Differences in Preoperative Health-Related Quality of Life between Women Receiving Mastectomy or Breast Conserving Surgery in a Prospectively Recruited Cohort of Breast Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Patient-Reported Outcomes
2.2.1. Patient-Health Questionnaire-9 (PHQ-9)
2.2.2. Generalized Anxiety Disorder (GAD-7)
2.2.3. Pain Intensity (P), Interference with Enjoyment of Life (E), and Interference with General Activity (G), PEG
2.2.4. EuroQoL EQ-5D-5L
2.2.5. Socioeconomic Status Variables
2.2.6. Breast-QTM
2.3. Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Regression Effect | PEG (Pain) | EQ-5D VAS (Health Status) | ||||
|---|---|---|---|---|---|---|
| Estimate | Standard Error | p-Value | Estimate | Standard Error | p-Value | |
| Intercept | 2.57 | 0.49 | <0.01 | 67.3 | 3.97 | <0.01 |
| Age (Years) | −0.01 | 0.01 | 0.68 | 0.05 | 0.05 | 0.32 |
| Charlson Index | ||||||
| 0 | −0.21 | 0.39 | 0.58 | 6.65 | 2.87 | 0.02 |
| 1–2 | −0.18 | 0.27 | 0.51 | 4.33 | 2.25 | 0.05 |
| 3+ | Reference | Reference | ||||
| Surgery type | ||||||
| Breast Conserving Surgery | −0.12 | 0.19 | 0.52 | 1.38 | 1.59 | 0.38 |
| Total mastectomy | Reference | Reference | ||||
| SES 5—Situational vulnerability | ||||||
| Q1 Least Vulnerable | −0.97 | 0.35 | <0.01 | 0.88 | 2.88 | 0.75 |
| Q2 | −0.5 | 0.34 | 0.14 | −0.43 | 2.83 | 0.87 |
| Q3 | −0.71 | 0.33 | 0.03 | −1.36 | 2.77 | 0.62 |
| Q4 | −0.94 | 0.33 | <0.01 | −1.08 | 2.72 | 0.69 |
| Q5 Most Vulnerable | Reference | Reference | ||||
| SES—Ethno-cultural composition | ||||||
| Q1 Least Diverse | −0.04 | 0.5 | 0.92 | 0.01 | 4.11 | 0.99 |
| Q2 | 0.56 | 0.4 | 0.16 | −4.52 | 3.3 | 0.17 |
| Q3 | 0.12 | 0.3 | 0.67 | −2.2 | 2.46 | 0.37 |
| Q4 | 0.32 | 0.22 | 0.15 | −0.69 | 1.86 | 0.7 |
| Q5 Most Diverse | Reference | Reference | ||||
References
- Fortin, J.; Leblanc, M.; Elgbeili, G.; Cordova, M.J.; Marin, M.-F.; Brunet, A. The mental health impacts of receiving a breast cancer diagnosis: A meta-analysis. Br. J. Cancer 2021, 125, 1582–1592. [Google Scholar] [CrossRef]
- Gu, J.; Delisle, M.; Engler-Stringer, R.; Groot, G. Mastectomy versus breast-conservation therapy: An examination of how individual, clinicopathologic, and physician factors influence decision-making. Curr. Oncol. 2019, 26, e522–e534. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Kuo, R.N.; Chung, K.P. Quality of life of breast cancer survivors following breast-conserving therapy versus mastectomy: A multicenter study in Taiwan. Jpn. J. Clin. Oncol. 2017, 47, 909–918. [Google Scholar] [CrossRef]
- Jagsi, R.; Li, Y.; Morrow, M.; Janz, N.; Alderman, A.; Graff, J.; Hamilton, A.; Katz, S.; Hawley, S. Patient-reported Quality of Life and Satisfaction With Cosmetic Outcomes After Breast Conservation and Mastectomy With and Without Reconstruction: Results of a Survey of Breast Cancer Survivors. Ann. Surg. 2015, 261, 1198–1206. [Google Scholar] [CrossRef]
- Nano, M.T.; Gill, P.G.; Kollias, J.; Bochner, M.A.; Malycha, P.; Winefield, H.R. Psychological impact and cosmetic outcome of surgical breast cancer strategies. ANZ J. Surg. 2005, 75, 940–947. [Google Scholar] [CrossRef]
- Nicholson, R.M.; Leinster, S.; Sassoon, E.M. A comparison of the cosmetic and psychological outcome of breast reconstruction, breast conserving surgery and mastectomy without reconstruction. Breast 2007, 16, 396–410. [Google Scholar] [CrossRef]
- Jay, M.; Creelman, B.; Baliski, C. Patient reported outcomes associated with surgical intervention for breast cancer. Am. J. Surg. 2020, 219, 816–822. [Google Scholar] [CrossRef]
- Li, X.; Meng, M.; Zhao, J.; Zhang, X.; Yang, D.; Fang, J.; Wang, J.; Han, L.; Hao, Y. Shared Decision-Making in Breast Reconstruction for Breast Cancer Patients: A Scoping Review. Patient Prefer Adherence 2021, 15, 2763–2781. [Google Scholar] [CrossRef]
- Mansano-Schlosser, T.C.; Ceolim, M.F.; Valerio, T.D. Poor sleep quality, depression and hope before breast cancer surgery. Appl. Nurs. Res. 2017, 34, 7–11. [Google Scholar] [CrossRef]
- Freeman, A.; Tyrovolas, S.; Koyanagi, A.; Chatterji, S.; Leonardi, M.; Ayuso-Mateos, J.L.; Tobiasz-Adamczyk, B.; Koskinen, S.; Rummel-Kluge, C.; Haro, J.M. The role of socio-economic status in depression: Results from the COURAGE (aging survey in Europe). BMC Public Health 2016, 16, 1098. [Google Scholar] [CrossRef]
- Hoebel, J.; Maske, U.E.; Zeeb, H.; Lampert, T. Social inequalities and depressive symptoms in adults: The role of objective and subjective socioeconomic status. PLoS ONE 2017, 12, e0169764. [Google Scholar] [CrossRef] [PubMed]
- Dorner, T.E.; Muckenhuber, J.; Stronegger, W.J.; Ràsky, E.; Gustorff, B.; Freidl, W. The impact of socio-economic status on pain and the perception of disability due to pain. Eur. J. Pain 2011, 15, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Kam, A.C.; Tong, M.C.; Van Hasselt, A. Cross-cultural adaption and validation of the Chinese Abbreviated Profile of Hearing Aid Benefit. Int. J. Audiol. 2011, 50, 334–339. [Google Scholar] [CrossRef]
- Montano, D. Socioeconomic status, well-being and mortality: A comprehensive life course analysis of panel data, Germany, 1984–2016. Arch. Public Health 2021, 79, 40. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Carrillo, G.; Alonso-Ferres, M.; Moya, M.; Valor-Segura, I. Socioeconomic Status and Psychological Well-Being: Revisiting the Role of Subjective Socioeconomic Status. Front. Psychol. 2020, 11, 1303. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Spitzer, R.; Kroenke, K.; Williams, J.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.; Monahan, P.O.; Löwe, B. Anxiety disorders in primary care: Prevalence, impairment, comorbidity, and detection. Arch. Intern. Med. 2007, 146, 317–325. [Google Scholar] [CrossRef]
- Krebs, E.E.; Lorenz, K.A.; Bair, M.J.; Damush, T.M.; Wu, J.; Sutherland, J.M.; Asch, S.M.; Kroenke, K. Development and Initial Validation of the PEG, a Three-item Scale Assessing Pain Intensity and Interference. J. Gen. Intern. Med. 2009, 24, 733–738. [Google Scholar] [CrossRef]
- EQ-5D-3L User Guide Basic Information on How to Use the EQ-5D-3L Instrument. Available online: https://euroqol.org/publications/user-guides/ (accessed on 31 October 2022).
- Statistics Canada. The Canadian Index of Multiple Deprivation—User Guide. Available online: https://www150.statcan.gc.ca/n1/pub/45-20-0001/452000012019002-eng.htm (accessed on 31 October 2022).
- Mundy, L.R.; Homa, K.; Klassen, A.F.; Pusic, A.L.; Kerrigan, C.L. Breast cancer and reconstruction: Normative data for interpreting the BREAST-Q. Plast. Reconstr. Surg. 2017, 139, 1046e–1055e. [Google Scholar] [CrossRef]
- BREAST-Q® | BREAST CANCER a User’s Guide for Researchers and Clinicians. November 2017. Available online: https://qportfolio.org/wp-content/uploads/2022/04/BREAST-Q-BREAST-CANCER-USER-GUIDE.pdf (accessed on 31 October 2022).
- Flanagan, M.R.; Zabor, E.C.; Romanoff, A.; Fuzesi, S.; Stempel, M.; Mehrara, B.J.; Morrow, M.; Pusic, A.L.; Gemignani, M.L. A Comparison of Patient-Reported Outcomes After Breast-Conserving Surgery and Mastectomy with Implant Breast Reconstruction. Ann. Surg. Oncol. 2019, 26, 3133–3140. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.; Kerr, J.; Schlesinger-Raab, A.; Sauer, H.; Hölzel, D. Quality of life following breast-conserving therapy or mastectomy: Results of a 5-year prospective study. Breast J. 2004, 10, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Aviado-Langer, J. Measuring preoperative anxiety in patients with breast cancer using the visual analog scale. Clin. J. Oncol. Nurs. 2014, 18, 489–491. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lagendijk, M.; Mittendorf, E.; King, T.A.; Gibbons, C.; Pusic, A.; Dominici, L.S. Incorporating Patient-Reported Outcome Measures into Breast Surgical Oncology: Advancing Toward Value-Based Care. Oncologist 2020, 25, 384–390. [Google Scholar] [CrossRef]
- Denieffe, S.; Cowman, S.; Gooney, M. Symptoms, clusters and quality of life prior to surgery for breast cancer. J. Clin. Nurs. 2014, 23, 2491–2502. [Google Scholar] [CrossRef]
- Katsohiraki, M.; Poulopoulou, S.; Fyrfiris, N.; Koutelekos, I.; Tsiotinou, P.; Adam, O.; Vasilopoulou, E.; Kapritsou, M. Evaluating Preoperative Anxiety Levels in Patients Undergoing Breast Cancer Surgery. Asia-Pac. J. Oncol. Nurs. 2020, 7, 361–364. [Google Scholar] [CrossRef]
- Mertz, B.G.; Bistrup, P.E.; Johansen, C.; Dalton, S.O.; Deltour, I.; Kehlet, H.; Kroman, N. Psychological distress among women with newly diagnosed breast cancer. Eur. J. Oncol. Nurs. 2012, 16, 439–443. [Google Scholar] [CrossRef]
- Barros, A.E.d.S.; Conde, C.R.; Lemos, T.M.R.; Kunz, J.A.; Ferreira, M.d.L.d.S.M. Feelings experienced by women when receiving the diagnoses of breast cancer. J. Nurs. 2018, 12, 102–113. [Google Scholar]
- Builes Ramírez, S.; Acea Nebril, B.; García Novoa, A.; Cereijo, C.; Bouzón, A.; Mosquera Oses, J. Evaluation of the preoperative perception of quality of life and satisfaction of women with breast cancer using the BREAST-Q™ questionnaire. Cir. Esp (Engl. Ed.) 2020, 98, 212–218. [Google Scholar] [CrossRef]
- Lim, D.W.; Retrouvey, H.; Kerrebijn, I.; Butler, K.; O’Neill, A.C.; Cil, T.D.; Zhong, T.; Hofer, S.O.P.; McCready, D.R.; Metcalfe, K.A. Longitudinal Study of Psychosocial Outcomes Following Surgery in Women with Unilateral Nonhereditary Breast Cancer. Ann. Surg. Oncol. 2021, 28, 5985–5998. [Google Scholar] [CrossRef]
- Parker, P.A.; Peterson, S.K.; Shen, Y.; Bedrosian, I.; Black, D.M.; Thompson, A.M.; Nelson, J.C.; DeSnyder, S.M.; Cook, R.L.; Hunt, K.K.; et al. Prospective Study of Psychosocial Outcomes of Having Contralateral Prophylactic Mastectomy among Women with Nonhereditary Breast Cancer. J. Clin. Oncol. 2018, 36, 2630–2638. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, G.H.; Schnur, J.B.; Erblich, J.; Diefenbach, M.A.; Bovbjerg, D.H. Pre-Surgery Psychological Factors Predict Pain, Nausea and Fatigue One Week Following Breast Cancer Surgery. J. Pain Symptom. Manag. 2010, 39, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Lau, G.J.; Smirnow, N.; Buono, A.T.; Cooke, A.; Gartshore, K.; Loiselle, C.G.; Johnson, K. A Multidisciplinary Preoperative Teaching Session for Women Awaiting Breast Cancer Surgery: A Quality Improvement Initiative. Rehabil. Process Outcome 2018, 7, 1179572718790937. [Google Scholar] [CrossRef]
- Sun, L.; Ang, E.; Ang, W.; Lopez, V. Losing the breast: A meta-synthesis of the impact in women breast cancer survivors. Psycho-oncology 2018, 27, 376–385. [Google Scholar] [CrossRef]
- Gopie, J.P.; Mureau, M.A.; Seynaeve, C.; Ter Kuile, M.M.; Menke-Pluymers, M.B.; Timman, R.; Tibben, A. Body image issues after bilateral prophylactic mastectomy with breast reconstruction in healthy women at risk for hereditary breast cancer. Fam. Cancer 2013, 12, 479–487. [Google Scholar] [CrossRef]
- Heidari, M.; Shahbazi, S.; Ghodusi, M. Evaluation of body esteem and mental health in patients with breast cancer after mastectomy. J. Midlife Health 2015, 6, 173–177. [Google Scholar]
- Morrow, M.; White, J.; Moughan, J.; Owen, J.; Pajack, T.; Sylvester, J.; Wilson, J.F.; Winchester, D. Factors predicting the use of breast-conserving therapy in stage I and II breast carcinoma. J. Clin. Oncol. 2001, 19, 2254–2262. [Google Scholar] [CrossRef]
- Dragun, A.E.; Huang, B.; Tucker, T.C.; Spanos, W.J. Increasing mastectomy rates among all age groups for early stage breast cancer: A 10-year study of surgical choice. Breast J. 2012, 18, 318–325. [Google Scholar] [CrossRef]
- Kummerow, K.L.; Du, L.; Penson, D.F.; Shyr, Y.; Hooks, M.A. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015, 150, 9–16. [Google Scholar] [CrossRef]
- Rosenberg, K. Mastectomy Rates Rising in Women who Don’t Require Mastectomy. AJN Am. J. Nurs. 2015, 115, 56–57. [Google Scholar] [CrossRef]
- Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; Godwin, J.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar]
- Anderson, S.J.; Wapnir, I.; Dignam, J.J.; Fisher, B.; Mamounas, E.P.; Jeong, J.H.; Geyer, C.E., Jr.; Wickerham, D.L.; Costantino, J.P.; Wolmark, N. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five national surgical adjuvant breast and bowel project protocols of node-negative breast cancer. J. Clin. Oncol. 2009, 27, 2466–2473. [Google Scholar] [CrossRef]
- Zumsteg, Z.S.; Morrow, M.; Arnold, B.; Zheng, J.; Zhang, Z.; Robson, M.; Traina, T.; McCormick, B.; Powell, S.; Ho, A.Y. Breast-conserving therapy achieves locoregional outcomes comparable to mastectomy in women with T1-2N0 triple-negative breast cancer. Ann. Surg. Oncol. 2013, 20, 3469–3476. [Google Scholar] [CrossRef]
- Anders, C.K.; Johnson, R.; Litton, J.; Phillips, M.; Bleyer, A. Breast Cancer Before Age 40 Years. Semin. Oncol. 2009, 36, 237–249. [Google Scholar] [CrossRef]
- Quan, M.L.; Olivotto, I.A.; Baxter, N.N.; Friedenreich, C.M.; Metcalfe, K.; Warner, E.; MacLennan, K.; Stephen, J.E.; Akbari, M.R.; Howell, D.; et al. RUBY site investigators. A pan-Canadian prospective study of young women with breast cancer: The rationale and protocol design for the RUBY study. Curr. Oncol. 2020, 27, e516–e523. [Google Scholar] [CrossRef]
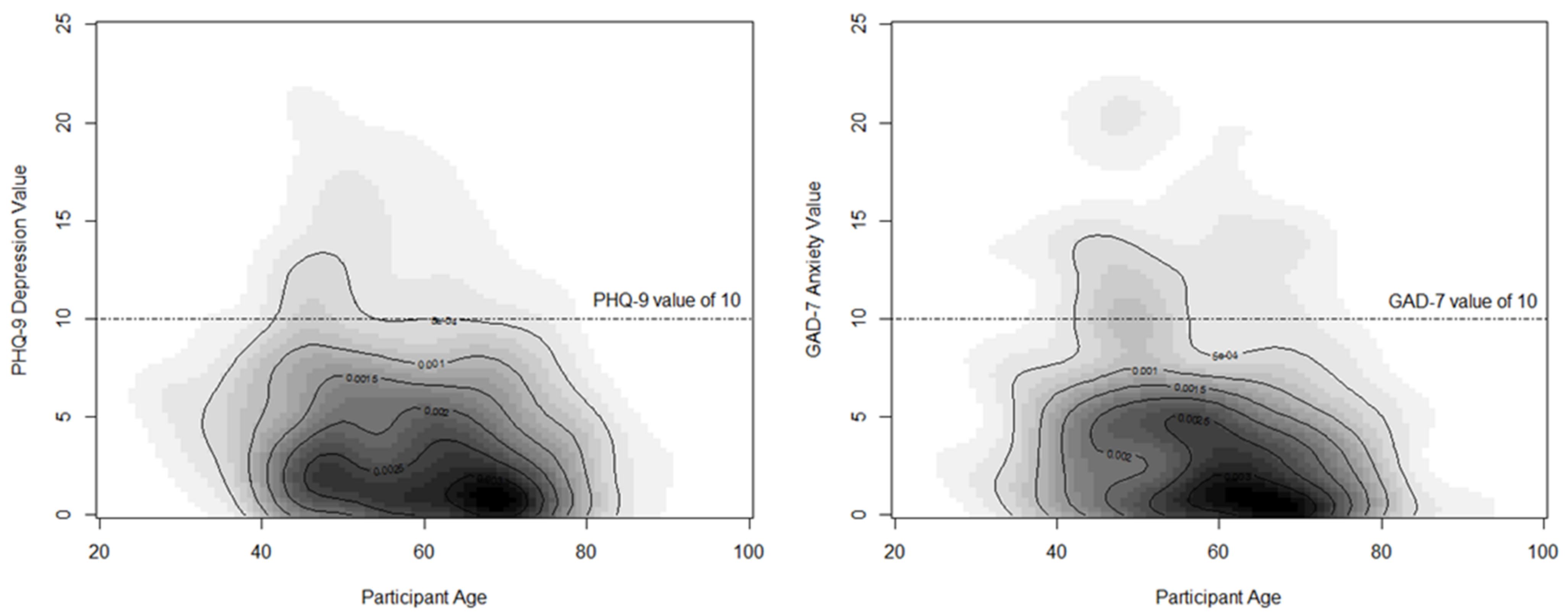
| Patient Characteristic | Overall Sample (N = 671) | Breast-Conserving Surgery (N = 443) | Total Mastectomy (N = 228) | p-Value |
|---|---|---|---|---|
| Age | ||||
| <49 | 27.57% | 20.54% | 41.34% | <0.01 |
| 50–59 | 23.25% | 20.99% | 27.63% | |
| 60–69 | 27.87% | 34.31% | 15.35% | |
| >70 | 21.31% | 24.15% | 15.79% | |
| Charlson Index | ||||
| 0 | 16.55% | 18.57% | 12.21% | <0.01 |
| 1–2 | 57.66% | 62.14% | 48.09% | |
| 3+ | 25.79% | 19.29% | 39.69% | |
| SES 1—Situational vulnerability | ||||
| Q1 Least Vulnerable | 27.75% | 28.54% | 26.22% | 0.86 |
| Q2 | 21.57% | 22.15% | 20.44% | |
| Q3 | 19.61% | 19.41% | 20.00% | |
| Q4 | 20.51% | 20.09% | 21.33% | |
| Q5 Most Vulnerable | 10.56% | 9.82% | 12.00% | |
| SES—Ethno-cultural composition | ||||
| Q1 Least Diverse | 3.32% | 2.97% | 4.00% | <0.01 |
| Q2 | 5.73% | 5.94% | 5.33% | |
| Q3 | 15.84% | 19.41% | 8.89% | |
| Q4 | 38.76% | 38.36% | 39.56% | |
| Q5 Most Diverse | 36.35% | 33.33% | 42.22% |
| Patient-Reported Outcome | Overall | Breast-Conserving Surgery | Total Mastectomy | F-Stat | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p-Value | |
| PHQ-9 2 | 4.64 | 4.7 | 4.36 | 4.48 | 5.18 | 5.06 | 0.03 |
| GAD-7 | 4.66 | 4.96 | 4.17 | 4.5 | 5.73 | 5.72 | <0.01 |
| PEG | 1.64 | 2.25 | 1.58 | 2.21 | 1.77 | 2.31 | 0.31 |
| EQ-5D VAS | 73.96 | 18.29 | 74.82 | 17.96 | 72.26 | 18.84 | 0.08 |
| Patient-Reported Outcome | Overall | Breast-Conserving Surgery | Total Mastectomy | Chi-Sq | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | p-Value | |
| PHQ-9 3 (depression) | |||||||
| ≥10, Moderate | 89 | 13.26% | 55 | 12.42% | 34 | 14.91% | 0.36 |
| ≥15, Severe | 36 | 5.37% | 18 | 4.06% | 18 | 7.89% | 0.03 |
| GAD-7 (anxiety) | |||||||
| ≥10, Moderate | 56 | 8.35% | 33 | 7.45% | 23 | 10.09% | 0.24 |
| ≥15, Severe | 19 | 2.83% | 8 | 1.81% | 11 | 4.82% | 0.02 |
| Regression Effect | PHQ-9 (Depression) | GAD-7 (Anxiety) | ||||
|---|---|---|---|---|---|---|
| Estimate | Standard Error | p-Value | Estimate | Standard Error | p-Value | |
| Intercept | 9.87 | 1.00 | <0.01 | 9.48 | 1.25 | <0.01 |
| Age (Years) | −0.08 | 0.01 | <0.01 | −0.09 | 0.01 | <0.01 |
| Charlson Index | ||||||
| 0 | −0.32 | 0.84 | 0.69 | −0.35 | 1.06 | 0.73 |
| 1–2 | −0.62 | 0.64 | 0.33 | −0.32 | 0.74 | 0.66 |
| 3+ | Reference | |||||
| Surgery type | ||||||
| Breast-conserving surgery | −0.17 | 0.40 | 0.67 | −0.74 | 0.50 | 0.14 |
| Total mastectomy | Reference | Reference | ||||
| SES 4—Situational vulnerability | ||||||
| Q1 Least Vulnerable | −0.33 | 0.71 | 0.63 | 0.64 | 0.93 | 0.49 |
| Q2 | 0.26 | 0.69 | 0.70 | 0.77 | 0.89 | 0.38 |
| Q3 | 0.80 | 0.68 | 0.23 | 1.15 | 0.86 | 0.18 |
| Q4 | 0.02 | 0.67 | 0.96 | 0.80 | 0.85 | 0.34 |
| Q5 Most Vulnerable | Reference | Reference | ||||
| SES—Ethno-cultural composition | ||||||
| Q1 Least Diverse | −0.02 | 1.02 | 0.98 | 0.42 | 1.32 | 0.74 |
| Q2 | 0.57 | 0.82 | 0.48 | 1.38 | 1.02 | 0.17 |
| Q3 | 0.30 | 0.61 | 0.61 | −0.43 | 0.78 | 0.58 |
| Q4 | 0.44 | 0.46 | 0.32 | 0.91 | 0.58 | 0.11 |
| Q5 Most Diverse | Reference | Reference | ||||
| Breast-QTM Scale: | Breast-Conserving Surgery | Total Mastectomy | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-Value | |
| Satisfaction with breasts | 67 | 21.9 | 62.5 | 22.2 | 0.014 |
| Psychosocial well-being | 72.8 | 19.9 | 68.4 | 21.9 | 0.011 |
| Physical well-being | 79.2 | 15.8 | 74.2 | 15.9 | <0.001 |
| Sexual well-being | 57.1 | 24.1 | 54.2 | 25.9 | 0.21 |
| Patient-Reported Outcome | Total Mastectomy with Immediate Breast Reconstruction | Total Mastectomy Alone | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-Value | |
| PHQ-9 2 | 6.03 | 5.55 | 3.84 | 3.85 | <0.01 |
| GAD-7 | 6.45 | 6.02 | 4.42 | 4.94 | 0.07 |
| PEG | 1.63 | 2.22 | 1.98 | 2.45 | 0.25 |
| EQ-5D VAS | 74.44 | 17.61 | 68.92 | 20.24 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKevitt, E.; Saleeb, M.; Liu, G.; Warburton, R.; Pao, J.-S.; Dingee, C.; Bazzarelli, A.; Tang, K.; Crump, T.; Sutherland, J.M. Differences in Preoperative Health-Related Quality of Life between Women Receiving Mastectomy or Breast Conserving Surgery in a Prospectively Recruited Cohort of Breast Cancer Patients. Curr. Oncol. 2023, 30, 118-129. https://doi.org/10.3390/curroncol30010010
McKevitt E, Saleeb M, Liu G, Warburton R, Pao J-S, Dingee C, Bazzarelli A, Tang K, Crump T, Sutherland JM. Differences in Preoperative Health-Related Quality of Life between Women Receiving Mastectomy or Breast Conserving Surgery in a Prospectively Recruited Cohort of Breast Cancer Patients. Current Oncology. 2023; 30(1):118-129. https://doi.org/10.3390/curroncol30010010
Chicago/Turabian StyleMcKevitt, Elaine, Maria Saleeb, Guiping Liu, Rebecca Warburton, Jin-Si Pao, Carol Dingee, Amy Bazzarelli, Katelynn Tang, Trafford Crump, and Jason M. Sutherland. 2023. "Differences in Preoperative Health-Related Quality of Life between Women Receiving Mastectomy or Breast Conserving Surgery in a Prospectively Recruited Cohort of Breast Cancer Patients" Current Oncology 30, no. 1: 118-129. https://doi.org/10.3390/curroncol30010010
APA StyleMcKevitt, E., Saleeb, M., Liu, G., Warburton, R., Pao, J.-S., Dingee, C., Bazzarelli, A., Tang, K., Crump, T., & Sutherland, J. M. (2023). Differences in Preoperative Health-Related Quality of Life between Women Receiving Mastectomy or Breast Conserving Surgery in a Prospectively Recruited Cohort of Breast Cancer Patients. Current Oncology, 30(1), 118-129. https://doi.org/10.3390/curroncol30010010






