hemoglobin with normal ALT and wbc: 13.7% (n = 7/51); Wide fluctuation in TGN level: 52.6% (n = 20/38) Overall detected by at least 1 method: 49% (n = 25/51) | No association between nonadherence and relapse risk Relapse: 11.3% (n = 7/62) 5-year DFS 78%, OS 91.7% |
| Lancaster et al., 1997 [30] | [O] Metabolite—TGN | NR | [O] Undetectable TGN level: 2% (n = 9/496) | |
| Landier et al., 2017 [31] | [S] Survey for parents and adolescents [O] Electronic—MEMS | Self-report 92.6% MEMS 91.0% | [O] MEMS <95%: 39.7% (n = 165/416) Perfect reporter (MEMS matched self-report): 12% (n = 50/416); Over reporter (Self-report > MEMS): 23.6% (n = 98/416) | Self-report overestimates intake, especially in nonadherent patients. 88% (n = 366/416) had self-report > MEMS at least some of the time. Nonadherent patients were more likely (OR9.4) to overestimate intake. Self-report sensitivity 52.7%, specificity 95.8% for detecting nonadherence |
| Landier et al., 2017 [32] | [O] Electronic—MEMS | MEMS 91% | [O] MEMS <95%: 48.3% (n = 193/441) | No association between relapse risk and ingestion habits Cumulative incidence of relapse at 5 years: 8.6%. No difference in taking with food, with dairy, in morning or evening |
| Lau et al., 1998 [34] | [O] Electronic—MEMS | NR | [O] MEMS <95%: 58% (n = 14/24); MEMS <90%: 33% (n = 8/24) | |
| Lennard et al., 1995 [35] | [O] Metabolite—TGN, MMP | NR | [O] TGN and MMP <25%ile: 10% (n = 32/237) | |
| Lennard et al., 2013 [36] | [O] Metabolite—TGN, MMP | NR | [O] Undetectable TGN and MMP: 2.7% (n = 20/744) | |
| Lennard et al., 2015 [37] | [O] Metabolite—TGN, MMP | NR | [O] Undetectable TGN and MMP: 2.8% (n = 20/707); TGN and MMP <25%ile: 10% (n = 71/707) | 5 year EFS 80%, OS 89%. No difference in EFS with nonadherence to 6MP. |
| MacDougall et al., 1992 [38] | [O] Drug level—urine 6MP | NR | Unable to detect nonadherence due to variability in 6MP urine excretion and unpredictable pattern of night-time voids Prevalence of adherence [O] Detectable urine 6MP in first morning voids of PM 6MP takers: 81% (n = 17/21) | |
| Pai et al., 2008 [40] | [S] Interview for patients [O] Metabolites—TGN, MMP | NR | [S] Reported missed dose in past week: 24.5% (n = 14/51); Missed dose in past 2 weeks: 45.1% (n = 23/51) [O] TGN and MMP <95%ile: 52.9% (n = 27/51) | Self-report at month 2 predicts nonadherence at month 4 (OR3.54, p < 0.05) |
| Psihogios et al., 2021 [42] | [S1] Survey—Text survey for patients (n = 18) [S2] Survey for physicians (n = 16) [O] Electronic—MEMS (n = 15) * Intervention: Text survey to assess adherence | Text 96.8%; Physician 97.8%; MEMS 90.7% # missed doses mean (SD): Text 0.89 (1.64); Provider 0.63 (0.96); MEMS 2.6 (3.09) | NR | Daily text messages feasible and reliable for delivering medication adherence assessment |
| Rohan et al., 2015 [43] | [O] Electronic—MEMS | Baseline 86.2%, decline to 83% in 1 month | [O] MEMS <95%: 44% (n = 58/139); MEMS <90%: 35% (n = 46/139) | |
| Rohan et al., 2017 [44] | [O1] Metabolite—TGN, MMP [O2] Electronic—MEMS | MEMS—low TGN/low MMP: 72–78%; Low TGN/high MMP 85–90%; high TGN/low MMP 86–89% (p = 0.008) | [O1] Low TGN and MMP cluster: 40.8% (n = 312/764) [O2] MEMS <95%: Low TGN/low MMP group (nonadherent metabolite) 60.3–74.2%; Low TGN/high MMP (adherent metabolite) 42.4–56.4% | |
| Wu et al., 2008 [48] | [O] Review of prescription claims | Medication possession ratio i 6MP 85% | NR | |
| Methotrexate | ||||
| Jaime-Perez et al., 2009 [25] | [S] Interview for parents [O1] Review of medical charts [O2] Drug level—serum MTX | NR | [S] Reported 2+ missed doses: 10% (n = 5/49) [O1] Record of 2+ missed doses: 16.3% (n = 8/49) [O2] Undetectable serum MTX level: 29% (n = 14/49) | |
| Kremeike et al., 2015 [28] | [S] Survey for parents | NR | [S] Reported non-exact medication intake: MTX 33% (n = 7/31) | |
| Schroder et al., 1986 [45] | [S] Interview for parents [O] Drug level—erythrocyte MTX | NR | [O] Undetectable eMTX level: 6% (n = 3/47) [S] Admitted nonadherence: 4% (n = 2/47) | |
| Schroder et al., 1987 [46] | [S] Interview for parents [O] Drug level—neutrophil MTX level | NR | [O] Undetectable nMTX level: 5% (n = 1/19) [S] Admitted nonadherence: 5% (n = 1/19) | |
| Wu et al., 2008 [48] | [O] Review of prescription claims | Medication possession ratio i MTX 81% | Did not provide information about prevalence of nonadherence | |
| Prednisone | ||||
| Lansky et al., 1983 [33] | [O] Metabolite—urine 17 kgs/Cr | NR | [O] Average urine 17-kgs/Cr value <18.7: 42% (n = 13/31) | |
| Smith et al., 1979 [47] | [O] Metabolite—urine 17-kgs/Cr | NR | [O] Average urine 17-kgs/Cr <18.7: 33% (n = 9/27) | |
| Not specified | ||||
| Farberman et al., 2021 [20] | [S] Survey—SMAQ h for parents, adolescents, physicians | NR | [S] SMAQ nonadherent: parents 25% (n = 48/194); adolescent 55% (n = 20/37); physician 18% (n = 37/203) | |
| Mancini et al., 2012 [39] | [S1] Survey (3 questions) and interview for parents and adolescents [S2] Survey for physicians | NR | [S1] MMAS-3 >1 or reported 1+ missed doses in past week: Overall 23% (n = 12/52); children 13% (n = 4/31); adolescents 33% (n = 4/12); adults 44% (n = 4/9) [S2] Physician reported missed dose: 11.5% (n = 6/52) | |
| Phillips et al., 2011 [41] | [O] Tablet count * Intervention: Home-based maintenance program | [O] Tablet count <97% adherence: After 3 months intervention 72% (n = 35/50); After remediation 22% (n = 11/50); After 2 years 45% (n = 23/50) * Remediation: program wide education, specific confrontation with parental intervention and directly observed medication therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.L.; Heneghan, M.B.; Badawy, S.M. Adherence to Oral Chemotherapy in Acute Lymphoblastic Leukemia during Maintenance Therapy in Children, Adolescents, and Young Adults: A Systematic Review. Curr. Oncol. 2023, 30, 720-748. https://doi.org/10.3390/curroncol30010056
Zeng XL, Heneghan MB, Badawy SM. Adherence to Oral Chemotherapy in Acute Lymphoblastic Leukemia during Maintenance Therapy in Children, Adolescents, and Young Adults: A Systematic Review. Current Oncology. 2023; 30(1):720-748. https://doi.org/10.3390/curroncol30010056
Chicago/Turabian StyleZeng, Xiaopei L., Mallorie B. Heneghan, and Sherif M. Badawy. 2023. "Adherence to Oral Chemotherapy in Acute Lymphoblastic Leukemia during Maintenance Therapy in Children, Adolescents, and Young Adults: A Systematic Review" Current Oncology 30, no. 1: 720-748. https://doi.org/10.3390/curroncol30010056
APA StyleZeng, X. L., Heneghan, M. B., & Badawy, S. M. (2023). Adherence to Oral Chemotherapy in Acute Lymphoblastic Leukemia during Maintenance Therapy in Children, Adolescents, and Young Adults: A Systematic Review. Current Oncology, 30(1), 720-748. https://doi.org/10.3390/curroncol30010056






