LKB1 Loss Assessed by Immunohistochemistry as a Prognostic Marker to First-Line Therapy in Advanced Non-Small-Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
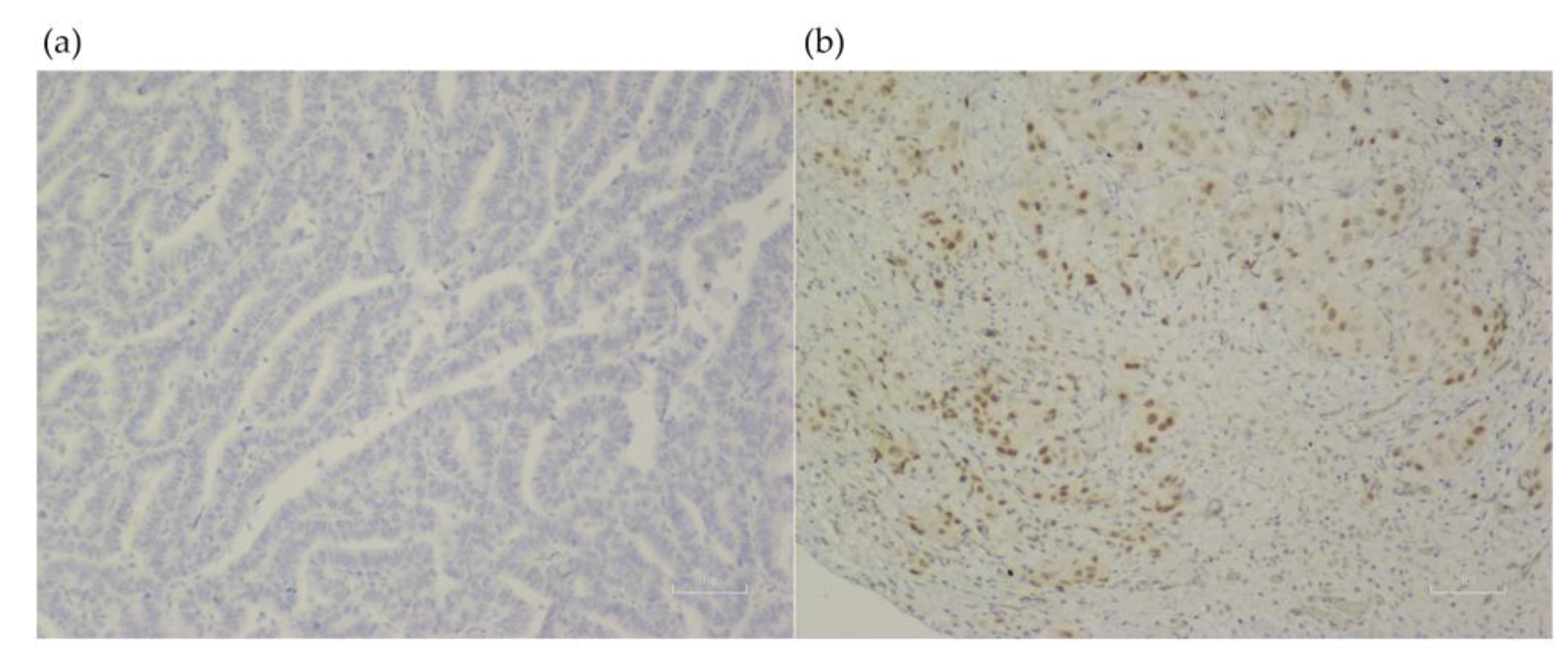
2.2. Immunohistochemistry Stain
2.3. Therapy
2.4. Statistical Analysis
3. Results
3.1. Patients
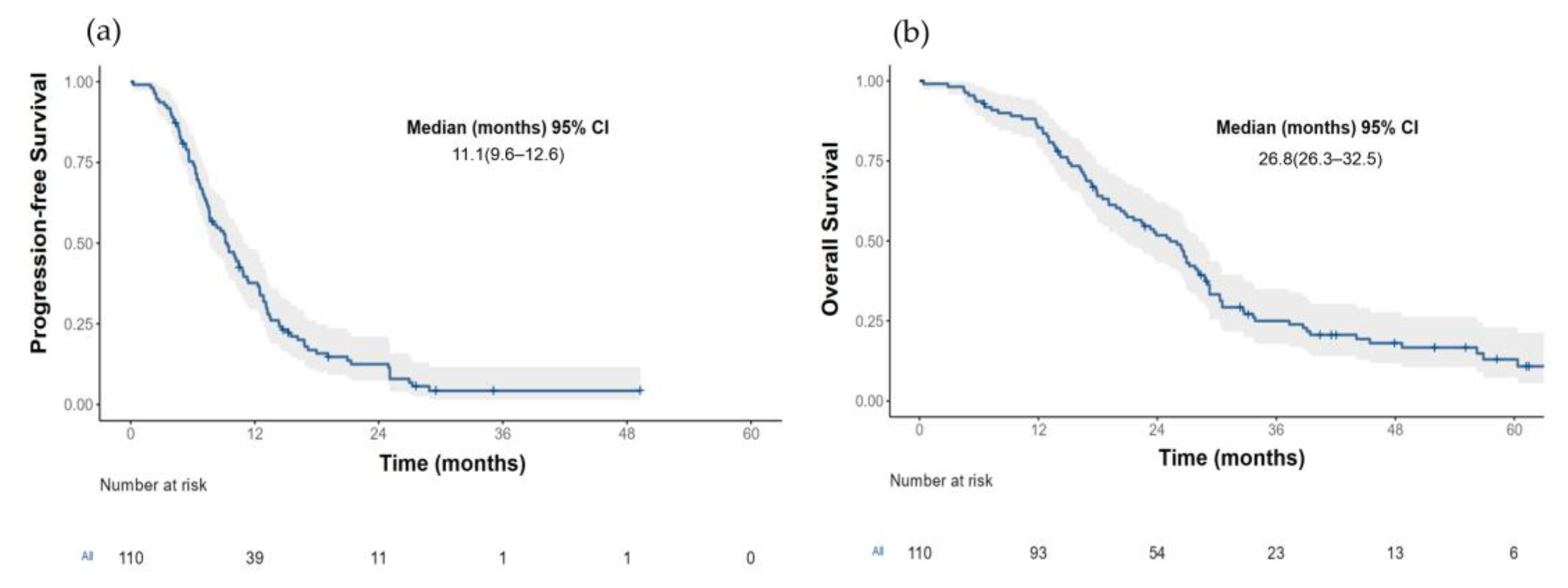
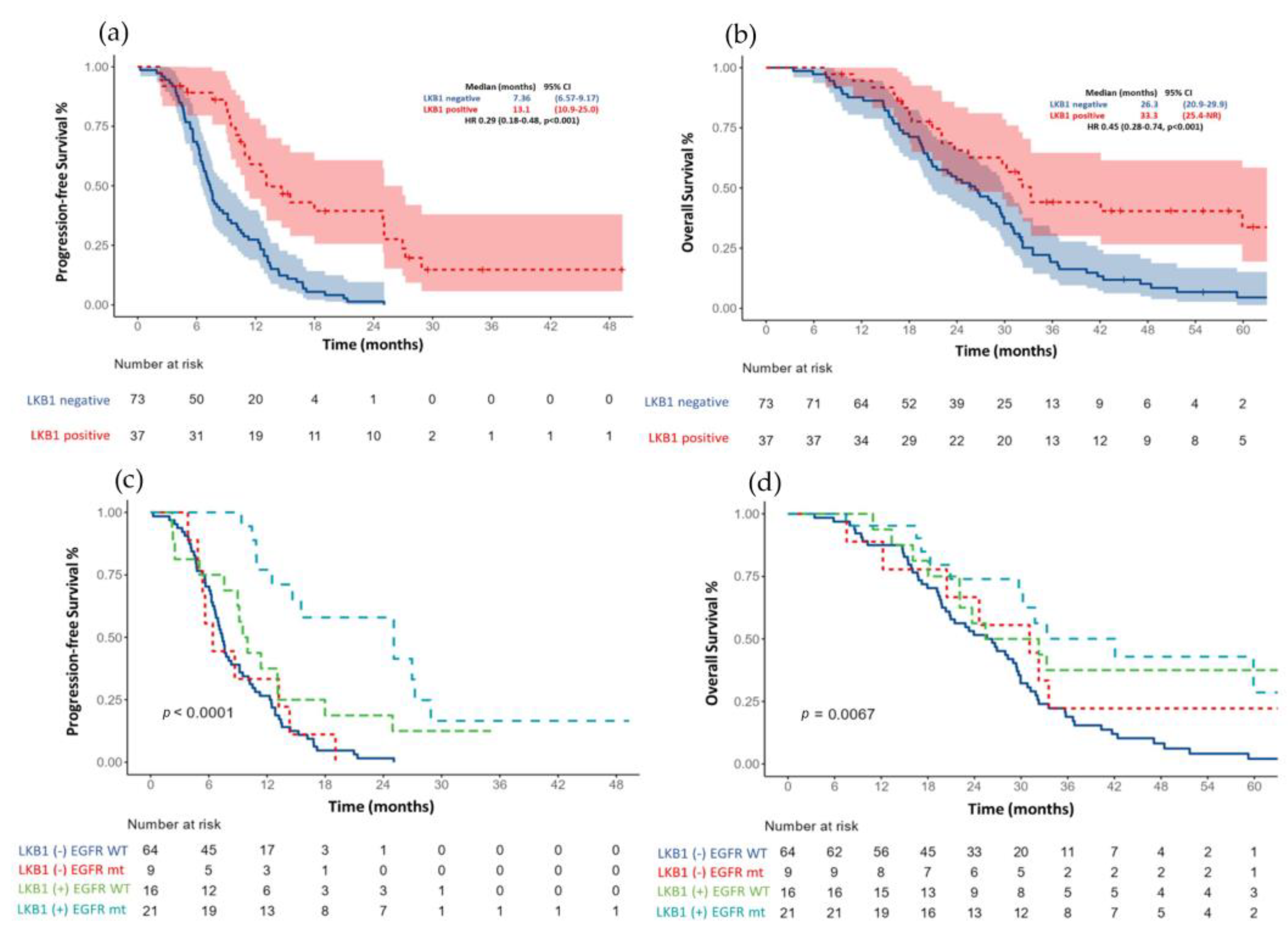
3.2. Progression-Free Survival According to LKB1 Status
3.3. Overall Survival according to LKB1 Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandez, P.; Carretero, J.; Medina, P.P.; Jimenez, A.I.; Rodriguez-Perales, S.; Paz, M.F.; Cigudosa, J.C.; Esteller, M.; Lombardia, L.; Morente, M.; et al. Distinctive gene expression of human lung adenocarcinomas carrying LKB1 mutations. Oncogene 2004, 23, 5084–5091. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, L.; Krahn, M.P. Controlling the master-upstream regulation of the tumor suppressor LKB1. Oncogene 2018, 37, 3045–3057. [Google Scholar] [CrossRef]
- Shaw, R.J.; Kosmatka, M.; Bardeesy, N.; Hurley, R.L.; Witters, L.A.; DePinho, R.A.; Cantley, L.C. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA 2004, 101, 3329–3335. [Google Scholar] [CrossRef] [PubMed]
- Mograbi, B.; Heeke, S.; Hofman, P. The Importance of STK11/ LKB1 Assessment in Non-Small Cell Lung Carcinomas. Diagnostics 2021, 11, 196. [Google Scholar] [CrossRef]
- Faubert, B.; Boily, G.; Izreig, S.; Griss, T.; Samborska, B.; Dong, Z.; Dupu, F.; Chambers, C.; Fuerth, B.J.; Viollet, B.; et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013, 17, 113–124. [Google Scholar] [CrossRef]
- Skoulidis, F.; Goldberg, M.E.; Greenawalt, D.M.; Hellmann, M.D.; Awad, M.M.; Gainor, J.F.; Schrock, A.B.; Hartmaier, R.J.; Trabucco, S.E.; Gay, L.; et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018, 8, 822–835. [Google Scholar] [CrossRef]
- Hollstein, P.E.; Eichner, L.J.; Brun, S.N.; Kamireddy, A.; Svensson, R.U.; Vera, L.I.; Ross, D.S.; Rymoff, T.J.; Hutchins, A.; Galvez, H.M.; et al. The AMPK-Related Kinases SIK1 and SIK3 Mediate Key Tumor-Suppressive Effects of LKB1 in NSCLC. Cancer Discov. 2019, 9, 1606–1627. [Google Scholar] [CrossRef]
- Murray, C.W.; Brady, J.J.; Tsai, M.K.; Li, C.; Winters, I.P.; Tang, R.; Andrejka, L.; Ma, R.K.; Kunder, C.A.; Chu, P.; et al. An LKB1-SIK Axis Suppresses Lung Tumor Growth and Controls Differentiation. Cancer Discov. 2019, 9, 1590–1605. [Google Scholar] [CrossRef]
- de Lima, V.C.C.; Corassa, M.; Saldanha, E.; Freitas, H.; Arrieta, O.; Raez, L.; Samtani, S.; Ramos, M.; Rojas, C.; Burotto, M.; et al. STK11 and KEAP1 mutations in non-small cell lung cancer patients: Descriptive analysis and prognostic value among Hispanics (STRIKE registry-CLICaP). Lung Cancer 2022, 170, 114–121. [Google Scholar] [CrossRef]
- Ghaffar, H.; Sahin, F.; Sanchez-Cepedes, M.; Su, G.H.; Zahurak, M.; Sidransky, D.; Westra, W.H. LKB1 Protein Expression in the Evolution of Glandular Neoplasia of the Lung. Clin. Cancer Res. 2003, 9, 2998–3003. [Google Scholar]
- Lin, C.; Lin, X.; Lin, K.; Tan, J.; Wei, C.; Liu, T. LKB1 expression and the prognosis of lung cancer: A meta-analysis. Medicine 2021, 100, e27841. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.G.; Di, G.H.; Shen, Z.Z.; Ding, J.; Shao, Z.M. Enhanced expression of LKB1 in breast cancer cells attenuates angiogenesis, invasion, and metastatic potential. Mol. Cancer Res. 2006, 4, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, O.; Cardona, A.F.; Bramuglia, G.F.; Gallo, A.; Campos-Parra, A.D.; Serrano, S.; Castro, M.; Avilés, A.; Amorin, E.; Kirchuk, R.; et al. Genotyping non-small cell lung cancer (NSCLC) in latin America. J. Thorac. Oncol. 2011, 6, 1955–1959. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, O.; Cardona, A.F.; Martín, C.; Más-López, L.; Corrales-Rodríguez, L.; Bramuglia, G.; Castillo-Fernandez, O.; Meyerson, M.; Amieva-Rivera, E.; Campos-Parra, A.D.; et al. Updated Frequency of EGFR and KRAS Mutations in NonSmall-Cell Lung Cancer in Latin America: The Latin-American Consortium for the Investigation of Lung Cancer (CLICaP). J. Thorac. Oncol. 2015, 10, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Raez, L.E.; Cardona, A.F.; Lopes, G.; Arrieta, O. Challenges in Genetic Testing and Treatment Outcomes among Hispanics with Lung Cancer. JCO Oncol. Pract. 2022, 18, 374–377. [Google Scholar] [CrossRef]
- Werutsky, G.; Barrios, C.H.; Cardona, A.F.; Albergaria, A.; Valencia, A.; Ferreira, C.G.; Rolfo, C.; de Azambuja, E.; Rabinovich, G.A.; Sposetti, G.; et al. Perspectives on emerging technologies, personalised medicine, and clinical research for cancer control in Latin America and the Caribbean. Lancet Oncol. 2021, 22, e488–e500. [Google Scholar] [CrossRef]
- Calles, A.; Sholl, L.M.; Rodig, S.J.; Pelton, A.K.; Hornick, J.L.; Butaney, M.; Lydon, C.; Dahlberg, S.E.; Oxnard, G.; Jackman, D.M.; et al. Immunohistochemical Loss of LKB1 Is a Biomarker for More Aggressive Biology in KRAS-Mutant Lung Adenocarcinoma. Clin. Cancer Res. 2015, 21, 2851–2860. [Google Scholar] [CrossRef]
- Imielinski, M.; Berger, A.H.; Hammerman, P.S.; Hernandez, B.; Pugh, T.J.; Hodis, E.; Cho, J.; Suh, J.; Capelletti, M.; Sivachenko, A.; et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell 2012, 150, 1107–1120. [Google Scholar] [CrossRef]
- Ding, L.; Getz, G.; Wheeler, D.A.; Mardis, E.R.; McLellan, M.D.; Cibulskis, K.; Sougnez, C.; Greulich, H.; Muzny, D.M.; Morgan, M.B.; et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 2008, 455, 1069–1075. [Google Scholar] [CrossRef]
- Sanchez-Cespedes, M. A role for LKB1 gene in human cancer beyond the Peutz-Jeghers syndrome. Oncogene 2007, 26, 7825–7832. [Google Scholar] [CrossRef]
- Koivunen, J.P.; Kim, J.; Lee, J.; Rogers, A.M.; Park, J.O.; Zhao, X.; Naoki, K.; Okamoto, I.; Nakagawa, K.; Yeap, B.Y.; et al. Mutations in the LKB1 tumour suppressor are frequently detected in tumours from Caucasian but not Asian lung cancer patients. Br. J. Cancer 2008, 99, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, O.; Ramírez-Tirado, L.-A.; Báez-Saldaña, R.; Peña, O.; Peña-Curiel, P.; Soca-Chafre, G.; Macedo-Perez, E.O. Different mutation profiles and clinical characteristics among Hispanic patients with non-small cell lung cancer could explain the “Hispanic paradox”. Lung Cancer 2015, 90, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Choi, J.E.; Na, Y.K.; Lee, E.J.; Lee, W.K.; Choi, Y.Y.; Yoon, G.S.; Jeon, H.S.; Kim, D.S.; Park, J.Y. Genetic and epigenetic alterations of the LKB1 gene and their associations with mutations in TP53 and EGFR pathway genes in Korean non-small cell lung cancers. Lung Cancer 2013, 81, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Shim, J.H.; Lee, B.; Cho, I.; Park, W.Y.; Kim, Y.; Lee, S.H.; Choi, Y.L.; Han, J.; Ahn, J.S.; et al. Paired genomic analysis of squamous cell carcinoma transformed from EGFR-mutated lung adenocarcinoma. Lung Cancer 2019, 134, 7–15. [Google Scholar] [CrossRef]
- Jiang, L.; Liang, X.; Liu, M.; Wang, W.; Ma, J.; Guo, Q.; Han, L.; Yang, C.; Nan, K. Reduced expression of liver kinase B1 and Beclin1 is associated with the poor survival of patients with non-small cell lung cancer. Oncol. Rep. 2014, 32, 1931–1938. [Google Scholar] [CrossRef]
- Bonanno, L.; Paoli Ad Zulato, E.; Esposito, G.; Calabrese, F.; Favaretto, A.; Santo, A.; Conte, A.; Chilosi, M.; Oniga, F.; Indraccolo, S.; et al. LKB1 Expression Correlates with Increased Survival in Patients with Advanced Non-Small Cell Lung Cancer Treated with Chemotherapy and Bevacizumab. Clin. Cancer Res. 2017, 23, 3316–3324. [Google Scholar] [CrossRef]
| Characteristics | Overall Population | LKB1 (+) 37 (33.6) | LKB1 (−) 73 (66.3) | p |
|---|---|---|---|---|
| Gender | ||||
| Female: | 75 (68.2) | 25 (33.3) | 50 (66.7) | 0.922 |
| Male: | 35 (31.8) | 12 (34.3) | 23 (65.7) | |
| Age | ||||
| <60 | 47 (42.7) | 16 (34.0) | 31 (66.0) | 0.938 |
| ≥60 | 63 (57.3) | 21 (33.3) | 42 (66.7) | |
| Smoking status | ||||
| Never | 88 (80.0) | 24 (27.3) | 64 (72.7) | 0.005 |
| Current or former | 22 (20.0) | 13 (59.1) | 9 (40.9) | |
| Wood smoke exposure | ||||
| No | 93 (84.5) | 25 (26.9) | 68 (73.1) | <0.001 |
| Yes | 17 (15.5) | 12 (70.6) | 5 (29.4) | |
| ECOG | ||||
| 0–1 | 99 (90.0) | 36 (36.4) | 63 (63.6) | 0.069 |
| 2 | 11 (10.0) | 1 (9.1) | 10 (90.9) | |
| Clinical stage | ||||
| IIIB | 9 (8.2) | 3 (33.3) | 6 (66.7) | 0.984 |
| IV | 101 (91.8) | 34 (33.7) | 67 (66.3) | |
| NSCLC subtype | ||||
| Non-squamous | 103 (93.6) | 37 (35.9) | 66 (64.1) | 0.052 |
| Squamous | 7 (6.4) | 0 (0) | 7 (100) | |
| Differentiation grade n = 95 | ||||
| Well-differentiated | 13 (11.8) | 3 (23.1) | 10 (76.9) | 0.081 |
| Moderately differentiated | 39 (35.5) | 17 (43.6) | 22 (56.4) | |
| Poorly differentiated | 40 (36.4) | 15 (37.5) | 25 (62.5) | |
| Other | 18 (16.4) | 2 (11.1%) | 16 (88.9) | |
| EGFR status | ||||
| Wild-type | 80 (72.7) | 16 (22.5) | 64 (77.5) | <0.001 |
| Mutation | 30 (27.3) | 21 (70.0) | 9 (30.0) | |
| Characteristics | HR (95% CI) | p | HR (95% CI) | p |
|---|---|---|---|---|
| Univariate | Multivariate | |||
| ECOG | ||||
| 0–1 | - | - | - | - |
| 2 | 2.20 (1.13–4.28) | 0.021 | 0.98 (0.48–2.02) | 0.966 |
| Smoking status | ||||
| Never | - | - | - | - |
| Current or former | 1.00 (0.61–1.64) | 0.993 | 3.40 (1.83–6.31) | <0.001 |
| Differentiation grade | ||||
| Well-differentiated | - | - | - | - |
| Moderately differentiated | 0.86 (0.45–1.67) | 0.660 | 1.06 (1.54–2.08) | 0.867 |
| Poorly differentiated | 0.97 (0.50–1.87) | 0.924 | 1.59 (0.81–3.15) | 0.181 |
| NOS | 1.58 (0.75–3.31) | 0.228 | 1.48 (0.68–3.21) | 0.320 |
| Clinical stage | ||||
| IIIB | 0.44 (0.20–0.96) | 0.039 | 0.32 (0.45–1.39) | 0.011 |
| IV | - | - | - | - |
| EGFR status | ||||
| Wild-type | - | - | - | - |
| Mutation | 0.39 (0.24–0.63) | <0.001 | 0.32 (0.17–0.59) | <0.001 |
| LKB1 expression | ||||
| Negative | - | - | ||
| Positive | 0.29 (0.18–0.48) | <0.001 | 0.20 (0.11–0.38) | <0.001 |
| Response to 1st line therapy | ||||
| No response | - | - | - | - |
| Partial or complete response | 0.52 (0.34–0.77) | 0.001 | 0.26 (0.16–0.43) | <0.001 |
| Characteristics | HR (95% CI) | p | HR (95% CI) | p |
|---|---|---|---|---|
| Univariate | Multivariate | |||
| Age | ||||
| <60 years | - | - | ||
| ≥60 years | 1.56 (1.02–2.40) | 0.041 | 1.63 (1.04–2.57) | 0.035 |
| Smoking status | ||||
| Never | - | - | ||
| Current or former | 0.78 (0.45–1.34) | 0.365 | 1.50 (0.73–3.09) | 0.265 |
| ECOG PS | ||||
| 0–1 | - | - | ||
| 2 | 3.27 (1.66–6.44) | 0.001 | 1.83 (0.87–3.84) | 0.112 |
| Differentiation grade | ||||
| Well-differentiated | - | - | ||
| Moderately differentiated | 2.28 (1.03–5.04) | 0.041 | 3.89 (1.55–9.75) | 0.004 |
| Poorly differentiated | 3.11 (1.39–6.94) | 0.006 | 3.95 (1.60–9.73) | 0.003 |
| NOS | 2.56 (1.08–6.05) | 0.033 | 2.53 (0.95–6.79) | 0.064 |
| EGFR status | ||||
| Wild-type | - | - | ||
| Mutation | 0.54 (0.33–0.90) | 0.019 | 0.79 (0.45–1.39) | 0.411 |
| LKB1 expression | ||||
| Negative | - | - | ||
| Positive | 0.45 (0.28–0.74) | 0.001 | 0.34 (0.18–0.65) | 0.001 |
| Response to 1st line therapy | ||||
| No response | - | - | ||
| Partial or complete response | 0.62 (0.41–0.95) | 0.027 | 0.54 (0.43–0.84) | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avilés-Salas, A.; Díaz-García, D.A.; Lara-Mejía, L.; Cardona, A.F.; Orozco-Morales, M.; Catalán, R.; Hernández-Pedro, N.Y.; Rios-Garcia, E.; Ramos-Ramírez, M.; Arrieta, O. LKB1 Loss Assessed by Immunohistochemistry as a Prognostic Marker to First-Line Therapy in Advanced Non-Small-Cell Lung Cancer. Curr. Oncol. 2023, 30, 333-343. https://doi.org/10.3390/curroncol30010027
Avilés-Salas A, Díaz-García DA, Lara-Mejía L, Cardona AF, Orozco-Morales M, Catalán R, Hernández-Pedro NY, Rios-Garcia E, Ramos-Ramírez M, Arrieta O. LKB1 Loss Assessed by Immunohistochemistry as a Prognostic Marker to First-Line Therapy in Advanced Non-Small-Cell Lung Cancer. Current Oncology. 2023; 30(1):333-343. https://doi.org/10.3390/curroncol30010027
Chicago/Turabian StyleAvilés-Salas, Alejandro, Diego A. Díaz-García, Luis Lara-Mejía, Andrés F. Cardona, Mario Orozco-Morales, Rodrigo Catalán, Norma Y. Hernández-Pedro, Eduardo Rios-Garcia, Maritza Ramos-Ramírez, and Oscar Arrieta. 2023. "LKB1 Loss Assessed by Immunohistochemistry as a Prognostic Marker to First-Line Therapy in Advanced Non-Small-Cell Lung Cancer" Current Oncology 30, no. 1: 333-343. https://doi.org/10.3390/curroncol30010027
APA StyleAvilés-Salas, A., Díaz-García, D. A., Lara-Mejía, L., Cardona, A. F., Orozco-Morales, M., Catalán, R., Hernández-Pedro, N. Y., Rios-Garcia, E., Ramos-Ramírez, M., & Arrieta, O. (2023). LKB1 Loss Assessed by Immunohistochemistry as a Prognostic Marker to First-Line Therapy in Advanced Non-Small-Cell Lung Cancer. Current Oncology, 30(1), 333-343. https://doi.org/10.3390/curroncol30010027





