Development and Validation of Prognostic Nomograms for Periampullary Neuroendocrine Neoplasms: A SEER Database Analysis
Abstract
1. Introduction
2. Materials and Methods
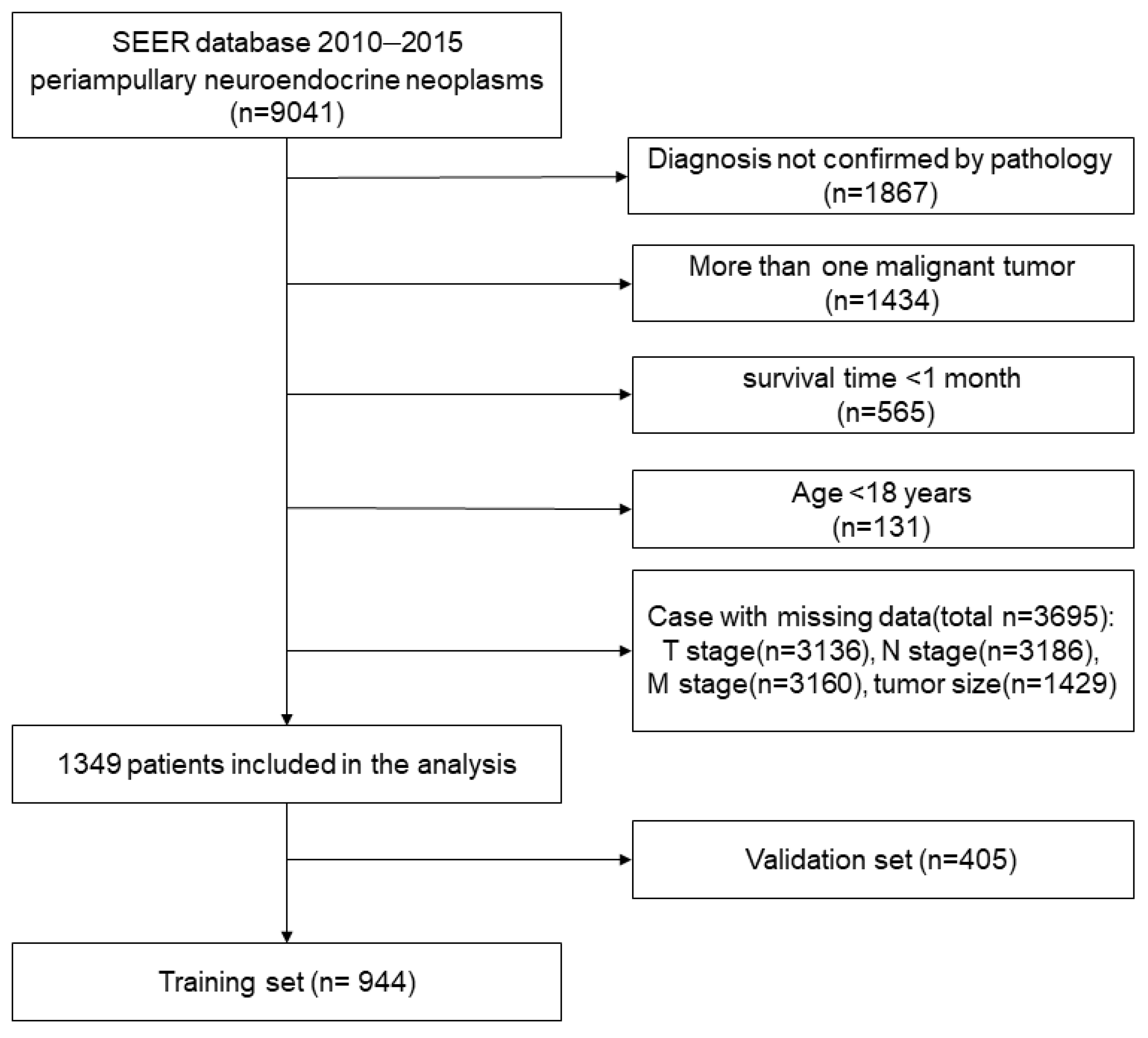
2.1. Data Resource and Patients
2.2. Variable Assessment and Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Independent Predictors in the Training Set and Survival Outcomes
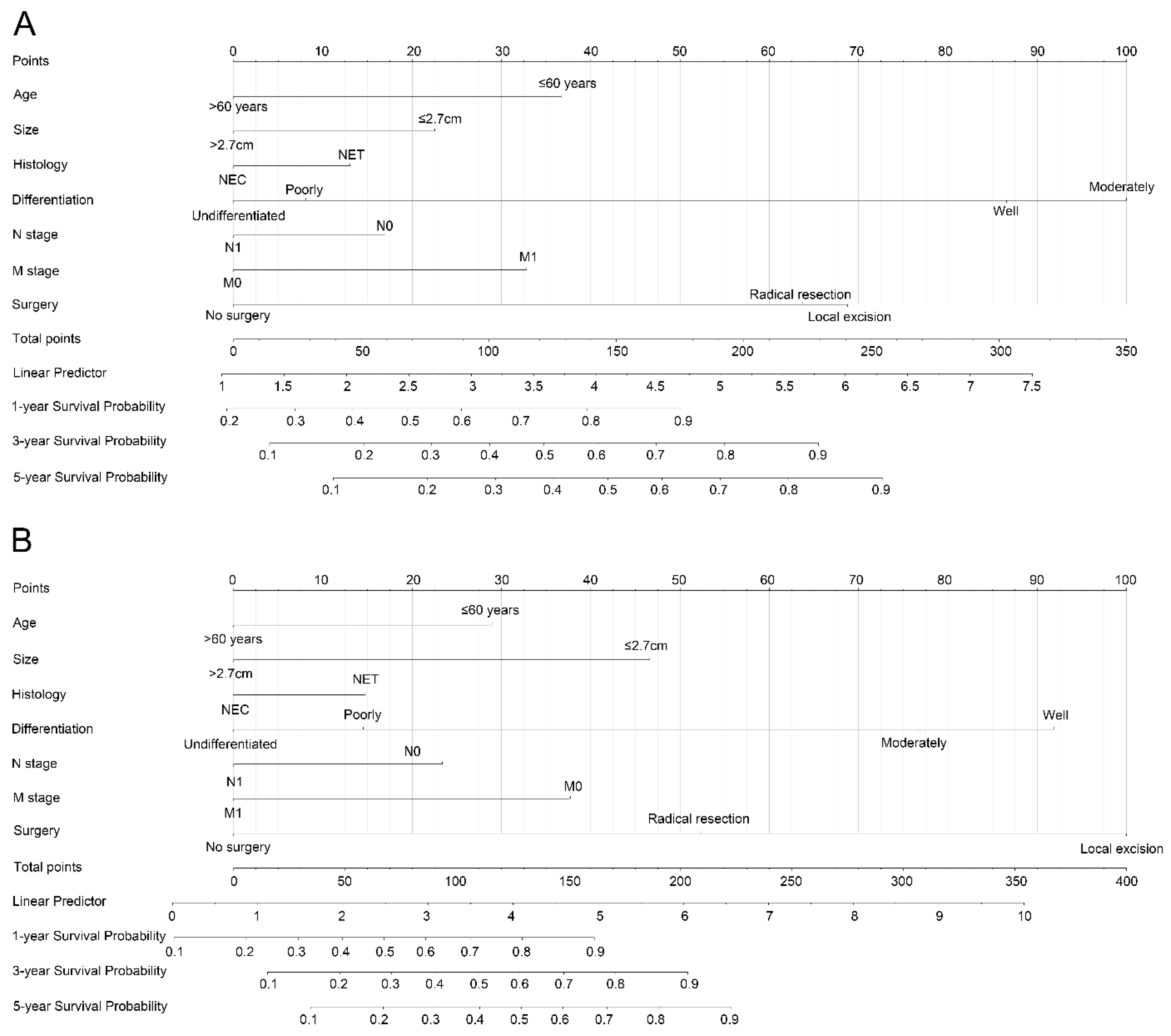
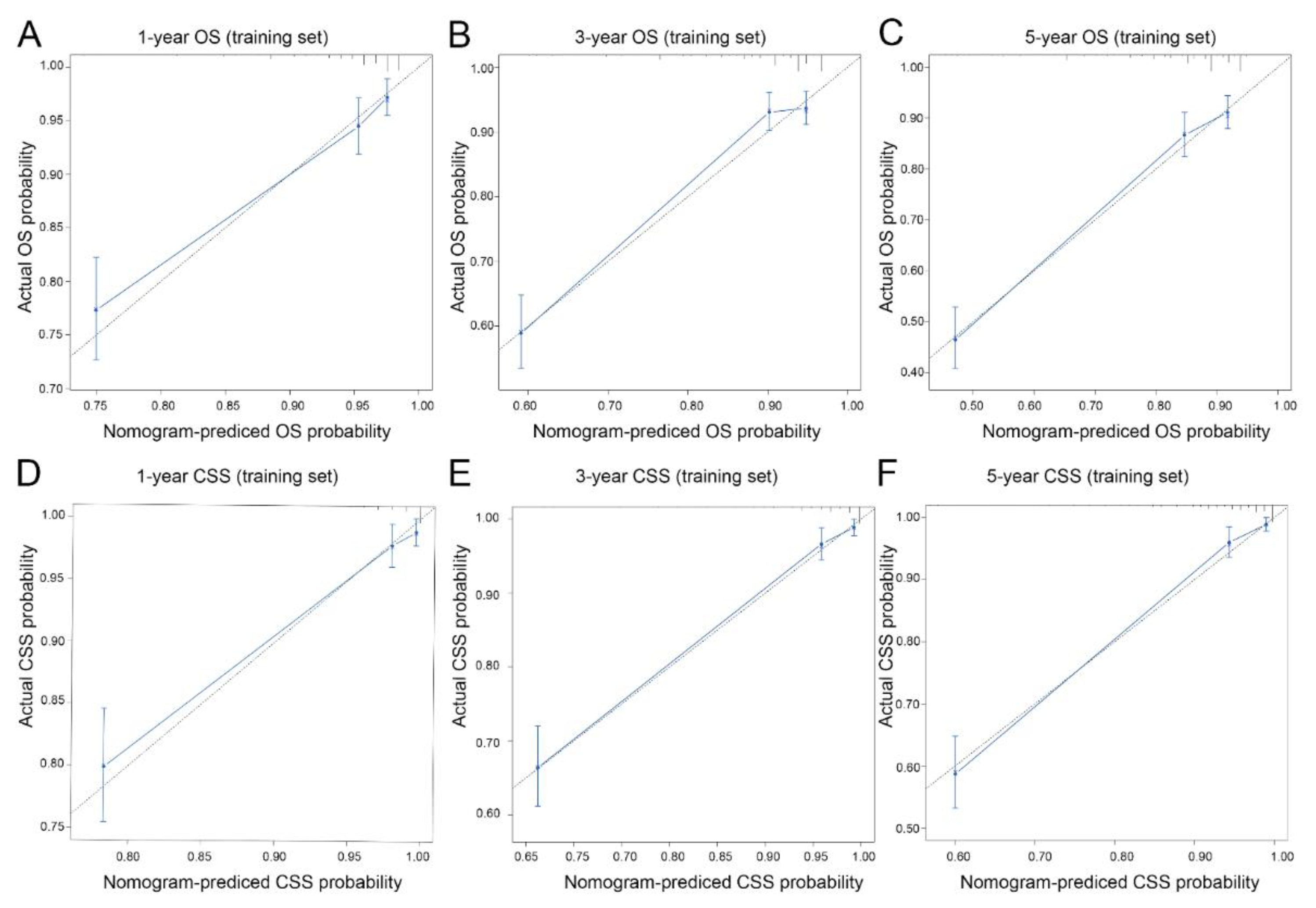
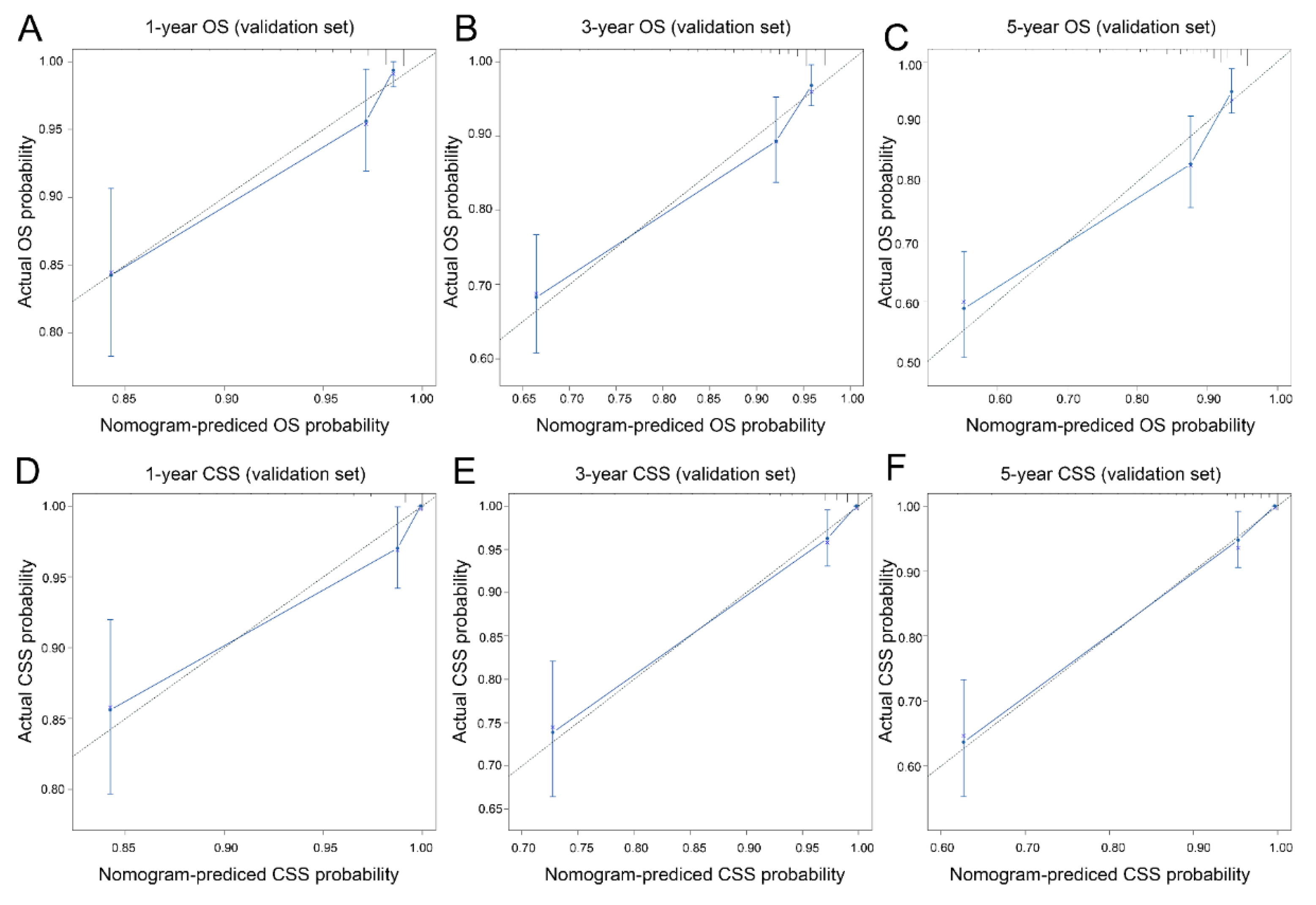
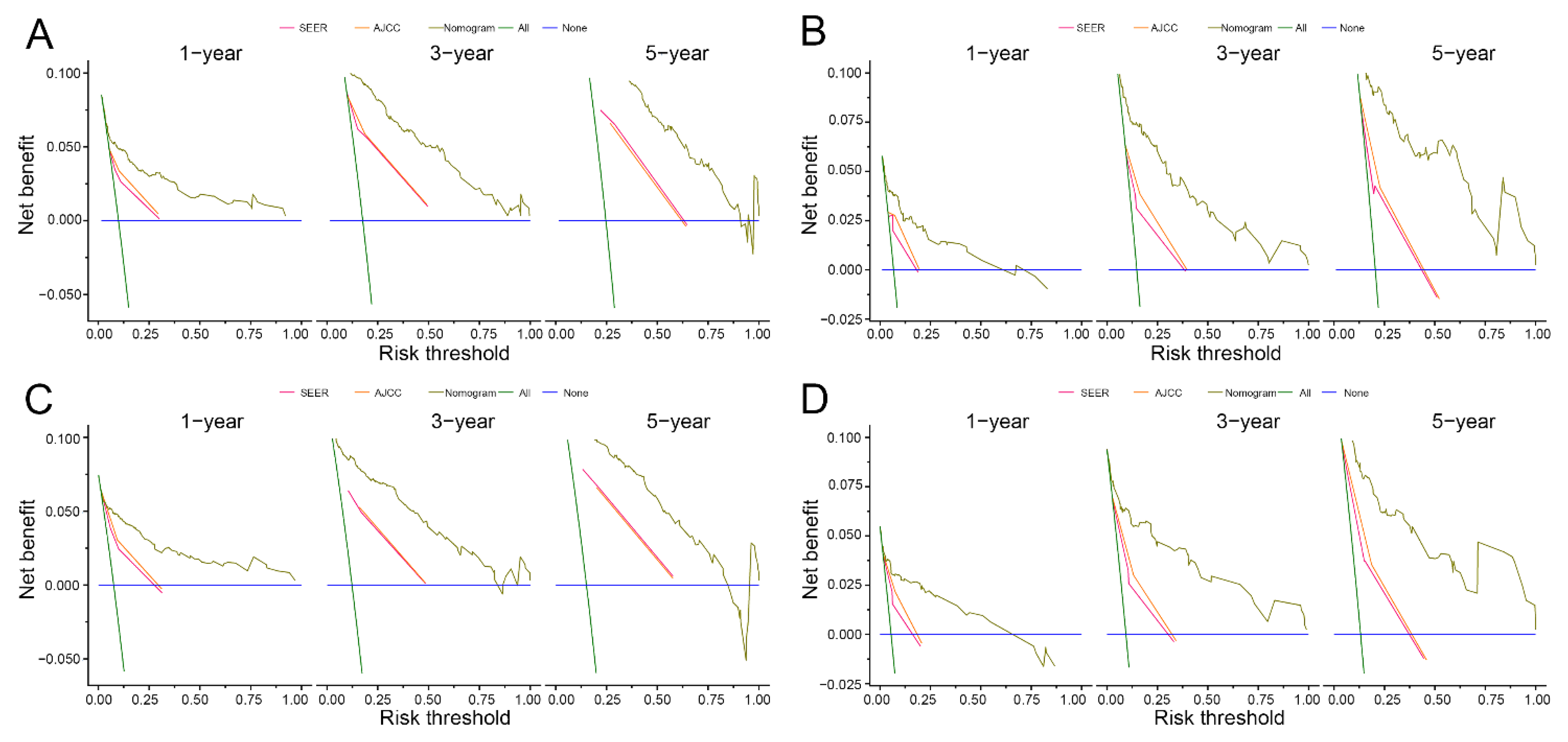
3.3. Nomogram Development and Validation
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sugita, R.; Furuta, A.; Ito, K.; Fujita, N.; Ichinohasama, R.; Takahashi, S. Periampullary Tumors: High-Spatial-Resolution MR Imaging and Histopathologic Findings in Ampullary Region Specimens. Radiology 2004, 231, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Neuroendocrine Tumors. Available online: https://www.cancer.net/cancer-types/neuroendocrine-tumor-gastrointestinal-tract/ (accessed on 9 September 2021).
- Fitzgerald, T.L.; Dennis, S.O.; Kachare, S.D.; Vohra, N.A.; Zervos, E.E. Increasing incidence of duodenal neuroendocrine tumors: Incidental discovery of indolent disease? Surgery 2015, 158, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.; Ding, Y.; Wang, S.; Jia, Y.; Cao, F.; Li, F. Head and body/tail pancreatic neuroendocrine tumors have different biological characteristics and clinical outcomes. J. Cancer Res. Clin. Oncol. 2020, 146, 3049–3061. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours, Digestive System Tumours; World Health Organization Press: Geneva, Switzerland, 2019.
- Hatzaras, I.; Palesty, J.A.; Abir, F.; Sullivan, P.; Kozol, R.A.; Dudrick, S.J.; Longo, W.E. Small-bowel tumors: Epidemiologic and clinical characteristics of 1260 cases from the connecticut tumor registry. Arch. Surg. 2007, 142, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, P.; Hammond, N.A.; Day, K.; Yaghmai, V.; Wood, C.G.; Mosbach, D.S.; Harmath, C.B.; Taffel, M.T.; Horowitz, J.M.; Berggruen, S.M.; et al. Imaging Features of Benign and Malignant Ampullary and Periampullary Lesions. RadioGraphics 2014, 34, 624–641. [Google Scholar] [CrossRef]
- Cameron, J.L.; He, J. Two Thousand Consecutive Pancreaticoduodenectomies. J. Am. Coll. Surg. 2015, 220, 530–536. [Google Scholar] [CrossRef]
- El Nakeeb, A.; Askar, W.; Atef, E.; El Hanafy, E.; Sultan, A.M.; Salah, T.; Shehta, A.; El Sorogy, M.; Hamdy, E.; El Hemly, M.; et al. Trends and outcomes of pancreaticoduodenectomy for periampullary tumors: A 25-year single-center study of 1000 consecutive cases. World J. Gastroenterol. 2017, 23, 7025–7036. [Google Scholar] [CrossRef]
- Dogeas, E.; Cameron, J.L.; Wolfgang, C.L.; Hirose, K.; Hruban, R.H.; Makary, M.A.; Pawlik, T.A.; Choti, M.A. Duodenal and Ampullary Carcinoid Tumors: Size Predicts Necessity for Lymphadenectomy. J. Gastrointest. Surg. 2017, 21, 1262–1269. [Google Scholar] [CrossRef]
- Ruff, S.M.; Standring, O.; Wu, G.; Levy, A.; Anantha, S.; Newman, E.; Karpeh, M.S., Jr.; Nealon, W.; Deutsch, G.B.; Weiss, M.J.; et al. Ampullary Neuroendocrine Tumors: Insight into a Rare Histology. Ann. Surg. Oncol. 2021, 28, 8318–8328. [Google Scholar] [CrossRef]
- Cattoni, M.; Vallières, E.; Brown, L.M.; Sarkeshik, A.A.; Margaritora, S.; Siciliani, A.; Filosso, P.L.; Guerrera, F.; Imperatori, A.; Rotolo, N.; et al. Improvement in TNM staging of pulmonary neuroendocrine tumors requires histology and regrouping of tumor size. J. Thorac. Cardiovasc. Surg. 2018, 155, 405–413. [Google Scholar] [CrossRef]
- Wu, L.; Chen, F.; Chen, S.; Wang, L. The Lymph Node Ratio Optimizes Staging in Patients with Small Intestinal Neuroendocrine Tumors. Neuroendocrinology 2018, 107, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER). Available online: http://www.seer.cancer.gov (accessed on 1 September 2021).
- Randle, R.W.; Ahmed, S.; Newman, N.A.; Clark, C.J. Clinical Outcomes for Neuroendocrine Tumors of the Duodenum and Ampulla of Vater:A Population-Based Study. J. Gastrointest. Surg. 2014, 18, 354–362. [Google Scholar] [CrossRef]
- Untch, B.R.; Bonner, K.P.; Roggin, K.K.; Reidy-Lagunes, D.; Klimstra, D.S.; Schattner, M.A.; Fong, Y.; Allen, P.J.; D’Angelica, M.I.; DeMatteo, R.P.; et al. Pathologic Grade and Tumor Size are Associated with Recurrence-Free Survival in Patients with Duodenal Neuroendocrine Tumors. J. Gastrointest. Surg. 2014, 18, 457–463; discussion 462–463. [Google Scholar] [CrossRef] [PubMed]
- Bornstein-Quevedo, L.; Gamboa-Domínguez, A. Carcinoid tumors of the duodenum and ampulla of vater: A clinicomorphologic, immunohistochemical, and cell kinetic comparison. Hum. Pathol. 2001, 32, 1252–1256. [Google Scholar] [CrossRef] [PubMed]
- Schmocker, R.K.; Ms, M.J.W.; Ding, D.; Javed, A.A.; Cameron, J.L.; Lafaro, K.; Burns, W.R.; He, J.; Wolfgang, C.L.; Burkhart, R.A. Duodenal, ampullary, and pancreatic neuroendocrine tumors: Oncologic outcomes are driven by tumor biology and tissue of origin. J. Surg. Oncol. 2021, 123, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Merath, K.; Bagante, F.; Beal, E.; Lopez-Aguiar, A.G.; Poultsides, G.; Makris, E.; Rocha, F.; Kanji, Z.; Weber, S.; Fisher, A.; et al. Nomogram predicting the risk of recurrence after curative-intent resection of primary non-metastatic gastrointestinal neuroendocrine tumors: An analysis of the U.S. Neuroendocrine Tumor Study Group. J. Surg. Oncol. 2018, 117, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Dasari, A.; Chu, Y.; Halperin, D.M.; Zhou, S.; Xu, Y.; Shih, Y.T.; Yao, J.C. Clinical, pathological, and demographic factors associated with development of recurrences after surgical resection in elderly patients with neuroendocrine tumors. Ann. Oncol. 2017, 28, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, X.; Tsilimigras, D.I.; Poultsides, G.; Rocha, F.; Abbott, D.E.; Fields, R.; Idrees, K.; Cho, C.; Maithel, S.K.; et al. Duodenal neuroendocrine tumors: Impact of tumor size and total number of lymph nodes examined. J. Surg. Oncol. 2019, 120, 1302–1310. [Google Scholar] [CrossRef]
- Delle Fave, G.; O’Toole, D.; Sundin, A.; Taal, B.; Ferolla, P.; Ramage, J.; Ferone, D.; Ito, T.; Weber, W.; Zheng-Pei, Z.; et al. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology 2016, 103, 119–124. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.I.; Jeon, S.W.; Moon, J.S.; Chung, I.-K.; Jee, S.-R.; Kim, H.U.; Seo, G.S.; Baik, G.H.; Lee, Y.C.; et al. Endoscopic resection for duodenal carcinoid tumors: A multicenter, retrospective study. J. Gastroenterol. Hepatol. 2014, 29, 318–324. [Google Scholar] [CrossRef]
- Masui, T.; Sato, A.; Nakano, K.; Uchida, Y.; Yogo, A.; Anazawa, T.; Nagai, K.; Kawaguchi, Y.; Takaori, K.; Uemoto, S. Comparison of Recurrence Between Pancreatic and Duodenal Neuroendocrine Neoplasms After Curative Resection: A Single-Institution Analysis. Ann. Surg. Oncol. 2018, 25, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Gay-Chevallier, S.; de Mestier, L.; Perinel, J.; Forestier, J.; Hervieu, V.; Ruszniewski, P.; Millot, I.; Valette, P.-J.; Pioche, M.; Lombard-Bohas, C.; et al. Management and Prognosis of Localized Duodenal Neuroendocrine Neoplasms. Neuroendocrinology 2021, 111, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Sung, J.K.; Cho, Y.S.; Bang, K.B.; Kang, S.H.; Kim, K.B.; Kim, S.H.; Moon, H.S.; Song, K.H.; Kim, S.M.; et al. Comparisons of therapeutic outcomes in patients with nonampullary duodenal neuroendocrine tumors (NADNETs): A multicenter retrospective study. Medicine 2019, 98, e16154. [Google Scholar] [CrossRef]
- Wu, L.; Sahara, K.; Tsilimigras, D.I.; Maithel, S.K.; Poultsides, G.A.; Rocha, F.G.; Weber, S.M.; Fields, R.C.; Idrees, K.; Cho, C.S.; et al. Therapeutic index of lymphadenectomy among patients with pancreatic neuroendocrine tumors: A multi-institutional analysis. J. Surg. Oncol. 2019, 120, 1080–1086. [Google Scholar] [CrossRef]
- Hollenbach, M.; Ali, E.A.; Auriemma, F.; Gulla, A.; Heise, C.; Regner, S.; Gaujoux, S. Study Protocol of the ESAP Study: Endoscopic Papillectomy vs. Surgical Ampullectomy vs. Pancreaticoduodenectomy for Ampullary Neoplasm—A Pancreas2000/EPC Study. Front. Med. 2020, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- Beger, H.G.; Mayer, B.; Rau, B.M. Parenchyma-Sparing, Limited Pancreatic Head Resection for Benign Tumors and Low-Risk Periampullary Cancer—A Systematic Review. J. Gastrointest. Surg. 2016, 20, 206–217. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Jang, J.-Y.; Kim, S.C.; Yoon, Y.-S.; Park, J.S.; Cho, C.K.; Park, S.-J.; Yang, J.D.; Lee, W.J.; Hong, T.H.; et al. Validation of the 8th AJCC Cancer Staging System for Pancreas Neuroendocrine Tumors Using Korean Nationwide Surgery Database. Cancer Res. Treat. 2019, 51, 1639–1652. [Google Scholar] [CrossRef]
- Gamboa, A.C.; Liu, Y.; Lee, R.M.; Zaidi, M.Y.; Staley, C.A.; Kooby, D.A.; Winer, J.H.; Shah, M.M.; Russell, M.C.; Cardona, K.; et al. Duodenal neuroendocrine tumors: Somewhere between the pancreas and small bowel? J. Surg. Oncol. 2019, 120, 1293–1301. [Google Scholar] [CrossRef]
- Chen, L.; Kaizhou, J.; Zhang, M.; Shang, L.; Zhang, P.; Wang, W.; Fang, C.; Li, J.; Xu, T.; Tan, H.; et al. Clinicopathological features and prognostic validity of WHO grading classification of SI-NENs. BMC Cancer 2017, 17, 521. [Google Scholar] [CrossRef]
- Albores-Saavedra, J.; Hart, A.; Chablé-Montero, F.; Henson, D.E. Carcinoids and high-grade neuroendocrine carcinomas of the ampulla of vater: A comparative analysis of 139 cases from the surveillance, epidemiology, and end results program—A population based study. Arch. Pathol. Lab. Med. 2010, 134, 1692–1696. [Google Scholar] [CrossRef]
- Rindi, G.; Klöppel, G.; Alhman, H.; Caplin, M.; Couvelard, A.; de Herder, W.W.; Erikssson, B.; Falchetti, A.; Falconi, M.; Komminoth, P.; et al. TNM staging of foregut (neuro)endocrine tumors: A consensus proposal including a grading system. Virchows Arch. 2006, 449, 395–401. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Whole Population [n = 1349 (%)] | Training Cohort [n = 944 (%)] | Validation Cohort [n = 405 (%)] | p-Value |
|---|---|---|---|---|
| Age | ||||
| ≤60 years | 659 (48.9) | 451 (47.8) | 208 (51.4) | 0.228 |
| >60 years | 690 (51.1) | 493 (52.2) | 197 (48.6) | |
| Sex | ||||
| Female | 678 (50.3) | 465 (49.3) | 213 (52.6) | 0.262 |
| Male | 671 (49.7) | 479 (50.7) | 192 (47.4) | |
| Race | ||||
| White | 954 (70.7) | 674 (71.4) | 280 (69.1) | 0.684 |
| Black | 253 (18.8) | 174 (18.4) | 79 (19.5) | |
| Other | 142 (10.5) | 96 (10.2) | 46 (11.4) | |
| Geographic region | ||||
| Rural/urban | 142 (10.5) | 90 (120.5) | 43 (10.5) | 0.943 |
| Metropolitan | 1207 (89.5) | 845 (89.5) | 362 (89.5) | |
| Income (USD) | ||||
| ≤60,000 | 484 (35.9) | 333 (35.3) | 151 (37.3) | 0.377 |
| 60,000–70,000 | 404 (29.9) | 304 (32.2) | 100 (24.7) | |
| >70,000 | 461 (34.2) | 307 (32.5) | 154 (38.0) | |
| Primary site | ||||
| Duodenum | 667 (49.4) | 473 (50.1) | 194 (47.9) | 0.672 |
| Ampulla | 57 (4.2) | 41 (4.3) | 16 (4.0) | |
| Pancreatic head | 625 (46.2) | 430 (45.6) | 195 (48.1) | |
| Tumor size | ||||
| ≤2.7 cm | 907 (67.2) | 637 (67.5) | 270 (66.7) | 0.771 |
| >2.7 cm | 442 (32.8) | 307 (32.5) | 135 (33.3) | |
| Histology | ||||
| Neuroendocrine tumors | 790 (58.6) | 563 (59.6) | 227 (56.0) | 0.220 |
| Neuroendocrine carcinomas | 559 (41.4) | 381 (40.4) | 178 (44.0) | |
| Differentiation | ||||
| Well differentiated | 1012 (75.0) | 709 (75.1) | 303 (74.8) | 0.677 |
| Moderately differentiated | 215 (15.9) | 146 (15.5) | 69 (17.0) | |
| Poorly differentiated | 86 (6.4) | 61 (6.4) | 25 (6.2) | |
| Undifferentiated | 36 (2.7) | 28 (3.0) | 8 (3.0) | |
| T stage | ||||
| T1 | 586 (43.4) | 410 (43.4) | 176 (43.4) | 0.819 |
| T2 | 393 (29.2) | 272 (28.8) | 121 (29.9) | |
| T3 | 302 (22.4) | 211 (22.4) | 91 (22.5) | |
| T4 | 68 (5.0) | 51 (5.4) | 17 (4.2) | |
| N stage | ||||
| N0 | 987 (73.2) | 687 (72.8) | 300 (74.1) | 0.622 |
| N1 | 362 (26.8) | 257 (27.2) | 105 (25.9) | |
| M stage | ||||
| M0 | 1175 (87.1) | 820 (86.9) | 355 (87.7) | 0.692 |
| M1 | 174 (12.9) | 124 (13.1) | 50 (12.3) | |
| TNM stage | ||||
| I | 655 (48.6) | 450 (47.7) | 205 (50.6) | 0.644 |
| II | 382 (28.3) | 268 (28.4) | 114 (28.1) | |
| III | 138 (10.2) | 102 (10.7) | 36 (8.9) | |
| IV | 174 (12.9) | 124 (13.2) | 50 (12.4) | |
| SEER stage | ||||
| Localized | 809 (60.0) | 565 (59.9) | 244 (60.2) | 0.772 |
| Regional | 357 (26.5) | 247 (26.2) | 110 (27.2) | |
| Distant | 183 (13.5) | 132 (13.9) | 51 (12.6) | |
| Surgery | ||||
| No surgery | 279 (20.7) | 195 (20.7) | 84 (20.7) | 0.889 |
| Local excision | 348 (25.8) | 252 (26.7) | 94 (23.7) | |
| Radical resection | 722 (53.5) | 497 (52.6) | 225 (55.6) | |
| Lymph node examination | ||||
| No | 704 (52.2) | 490 (51.9) | 214 (52.8) | 0.753 |
| Yes | 645 (47.8) | 454 (48.1) | 191 (47.2) |
| Survival Types | Tumor Stage Types | Training Set | Validation Set | |||||
|---|---|---|---|---|---|---|---|---|
| AUC | 95% CI | p | AUC | 95% CI | p | |||
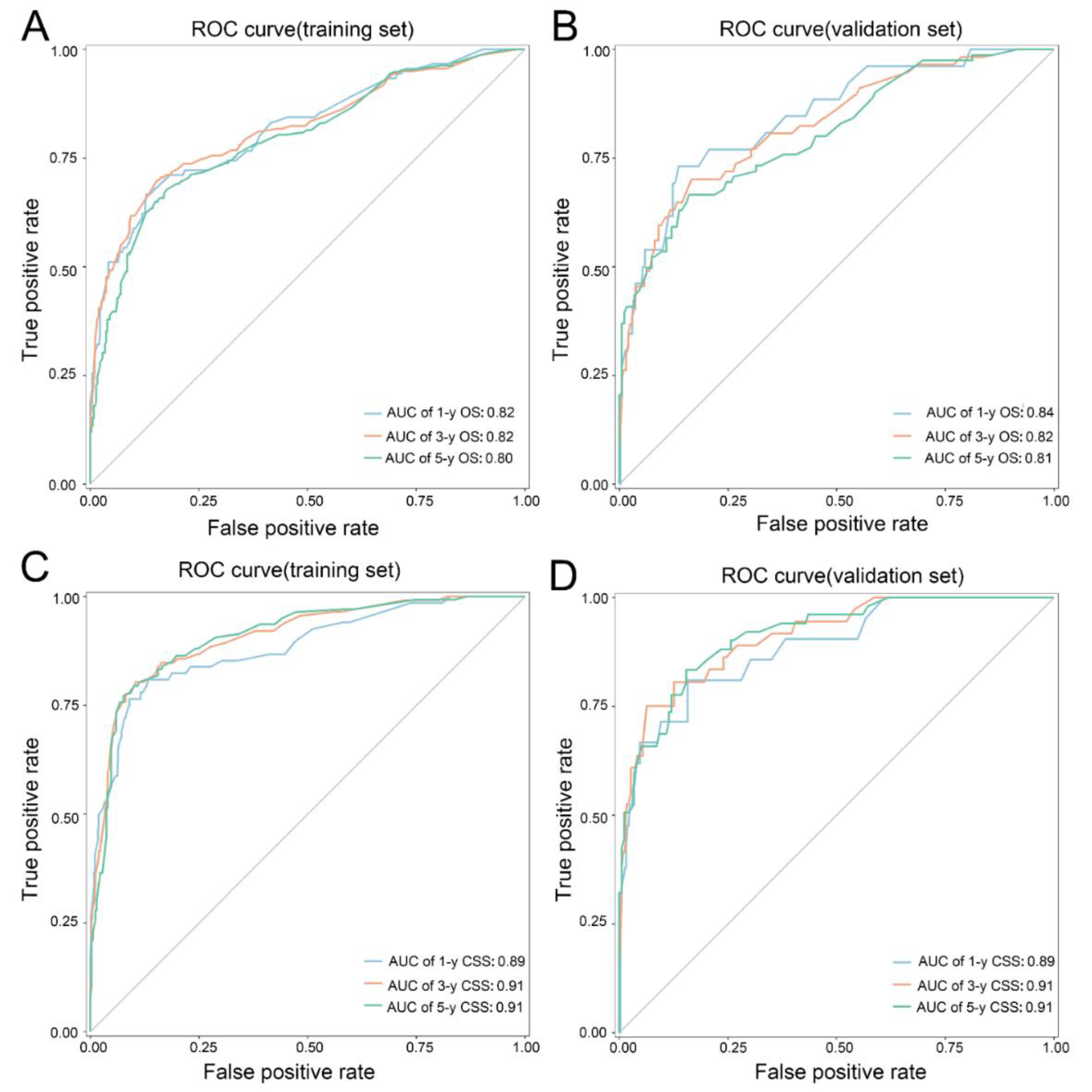
| OS | 1-year | Nomogram | 0.817 | 0.764–0.869 | 0.845 | 0.763–0.927 | ||
| SEER stage | 0.716 | 0.659–0.773 | <0.001 | 0.740 | 0.651–0.829 | 0.019 | ||
| TNM stage 7th | 0.686 | 0.625–0.746 | <0.001 | 0.720 | 0.629–0.829 | 0.023 | ||
| 3-year | Nomogram | 0.817 | 0.776–0.859 | 0.825 | 0.763–0.887 | |||
| SEER stage | 0.728 | 0.684–0.772 | <0.001 | 0.690 | 0.627–0.763 | <0.001 | ||
| TNM stage 7th | 0.713 | 0.667–0.760 | <0.001 | 0.661 | 0.554–0.705 | <0.001 | ||
| 5-year | Nomogram | 0.796 | 0.756–0.836 | 0.806 | 0.744–0.868 | |||
| SEER stage | 0.683 | 0.639–0.727 | <0.001 | 0.651 | 0.580–0.723 | <0.001 | ||
| TNM stage 7th | 0.667 | 0.631–0.723 | <0.001 | <0.001 | ||||
| CSS | 1-year | Nomogram | 0.889 | 0.843–0.934 | 0.885 | 0.808–0.962 | ||
| SEER stage | 0.805 | 0.754–0.857 | <0.001 | 0.790 | 0.711–0.869 | 0.004 | ||
| TNM stage 7th | 0.779 | 0.724–0.833 | <0.001 | 0.749 | 0.660–0.838 | <0.001 | ||
| 3-year | Nomogram | 0.913 | 0.883–0.943 | 0.911 | 0.861–0.961 | |||
| SEER stage | 0.834 | 0.796–0.873 | <0.001 | 0.795 | 0.724–0.866 | <0.001 | ||
| TNM stage 7th | 0.819 | 0.778–0.859 | <0.001 | 0.764 | 0.690–0.838 | <0.001 | ||
| 5-year | Nomogram | 0.911 | 0.882–0.940 | 0.913 | 0.870–0.956 | |||
| SEER stage | 0.838 | 0.801–0.876 | <0.001 | 0.753 | 0.679–0.828 | <0.001 | ||
| TNM stage 7th | 0.828 | 0.788–0.868 | <0.001 | 0.731 | 0.653–0.810 | <0.001 | ||
| Survival Types | Tumor Stage Types | Training Set | Validation Set | ||||
|---|---|---|---|---|---|---|---|
| C-Index | 95% CI | p | C-Index | 95% CI | p | ||
| OS | Nomogram | 0.888 | 0.860–0.917 | 0.881 | 0.836–0.925 | ||
| SEER stage | 0.906 | 0.876–0.933 | 0.882 | 0.861 | 0.805–0.918 | 0.226 | |
| TNM stage 7th | 0.868 | 0.834–0.902 | 0.097 | 0.814 | 0.749–0.878 | 0.016 | |
| CSS | Nomogram | 0.890 | 0.872–0.925 | 0.897 | 0.856–0.938 | ||
| SEER stage | 0.904 | 0.876–0.933 | 0.695 | 0.861 | 0.805–0.918 | 0.063 | |
| TNM stage 7th | 0.868 | 0.834–0.902 | 0.014 | 0.814 | 0.749–0.879 | 0.002 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Chen, Q.; Deng, Y.; Jiang, Y.; Huang, Z.; Zhou, J.; Zhao, H.; Cai, J. Development and Validation of Prognostic Nomograms for Periampullary Neuroendocrine Neoplasms: A SEER Database Analysis. Curr. Oncol. 2023, 30, 344-357. https://doi.org/10.3390/curroncol30010028
Chen J, Chen Q, Deng Y, Jiang Y, Huang Z, Zhou J, Zhao H, Cai J. Development and Validation of Prognostic Nomograms for Periampullary Neuroendocrine Neoplasms: A SEER Database Analysis. Current Oncology. 2023; 30(1):344-357. https://doi.org/10.3390/curroncol30010028
Chicago/Turabian StyleChen, Jinghua, Qichen Chen, Yiqiao Deng, Yujuan Jiang, Zhen Huang, Jianguo Zhou, Hong Zhao, and Jianqiang Cai. 2023. "Development and Validation of Prognostic Nomograms for Periampullary Neuroendocrine Neoplasms: A SEER Database Analysis" Current Oncology 30, no. 1: 344-357. https://doi.org/10.3390/curroncol30010028
APA StyleChen, J., Chen, Q., Deng, Y., Jiang, Y., Huang, Z., Zhou, J., Zhao, H., & Cai, J. (2023). Development and Validation of Prognostic Nomograms for Periampullary Neuroendocrine Neoplasms: A SEER Database Analysis. Current Oncology, 30(1), 344-357. https://doi.org/10.3390/curroncol30010028




