Abstract
Background: Rectal gastrointestinal stromal tumours (GISTs) have many treatment options, but uncertainty remains regarding the best treatment regimen for this rare pathology. The aim of this review is to assess the optimal management approach including timing of chemotherapy. Methods: PubMed, EMBASE, and Cochrane databases were searched for relevant articles comparing the impact of radical vs. local excision, and neoadjuvant vs. adjuvant therapy had on outcomes in the management of rectal GISTs. We specifically evaluated the influence that the aforementioned factors had on margins, recurrence, overall survival, 5-year disease-free survival, and hospital length of stay. Results: Twenty-eight studies met our predefined criteria and were included in our study, twelve of which were included in the quantitative synthesis. When comparing neoadjuvant versus adjuvant chemotherapy, our meta-analysis noted no significance in terms of margin negativity (R0) (odds ratio [OR] 2.01, 95% confidence interval [CI], 0.7–5.79, p = 0.20) or recurrence rates (OR 0.22, 95% CI, 0.02–1.91, p = 0.17). However, there was a difference in overall 5-year survival in favour of neoadjuvant therapy (OR 3.19, 95% CI, 1.37–7.40, * p = 0.007). Comparing local excision versus radical excision, our meta-analysis observed no significance in terms of overall 5-year survival (OR1.31, 95% CI, 0.81–2.12, p = 0.26), recurrence (OR 0.67, 95% CI, 0.40–1.13, p = 0.12), or 5-year disease-free survival (OR 1.10, 95% CI, 0.55–2.19, p = 0.80). There was a difference in length of hospital stay with a reduced mean length of stay in local excision group (mean difference [MD] 6.74 days less in the LE group; 95% CI, −6.92–−6.56, * p =< 0.00001) as well as a difference in R0 rates in favour of radical resection (OR 0.68, 95% CI, 0.47–0.99, * p = 0.05). Conclusion: Neoadjuvant chemotherapy is associated with improved overall 5-year survival, while local excision is associated with reduced mean length of hospital stay. Further large-volume, prospective studies are required to further define the optimal treatment regimen in this complex pathology.
1. Introduction
While gastrointestinal stromal tumours (GISTs) have the highest incidence among mesenchymal tumours, their presence in the rectum is rare, accounting for only 5% of all GISTs [1,2,3]. Surgery has long been the mainstay of treatment; however, the challenges faced in resection due to location and disease biology combined with advances in therapeutic modalities has led to an evolution in the management of rectal GIST [4].
Due to the challenging anatomy of the rectum, in the sense that complete tumour resection must be achieved while preserving sphincter function, a wide variety of surgical approaches have been established [5]. Historically, when tumours were large and encompassed a significant portion of the rectal lumen, there were few options available beyond radical resections such as abdominoperineal resection (APR), and in extreme cases total pelvic exenteration (TPE) [6]. While this was curative, it was associated with significant morbidity [7]. Once it was established that lymph node spread is negligible, more conservative sphincter-sparing surgeries involving local excision became more popular [8]. As surgical techniques progressed, with a shift towards minimally invasive surgery (MIS), these techniques (such as transanal minimally invasive surgery—TAMIS) were also employed to treat rectal GISTs [9].
A breakthrough in treatment options for rectal GIST was the introduction of tyrosine kinase inhibitors (TKIs) [10]. Imatinib was initially shown to reduce the risk of disease recurrence and was subsequently used as a method of reducing tumour burden preoperatively to facilitate MIS options [11]. The efficacy of neoadjuvant treatment was significant enough, in that it allowed tumours which were previously deemed unresectable to become resectable, often using sphincter-sparing methods and with lower morbidity rates [12]. Despite the development of new treatment modalities for rectal GISTs, there is an appreciable gap in the literature on the best regimen of choice due to the rarity of this condition. Our paper aims to collect and analyse data in the literature to determine the optimal treatment approach in the management of rectal GIST.
2. Methods
This systematic review and meta-analysis was conducted in accordance with the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) extension statement for reporting of systematic reviews incorporating network meta-analyses of healthcare interventions [13]. Local institutional ethical approval was not sought as all included data were obtained from previously published studies. The study was registered with the PROSPERO database (ID: CRD42022331856)
2.1. Study Selection Strategy
A formal systematic search was performed of the PubMed, EMBASE, and Cochrane databases to identify relevant titles. The following search terms were used: “gastrointestinal stromal tumours”, “GISTs”, “rect*”, “(neo)adjuvant therapy”, “local excision”, “radical excision”, and “survival”. The symbol “*” was used to allow variations on a word stem to be included in the search results. Furthermore, the following MeSH (medical subject headings) were used: GIST[MeSH], resection[MeSH], chemotherapy[MeSH], and survival[MeSH]. The grey literature (academic papers, research and committee reports, conference papers, and ongoing research) was also searched to further identify ongoing works of literature. This search was performed by two independent reviewers (S.I.K. and N.O.S.), using a predetermined search strategy that was designed by the senior authors. Details in relation to the search strategy can be found in Supplementary File S1. Manual cross-referencing of reference lists from previous review articles and included trials was undertaken. Manual removal of duplicate studies was performed before all titles were screened. Thereafter, studies considered to be appropriate had their abstracts and/or full text reviewed. Retrieved studies were reviewed to ensure inclusion criteria were met for the primary outcome at a minimum, with discordances in opinion resolved through consultation with a third author (M.K.). Data extraction was also performed by two independent reviewers (S.I.K. and N.O.S.), with study details, basic patient clinicopathological characteristics and surgical data all recorded. Furthermore, information extracted was based on the PICOTS framework (population, intervention, comparator, outcomes, timing, and setting). The final search was performed on 1 March 2022. A grey literature search was also conducted to further identify ongoing works of literature.
2.2. Inclusion Criteria
All original studies, irrespective of design, which compared outcomes between patient cohorts receiving any form of surgical treatment for rectal GISTs and which reported on at least one of the predefined outcomes of interest including overall survival (OS), disease-free survival (DFS), and recurrence were included in the review. In addition, we included only studies from the year 2000 onwards, studies which reported on ≥10 patients, and only studies that were written in English.
2.3. Exclusion Criteria
Studies published prior to the year 2000, reporting on <10 patients and in languages other than English were excluded from analysis.
2.4. Data Extraction and Critical Appraisal
Data extracted from each study included: year of publication, journal of publication, primary authors name, study design, period of study, number of patients included, type of surgery (primarily local resection vs. radical resection), use of neoadjuvant and adjuvant therapy, and long-term outcomes (overall survival, disease-free survival, and recurrence). Additional data collected included margin status, length of stay in hospital, and intraoperative tumour rupture rates.
Data were collected by two reviewers independently, using the following headings: study details, study design, population, intervention, comparison groups, and outcomes. Conflicts between the two reviewers were resolved following a discussion and final decision by the senior author.
The quality of the studies included in this systematic review was assessed using the Newcastle Ottawa scale. Furthermore, the certainty of evidence was assessed using the grading of recommendations, assessment, development, and evaluations (GRADE) tool for grading quality of evidence [14]. The quality score rating was determined for each publication and recorded.
2.5. Outcomes of Interest
The following outcomes were used in the analysis to compare the effect of neoadjuvant versus adjuvant therapy and local excision versus radical excision.
2.5.1. Primary Outcomes
The primary outcome of interest was the impact that surgical strategy had on survival outcomes (5-year overall survival, 5-year disease-free survival, recurrence and negative margins (R0) rates for the management of rectal GISTs). Specifically comparing neoadjuvant versus adjuvant therapy and local excision versus radical excision.
2.5.2. Secondary Outcomes
Alongside the primary outcomes, length of stay and intraoperative tumour rupture between local excision versus radical excision was analysed.
2.6. Statistical Analysis
Statistical analysis was performed using Revman Statistical Software (Ver. 5, Copenhagen, Denmark). Binary outcome data were reported as odd ratios (OR) and 95% confidence interval (95% CI) were estimated using the Mantel–Haenszel method. For continuous data, mean differences and 95% CI were estimated using inverse variance weighting. Outcome measures (mean + standard deviation and median + interquartile range) were recorded. If needed, outcome variables (mean and SD) were estimated from the median and range using formula described by Hozo et al. [15]. Heterogeneity was assessed by I-squared statistics, with >50% being considered as considerable heterogeneity. Statistical significance was attributed to p-value < 0.05.
3. Results:
3.1. Search Results
Our initial search produced 1797 results in total. After removing duplicate articles, the remaining 552 articles were screened. From these articles, 51 articles had their abstracts reviewed for eligibility, of which sixteen articles were removed due to not meeting the inclusion/exclusion criteria. Ultimately, we identified 28 studies which met our predefined criteria and reported our desired outcomes, and 12 of these studies were included in our meta-analysis (Supplementary File S1).
3.2. Methodological Characteristics and Quality of Studies
All 28 of the identified studies are retrospective, cohort studies with more than ten patients included [1,5,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. Only articles published in English were accepted. A summary of Table 1 summarises the methodological characteristics of the included studies. The methodological quality of the included studies was generally good and can be found in Supplementary Table S1. Nine studies achieved a rating of 7 or higher on the Newcastle Ottawa Scale (NOS), meeting criteria for ”high quality” studies. The GRADE certainty of evidence ranged from very low to low and is presented in the Supplementary Table S2.

Table 1.
Methodological characteristics of the included studies.
3.3. Participant Characteristics
The total number of participants who underwent surgery from the twenty-eight studies included was 1654. Of these patients, 813 underwent local excision, while 740 patients underwent radical excision procedures, and it was unspecified in 101 patients. Overall, 17/28 reported on initial diagnosis tumour size, with an overall mean of 5.32 cm (±3.77). Overall, 8/28 studies (293 patients) report on which specific surgical approach was undertaken. Laparotomy was most commonly performed (59.0% (173/293) of patients), followed by transanal (29.4% (86/293)) and laparoscopic (11.6% (34/293)). A summary of surgical details, including tumour size and margin status data, can be found in Table 2.

Table 2.
Summary of Surgical Detail.
3.4. Neoadjuvant Versus Adjuvant Outcomes
3.4.1. Chemotherapy Characteristics
Overall, 23 studies reported on neoadjuvant chemotherapy. In total, 40.9% (539/1316) patients underwent neoadjuvant chemotherapy. All studies which reported on neoadjuvant therapy utilised Imatinib (99.6% (537/539)), with only 1/23 studies reporting Sunitinib (1/539) and Adriamycin plus ifosfamide (1/539) use. The overall median time of neoadjuvant therapy was 7.7 months (range: 1–102 months). Overall, 22 studies reported on adjuvant chemotherapy, with 39.4% (491/1246) of patients undergoing adjuvant therapy. All studies that reported on adjuvant therapy utilized Imatinib only. The overall median time of adjuvant therapy was 18 months (range: 0–112 months). A summary of chemotherapy details can be found in Table 3.

Table 3.
Summary of Chemotherapy Details.
3.4.2. Recurrence
Four studies reported recurrence rates between neoadjuvant and adjuvant therapy groups. The recurrence rate was 18.75% in the neoadjuvant group and 45.8% in the adjuvant therapy group. A meta-analysis of the included studies using an M-H random effects model showed no significant difference between the two groups in regard to recurrence rates (OR 0.22, 95% CI, 0.02–1.91, p = 0.17), with significant heterogeneity between studies (I2 = 83%) (Figure 1A).
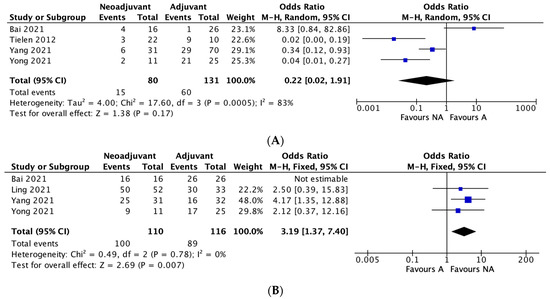
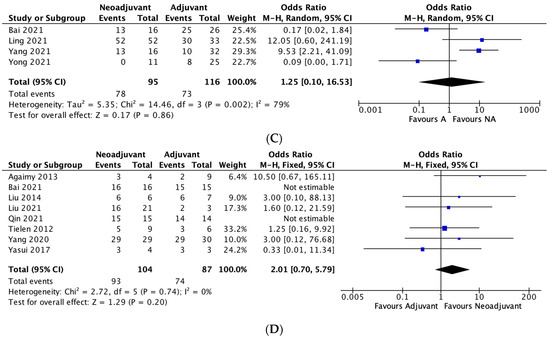
Figure 1.
(A–D): Neoadjuvant vs. adjuvant chemotherapy meta-analysis outcomes. (A) Recurrence, (B) Five-year overall survival, (C) Five-year disease-free survival, (D) Negative margin (R0) rates.
3.4.3. Five-Year Overall Survival
Four studies reported overall 5-year survival rates between the two groups. The 5-year survival rate was 90.9% in the neoadjuvant group and 76.7% in the adjuvant group. A meta-analysis of the included studies using an M-H fixed effects model showed a significant difference between the two groups in terms of overall 5-year survival rates, in favour of neoadjuvant therapy (OR 3.19, 95% CI, 1.37–7.40, * p = 0.007), with no heterogeneity between studies (I2 = 0%). (Figure 1B).
3.4.4. Five-Year Disease-Free Survival
Four studies reported on disease-free survival rates between the two groups. The 5-year disease-free survival rate was 82.1% in the neoadjuvant group and 62.9% in the adjuvant group. The meta-analysis demonstrated no significant difference between the two groups in terms of 5-year disease-free survival rates (OR 1.25, 95% CI, 0.10–16.53, p = 0.86), with significant heterogeneity between studies (I2 = 79%). (Figure 1C)
3.4.5. Negative Margin (R0) Rates
Eight studies reported on R0 rates between the two groups. The R0 rate was 89.4% in the neoadjuvant group and 85.1% in the adjuvant group. The meta-analysis demonstrated no significant difference between the two groups in terms of R0 rates (OR 2.01, 95% CI, 0.7–5.79, p = 0.20), with no heterogeneity between studies (I2 = 0%). (Figure 1D)
3.5. Local Excision vs. Radical Resection Outcomes
3.5.1. Recurrence
Six studies reported on overall recurrence rates between the two groups. The overall recurrence rate was 25% in the local excision group and 32.3% in the radical excision group. A meta-analysis performed using the M-H fixed effects model demonstrated no significant difference between the two groups in terms of overall recurrence (OR 0.67, 95% CI, 0.40–1.13, p = 0.12), with moderate heterogeneity between studies (I2 = 42%). (Figure 2A).
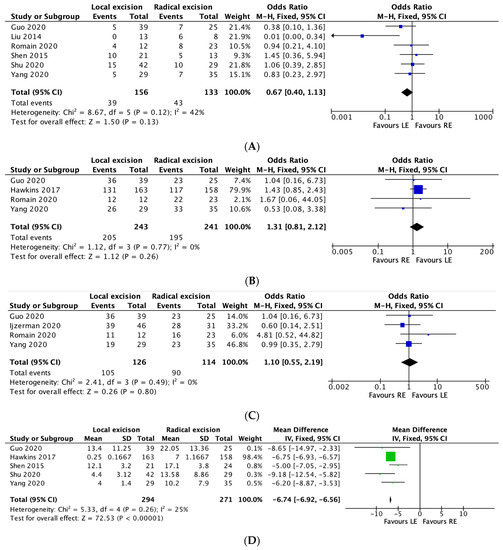

Figure 2.
(A–F): Local excision vs. radical excision meta-analysis results. (A) Recurrence, (B) Five-year Overall Survival, (C) Five-year Disease-free Survival, (D) Length of Stay, (E) Intraoperative Tumour Perforation, (F) R0 Rates.
3.5.2. Five-Year Overall Survival:
Four studies reported overall 5-year survival rates. The rate was 84.4% in the local excision group and 80.9% in the radical excision group. The meta-analysis noted no significant difference between the two groups in terms of 5-year survival rates (OR 1.31, 95% CI, 0.81–2.12, p = 0.26), with no heterogeneity between studies (I2 = 0%). (Figure 2B)
3.5.3. Five-Year Disease-Free Survival:
Four studies reported 5-year disease-free survival between the two groups. The 5-year disease-free survival rate was 83.3% in the local excision group and 78.9% in the radical excision group. The meta-analysis demonstrated no significant difference between the two groups in relation to 5-year disease-free survival rates (OR 1.10, 95% CI, 0.55–2.19, p = 0.80), with no heterogeneity between studies (I2 = 0%). (Figure 2C).
3.5.4. Length of Stay
Five studies reported on length of stay (days) between the two groups. The meta-analysis performed using the fixed-effects model demonstrated a reduced length of stay in the local excision group (MD 6.74 days less in the LE group; 95% CI, −6.92–−6.56, * p =< 0.00001), with low heterogeneity between studies (I2 = 25%). (Figure 2D).
3.5.5. Intraoperative Tumour Perforation:
Five studies reported on tumour perforation rates between the two groups. The tumour perforation rate was 5.6% in the local excision group and 5.3% in the radical excision group. Meta-analysis revealed no significant difference between the two groups in terms of tumour perforation rates (OR 0.90, 95% CI, 0.35–2.34, p = 0.83), with no heterogeneity between studies (I2 = 0%). (Figure 2E)
3.5.6. R0 Rates
Twelve studies reported R0 rates between the two groups. The R0 rate was 78.5% in the local excision group and 83.3% in the radical resection group. Meta-analysis demonstrated a significant difference in R0 rates between the two groups, in favour of radical resection (OR 0.68, 95% CI, 0.47–0.99, * p = 0.05), with moderate heterogeneity reported across the studies (I2 = 43%). (Figure 2F)
4. Discussion
Our systematic review observed how the timing of chemotherapy and radicalness of surgery impacts on the management of rectal GISTs. While case reports on the topic are common, large prospective studies are limited, and there is a considerable lack of high-level evidence regarding the optimal management of this rare entity. To the best of our knowledge, our study is the first meta-analysis comparing local and radical excision, as well as neoadjuvant vs. adjuvant therapy in the management of rectal GISTs.
Our review demonstrated no significant difference between neoadjuvant and adjuvant therapy in terms of recurrence and 5-year disease-free survival rates. It did however reveal a significant overall survival benefit in favour of neoadjuvant therapy. These results demonstrate that the use of neoadjuvant chemotherapy in rectal GISTs patients might not play a role in preventing local recurrence, but potentially does impact overall survival (90.9% in the neoadjuvant group vs. 76.7% in the adjuvant group). Similarly, when comparing local vs. radical excision there was no difference in 5-year disease free survival or recurrence rates. In addition, the method of excision did not influence overall survival either (84.4% in the local excision group vs. 80.9% in the radical excision group). However, local excision did have some minor benefits such as reduced hospital stay. These results suggest that local excision when applicable should be utilised as it does not have inferior oncological outcomes, and would likely reduce overall stoma rate or morbidity that is associated with radical rectal surgery.
Recent studies have highlighted the benefits of neoadjuvant therapy in tumour down-sizing resulting in R0 rates after resection as well as improved anal sphincter preservation [12]. This can often be challenging, as rectal GIST’s can be of a large size within the confines of a narrow pelvis [5]. Unfortunately, data in this study were limited in terms of tumour response to neoadjuvant therapies; however, they did show an overall survival benefit with neoadjuvant treatment. The benefit in overall survival may be attributed to the fact that neoadjuvant therapy in general is associated with improved patient compliance when compared to that of adjuvant therapy [42]. Neoadjuvant radiotherapy has also been reported to have fewer significant side effects [42]. However, due to significant heterogeneity between studies in terms of neoadjuvant and adjuvant regimens, it would be prudent to suggest the benefit of one modality over another without further large-scale randomized trials. Due to variation in the chemotherapeutic regimens across different studies, there is a lack of comparability and results should therefore be interpreted with caution.
Regardless of treatment choice, it is imperative to take patient quality of life (QoL) into consideration when selecting patients for radical therapy. Our data provide important information regarding the non-inferiority of local excision in terms of recurrence or survival, which may reduce the necessity of radical resection and its associated morbidity moving forward. To date, there is a considerable lack of QoL data from patients being managed for rectal GISTs. Additionally, the introduction of TKIs, such as Imatinib, has revolutionized the management of primary and recurrent diseases, particularly via tumour downsizing and a reduction in mitotic activity, morbidity, and recurrence [12]. This method of chemo-reduction is particularly useful for distal tumours, where conventional resection may compromise the anal sphincter [16], resulting in significant long-term morbidity. Few studies have directly compared quality of life in patients undergoing radical or sphincter-preserving surgery; however, evidence does point towards improved functional outcomes without compromising oncological outcomes [43,44].
The authors acknowledge that the review does have some limitations. The rarity of this condition, combined with the heterogeneity in management between studies, prevents large-volume analysis. It is unlikely that an RCT could ever recruit adequately and compare several treatment approaches. Despite this, our study provides important data for the shared decision-making process. Future studies should also focus on quality-of-life outcomes in patients undergoing local or radical excision of rectal GIST, incorporating outcome data on surgical approach (open versus minimally invasive platforms). Nonetheless, our study will impact clinical practice by allowing clinicians and surgeons to counsel patients on the optimal managements options and inform them on expected outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/curroncol30010034/s1, File S1: Study selection. A PRISMA Flowchart of the selection of relevant publications included in this review; Table S1: Newcastle Ottawa Scale (NOS) risk of bias assessment for non-randomised studies; Table S2: GRADE Certainty of Evidence table.
Author Contributions
Conceptualization, S.I.K., N.J.O. and M.E.K.; methodology, S.I.K., N.J.O. and H.C.T.; software, N.J.O.; validation, E.R., B.J.M. and P.M.; formal analysis, N.J.O.; investigation, S.I.K.; resources, S.I.K. and H.C.T.; data curation, S.I.K., H.C.T. and E.R.; writing—original draft preparation, S.I.K. and N.J.O.; writing—review and editing, N.J.O., B.J.M., P.M., J.O.L., D.O.K. and M.E.K.; visualization, S.I.K. and N.J.O.; supervision, B.J.M., P.M., J.O.L., D.O.K. and M.E.K.; project administration, B.J.M., P.M., J.O.L., D.O.K. and M.E.K.; funding acquisition, N/A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, H.; Yan, Z.; Liao, G.; Yin, H. Treatment strategy of rectal gastrointestinal stromal tumor (GIST). J. Surg. Oncol. 2014, 109, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Sarlomo-Rikala, M.; Lasota, J. Gastrointestinal stromal tumors: Recent advances in understanding of their biology. Hum. Pathol. 1999, 30, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Søreide, K.; Sandvik, O.M.; Søreide, J.A.; Giljaca, V.; Jureckova, A.; Bulusu, V.R. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016, 40, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, H.; Kanda, T.; Tajima, Y.; Shimada, Y.; Ichikawa, H.; Hanyu, T.; Ishikawa, T.; Wakai, T. Management of rectal gastrointestinal stromal tumor. Transl. Gastroenterol. Hepatol. 2018, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Tielen, R.; Verhoef, C.; van Coevorden, F.; Reyners, A.K.; van der Graaf, W.T.A.; Bonenkamp, J.J.; van Etten, B.; de Wilt, J.H.W. Surgical management of rectal gastrointestinal stromal tumors. J. Surg. Oncol. 2013, 107, 320–323. [Google Scholar] [CrossRef]
- Nagasaki, T.; Fukunaga, Y.; Akiyoshi, T.; Konishi, T.; Fujimoto, Y.; Nagayama, S.; Ueno, M.; Yamaguchi, T. Laparoscopic Total Pelvic Exenteration after Neoadjuvant Imatinib Therapy for Gastrointestinal Stromal Tumor of the Rectum: A Case Report. Int. Surg. 2017, 102, 205–209. [Google Scholar] [CrossRef]
- Farid, M.; Lee, M.J.; Chew, M.H.; Ong, W.S.; Sairi, A.N.H.; Foo, K.F.; Choo, S.P.; Koo, W.H.; Ong, S.; Koh, P.K.; et al. Localized gastrointestinal stromal tumor of the rectum: An uncommon primary site with prominent disease and treatment-related morbidities. Mol. Clin. Oncol. 2013, 1, 190–194. [Google Scholar] [CrossRef][Green Version]
- Pierie, J.-P.E.N.; Choudry, U.; Muzikansky, A.; Yeap, B.Y.; Souba, W.W.; Ott, M.J. The Effect of Surgery and Grade on Outcome of Gastrointestinal Stromal Tumors. Arch. Surg. 2001, 136, 383–389. [Google Scholar] [CrossRef]
- Nepal, P.; Mori, S.; Kita, Y.; Tanabe, K.; Baba, K.; Uchikado, Y.; Kurahara, H.; Arigami, T.; Sakoda, M.; Maemura, K.; et al. Management of a case of high-risk gastrointestinal stromal tumor in rectum by transanal minimal invasive surgery. World J. Surg. Oncol. 2018, 16, 165. [Google Scholar] [CrossRef]
- DeMatteo, R.P.; Ballman, K.V.; Antonescu, C.R.; Maki, R.G.; Pisters, P.W.T.; Demetri, G.D.; Blackstein, M.E.; Blanke, C.D.; von Mehren, M.; Brennan, M.F.; et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: A randomised, double-blind, placebo-controlled trial. Lancet 2009, 373, 1097–1104. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Akiyoshi, T.; Konishi, T.; Nagayama, S.; Fukunaga, Y.; Ueno, M. Laparoscopic sphincter-preserving surgery (intersphincteric resection) after neoadjuvant imatinib treatment for gastrointestinal stromal tumor (GIST) of the rectum. Int. J. Color. Dis. 2014, 29, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Emoto, S.; Murono, K.; Sonoda, H.; Hiyoshi, M.; Sasaki, K.; Shuno, Y.; Nishikawa, T.; Tanaka, T.; Hata, K.; et al. Neoadjuvant imatinib therapy in rectal gastrointestinal stromal tumors. Surg. Today 2019, 49, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffman, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schunemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Yong, Z.Z.; Wong, J.S.M.; Teo, M.C.C.; Chia, C.S.; Ong, C.J.; Farid, M.; Tan, C.S.C. Neoadjuvant tyrosine kinase inhibitors in rectal gastrointestinal stromal tumours: A provision for enhanced oncological and functional outcomes. Int. J. Clin. Oncol. 2021, 26, 913–921. [Google Scholar] [CrossRef]
- Yang, H.; Shen, C.; Yin, X.; Cai, Z.; Wang, Q.; Zhang, B. Clinicopathological features, clinical efficacy on 101 cases of rectal gastrointestinal stromal tumors, and the significance of neoadjuvant therapy. BMC Surg. 2021, 21, 400. [Google Scholar] [CrossRef]
- Qin, X.; Li, C.; Yang, Z.; Guo, W.; Guo, H.; Chen, C.; Huang, R.; Zhang, D.; Wang, H.; Wang, H. Transsacrococcygeal approach in rectal gastrointestinal stromal tumour resection: 10-year experience at a single centre. Ann. Transl. Med. 2021, 9, 341. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, W.; Tang, W.; Wei, Y.; Liu, T.; Chen, Y.; Ji, M.; Liang, F.; Ren, L.; Xu, J. The Combination of Neoadjuvant Therapy and Surgical Resection: A Safe and Effective Treatment for Rectal Gastrointestinal Stromal Tumors. Cancer Manag. Res. 2021, 13, 4671–4678. [Google Scholar] [CrossRef]
- Ling, J.Y.; Ding, M.M.; Yang, Z.F.; Zhai, Y.D.; Xie, X.Y.; Shi, L.S.; Wang, H.M.; Cao, W.T.; Zhang, J.W.; Hu, H.B.; et al. Comparison of outcomes between neoadjuvant imatinib and upfront surgery in patients with localized rectal GIST: An inverse probability of treatment weighting analysis. J. Surg. Oncol. 2021, 124, 1442–1450. [Google Scholar] [CrossRef]
- Emoto, S.; Akiyoshi, T.; Mukai, T.; Yamaguchi, T.; Nagasaki, T.; Konishi, T.; Fukunaga, Y. Surgical Outcomes of Rectal Gastrointestinal Stromal Tumor in the Era of Imatinib. J. Gastrointest. Surg. 2021, 25, 2963–2965. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhou, W.; Li, Y.; Lin, G. Transanal endoscopic microsurgery with alternative neoadjuvant imatinib for localized rectal gastrointestinal stromal tumor: A single center experience with long-term surveillance. Surg. Endosc. 2021, 35, 3607–3617. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Guo, W.; Huang, R.; Hu, M.; Wang, H.; Wang, H. Transanal versus nontransanal surgery for the treatment of primary rectal gastrointestinal stromal tumors: A 10-year experience in a high-volume center. Ann. Transl. Med. 2020, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Shu, P.; Sun, X.F.; Fang, Y.; Gao, X.D.; Hou, Y.A.Y.; Shen, K.T.; Qin, J.; Sun, Y.H.; Qin, X.Y.; Xue, A.W.; et al. Clinical outcomes of different therapeutic modalities for rectal gastrointestinal stromal tumor: Summary of 14-year clinical experience in a single center. Int. J. Surg. 2020, 77, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Romain, B.; Delhorme, J.B.; Manceau, G.; Lefevre, J.H.; Tresallet, C.; Mariani, P.; Iannelli, A.; Rouanet, P.; Piessen, G.; Brigand, C.; et al. Is nonanatomic rectal resection a valid therapeutic option for rectal gastrointestinal stromal tumors? A proposed decision algorithm. J. Surg. Oncol. 2020, 122, 1639–1646. [Google Scholar] [CrossRef]
- NS, I.J.; Mohammadi, M.; Tzanis, D.; Gelderblom, H.; Fiore, M.; Fumagalli, E.; Rutkowski, P.; Bylina, E.; Zavrakidis, I.; Steeghs, N.; et al. Quality of treatment and surgical approach for rectal gastrointestinal stromal tumour (GIST) in a large European cohort. Eur. J. Surg. Oncol. 2020, 46, 1124–1130. [Google Scholar]
- Guo, W.; Yang, Z.; Wei, Y.; Qin, X.; Li, C.; Huang, R.; Hu, M.; Zeng, Z.; Wang, H.; Wang, H. Radical excision versus local resection for primary rectal gastrointestinal stromal tumors. Cohort Study. Int. J. Surg. 2020, 77, 190–197. [Google Scholar] [CrossRef]
- Stuart, E.; Banerjee, S.; de la Torre, J.; Wang, Y.; Scherzer, N.; Burgoyne, A.M.; Parry, L.; Fanta, P.T.; Ramamoorthy, S.; Sicklick, J.K. Frequent rectal gastrointestinal stromal tumor recurrences in the imatinib era: Retrospective analysis of an International Patient Registry. J. Surg. Oncol. 2019, 120, 715–721. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, F.; Grisotti, G.; Perez-Irizarry, J.; Cha, C.H.; Johnson, C.H.; Boffa, D.J.; Han, D.; Johung, K.L.; Zhang, Y.; et al. Distinctive features of gastrointestinal stromal tumors arising from the colon and rectum. J. Gastrointest. Oncol. 2018, 9, 231–240. [Google Scholar] [CrossRef]
- Yasui, M.; Tsujinaka, T.; Mori, M.; Takahashi, T.; Nakashima, Y.; Nishida, T. Characteristics and prognosis of rectal gastrointestinal stromal tumors: An analysis of registry data. Surg. Today 2017, 47, 1188–1194. [Google Scholar] [CrossRef]
- Hawkins, A.T.; Wells, K.O.; Krishnamurty, D.M.; Hunt, S.R.; Mutch, M.G.; Glasgow, S.C.; Wise, P.E.; Silviera, M.L. Preoperative Chemotherapy and Survival for Large Anorectal Gastrointestinal Stromal Tumors: A National Analysis of 333 Cases. Ann. Surg. Oncol. 2017, 24, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Cavnar, M.J.; Wang, L.; Balachandran, V.P.; Antonescu, C.R.; Tap, W.D.; Koehan, M.; Singer, S.; Temple, L.; Nash, G.M.; Weiser, M.R.; et al. Rectal Gastrointestinal Stromal Tumor (GIST) in the Era of Imatinib: Organ Preservation and Improved Oncologic Outcome. Ann. Surg. Oncol. 2017, 24, 3972–3980. [Google Scholar] [CrossRef] [PubMed]
- Zanwar, S.; Ostwal, V.; Sahu, A.; Jain, D.; Ramaswamy, A.; Saklani, A.; Ramadwar, M.; Shetty, N.; Shrikande, S.V. Rectal GIST-Outcomes and viewpoint from a tertiary cancer center. Indian J. Gastroenterol. 2016, 35, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.J.; Fitzgerald, J.E.; Strauss, D.C.; Hayes, A.J.; Thomas, J.M.; Messiou, C.; Fisher, C.; Benson, C.; Tekkis, P.P.; Judson, I. Surgical treatment of gastrointestinal stromal tumour of the rectum in the era of imatinib. Br. J. Surg. 2015, 102, 965–971. [Google Scholar] [CrossRef]
- Shen, C.; Chen, H.; Yin, R.; Yin, Y.; Chen, J.; Han, L.; Zhang, B.; Chen, Z.; Chen, J. Clinicopathologic, surgical characteristics and survival outcomes of rectal gastrointestinal stromal tumors. Neoplasma 2015, 62, 610–617. [Google Scholar] [CrossRef]
- Huynh, T.K.; Meeus, P.; Cassier, P.; Bouche, O.; Lardiere-Deguelte, S.; Adenis, A.; Andre, T.; Mancici, J.; Collard, O.; Montemurro, M.; et al. Primary localized rectal/pararectal gastrointestinal stromal tumors: Results of surgical and multimodal therapy from the French Sarcoma group. BMC Cancer 2014, 14, 156. [Google Scholar] [CrossRef]
- Xiao, C.C.; Zhang, S.; Wang, M.H.; Huang, L.Y.; Wu, P.; Xu, Y.; Zhu, X.L.; Sheng, W.Q.; Du, C.Y.; Shi, Y.Q.; et al. Clinicopathological features and prognostic factors of rectal gastrointestinal stromal tumors. J. Gastrointest. Surg. 2013, 17, 793–798. [Google Scholar] [CrossRef]
- Agaimy, A.; Vassos, N.; Märkl, B.; Meidenbauer, N.; Kohler, J.; Spatz, J.; Hohenberger, W.; Haller, F.; Croner, R.S.; Schneider-Stock, R.; et al. Anorectal gastrointestinal stromal tumors: A retrospective multicenter analysis of 15 cases emphasizing their high local recurrence rate and the need for standardized therapeutic approach. Int. J. Color. Dis. 2013, 28, 1057–1064. [Google Scholar] [CrossRef]
- Chen, D.; Cui, J.-H.; Yang, X.-J.; Mei, K.; Bo, W.; Chen-Fe, J.; Wei-Li, Y. Gastrointestinal stromal tumors of the rectum: Clinical, pathologic, immunohistochemical characteristics and prognostic analysis. Scand. J. Gastroenterol. 2007, 42, 1221–1229. [Google Scholar]
- Hassan, I.; You, Y.N.; Dozois, E.J.; Shayyan, R.; Smyrk, T.C.; Okuno, S.H.; Donohue, J.H. Clinical, pathologic, and immunohistochemical characteristics of gastrointestinal stromal tumors of the colon and rectum: Implications for surgical management and adjuvant therapies. Dis. Colon Rectum 2006, 49, 609–615. [Google Scholar] [CrossRef]
- Changchien, C.R.; Wu, M.C.; Tasi, W.S.; Tang, R.; Chiang, J.M.; Chen, J.S.; Huang, S.F.; Wang, J.Y.; Yeh, C.Y. Evaluation of prognosis for malignant rectal gastrointestinal stromal tumor by clinical parameters and immunohistochemical staining. Dis. Colon Rectum 2004, 47, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Popek, S.; Tsikitis, V.L. Neoadjuvant vs. adjuvant pelvic radiotherapy for locally advanced rectal cancer: Which is superior? World J. Gastroenterol. 2011, 17, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-B.; Cho, J.R.; Jeong, S.-Y.; Oh, J.H.; Ahn, S.; Choi, S.; Kim, D.W.; Lee, B.H.; Youk, E.G.; Park, S.C.; et al. Quality of life after sphincter preservation surgery or abdominoperineal resection for low rectal cancer (ASPIRE): A long-term prospective, multicentre, cohort study. Lancet Reg. Health West. Pac. 2021, 6, 100087. [Google Scholar] [CrossRef] [PubMed]
- How, P.; Stelzner, S.; Branagan, G.; Bundy, K.; Chandrakumaran, K.; Heald, R.J.; Moran, B. Comparative quality of life in patients following abdominoperineal excision and low anterior resection for low rectal cancer. Dis. Colon Rectum 2012, 55, 400. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).