Complete Surgical Excision Is Necessary following Vacuum-Assisted Biopsy for Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Identification
2.2. Statistical Analysis
3. Results
3.1. Clincopathological Characteristics of the Study Patients
3.2. Diagnostic Performances of Imaging Modalities for Detecting Remnant Lesions
3.3. Sentinel Lymph Node Biopsy
3.4. The Association of Tumor Size on VABB Specimen and Remnant Tumor on Pathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, E.T.; Ang, R.Z.; Tran, B.X.; Ho, C.S.; Zhang, Z.; Tan, W.; Bai, Y.; Zhang, M.; Tam, W.W.; Ho, R.C. Comparing Quality of Life in Breast Cancer Patients Who Underwent Mastectomy Versus Breast-Conserving Surgery: A Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 4970. [Google Scholar] [CrossRef] [PubMed]
- Shubeck, S.P.; Morrow, M.; Dossett, L.A. De-escalation in breast cancer surgery. NPJ Breast Cancer 2022, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-L.; Kim, K.Y.; Park, J.S.; Shin, J.-E.; Kim, H.-R.; Yang, B.; Kim, J.-Y.; Shim, J.Y.; Shin, E.-A.; Noh, S.-M. Clinicopathological Analysis of Ultrasound-guided Vacuum-assisted Breast Biopsy for the Diagnosis and Treatment of Breast Disease. Anticancer Res. 2018, 38, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Rageth, C.J.; O’Flynn, E.A.M.; Pinker, K.; Kubik-Huch, R.A.; Mundinger, A.; Decker, T.; Tausch, C.; Dammann, F.; Baltzer, P.A.; Fallenberg, E.M.; et al. Second International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast Cancer Res. Treat. 2018, 174, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Massone, E.; Gristina, L.; Fregatti, P.; Rescinito, G.; Villa, A.; Friedman, D.; Calabrese, M.; Simone, S.; Elena, M.; et al. Vacuum assisted breast biopsy (VAB) excision of subcentimeter microcalcifications as an alternative to open biopsy for atypical ductal hyperplasia. Br. J. Radiol. 2018, 91, 20180003. [Google Scholar] [CrossRef] [PubMed]
- Garlaschi, A.; Valente, I.; Brunetti, N.; De Giorgis, S.; Massa, B.; Calabrese, M.; Tagliafico, A.S. Is 9-G DBT-Guided Vacuum-Assisted Breast Biopsy Sufficient to Completely Remove T1 Breast Cancers (below 20 mm)? Analysis of 146 Patients with Histology as Reference Standard. Breast Care 2022, 17, 5. [Google Scholar] [CrossRef]
- Krivorotko, P.; Yerechshenko, S.; Emelyanov, A.; Busko, E.; Tabagua, T.; Novikov, S.; Artemyeva, A.; Krzhivitskiy, P.; Zhiltsova, E.; Komyahov, A.; et al. 125P De-escalation of breast cancer surgery after neoadjuvant systemic therapy in cCR/pCR patients confirmed by vacuum-assisted biopsy (VAB) and SLNB: A first report of the prospective non-randomized trial results. Ann. Oncol. 2022, 33, S180. [Google Scholar] [CrossRef]
- Pilewskie, M.; Morrow, M. Margins in breast cancer: How much is enough? Cancer 2018, 124, 1335–1341. [Google Scholar] [CrossRef]
- He, X.-F.; Ye, F.; Wen, J.-H.; Li, S.-J.; Huang, X.-J.; Xiao, X.-S.; Xie, X.-M. High Residual Tumor Rate for Early Breast Cancer Patients Receiving Vacuum-assisted Breast Biopsy. J. Cancer 2017, 8, 490–496. [Google Scholar] [CrossRef]
- Krischer, B.; Forte, S.; Singer, G.; Kubik-Huch, R.A.; Leo, C. Stereotactic Vacuum-Assisted Breast Biopsy in Ductal Carcinoma in situ: Residual Microcalcifications and Intraoperative Findings. Breast Care 2019, 15, 386–391. [Google Scholar] [CrossRef]
- Penco, S.; Rizzo, S.; Bozzini, A.C.; Latronico, A.; Menna, S.; Cassano, E.; Bellomi, M. Stereotactic Vacuum-Assisted Breast Biopsy Is Not a Therapeutic Procedure Even When All Mammographically Found Calcifications Are Removed: Analysis of 4086 Procedures. Am. J. Roentgenol. 2010, 195, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Vag, T.; Pfleiderer, S.O.R.; Böttcher, J.; Wurdinger, S.; Gajda, M.; Camara, O.; Kaiser, W.A. Ultrasound-guided breast biopsy using a 10-gauge self-contained vacuum-assisted device. Eur. Radiol. 2007, 17, 3100–3102. [Google Scholar] [CrossRef] [PubMed]
- Gajdos, C.; Levy, M.; Herman, Z.; Herman, G.; Bleiweiss, I.J.; Tartter, P.I. Complete removal of nonpalpable breast malignancies with a stereotactic percutaneous vacuum-assisted biopsy instrument. J. Am. Coll. Surg. 1999, 189, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Liberman, L.; Dershaw, D.D.; Rosen, P.P.; Morris, E.; Abramson, A.F.; Borgen, P. Percutaneous removal of malignant mammographic lesions at stereotactic vacuum-assisted biopsy. Radiology 1998, 206, 711–715. [Google Scholar] [CrossRef]
- Liberman, L.; Zakowski, M.F.; Avery, S.; Hudis, C.; Morris, E.; Abramson, A.F.; LaTrenta, L.R.; Glassman, J.R.; Dershaw, D.D. Complete percutaneous excision of infiltrating carcinoma at stereotactic breast biopsy: How can tumor size be assessed? Am. J. Roentgenol. 1999, 173, 1315–1322. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krishnamurthy, K.; Febres-Aldana, C.A.; Alghamdi, S.; Mesko, T.; Paramo, J.; Poppiti, R.J. Comparative analysis of margin status in breast conservation surgery and its correlation with subsequent re-excision findings. Pathologica 2019, 111, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Youn, I.; Kim, M.J.; Moon, H.J.; Kim, E.-K. Absence of Residual Microcalcifications in Atypical Ductal Hyperplasia Diagnosed via Stereotactic Vacuum-Assisted Breast Biopsy: Is Surgical Excision Obviated? J. Breast Cancer 2014, 17, 265–269. [Google Scholar] [CrossRef][Green Version]
- Chae, E.Y.; Cha, J.H.; Kim, H.H.; Shin, H.J.; Kim, H.; Lee, J.; Cheung, J.Y. Evaluation of Residual Disease Using Breast MRI After Excisional Biopsy for Breast Cancer. Am. J. Roentgenol. 2013, 200, 1167–1173. [Google Scholar] [CrossRef]
- Michalopoulos, N.V.; Zagouri, F.; Sergentanis, T.N.; Pararas, N.; Koulocheri, D.; Nonni, A.; Filippakis, G.M.; Chatzipantelis, P.; Bramis, J.; Zografos, G.C. Needle tract seeding after vacuum-assisted breast biopsy. Acta Radiol. 2008, 49, 267–270. [Google Scholar] [CrossRef]
- Perretta, T.; Meucci, R.; Pistolese, C.A.; Manenti, G.; Di Stefano, C.; Vanni, G.; Anemona, L.; Ferrari, D.; Lamacchia, F.; De Stasio, V.; et al. Ultrasound-Guided Laser Ablation After Excisional Vacuum-Assisted Breast Biopsy for Small Malignant Breast Lesions: Preliminary Results. Technol. Cancer Res. Treat. 2021, 20, 1533033820980089. [Google Scholar] [CrossRef]
- Papapanagiotou, I.K.; Koulocheri, D.; Kalles, V.; Liakou, P.; Michalopoulos, N.V.; Al-Harethee, W.; Georgiou, G.; Matiatou, M.; Nonni, A.; Pazaiti, A.; et al. Margin-free excision of small solid breast carcinomas using the Intact Breast Lesion Excision System®: Is it feasible? Breast Cancer 2017, 25, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Kotani, H.; Yoshimura, A.; Adachi, Y.; Ishiguro, J.; Hisada, T.; Ichikawa, M.; Gondou, N.; Hattori, M.; Kondou, N.; Sawaki, M.; et al. Sentinel lymph node biopsy is not necessary in patients diagnosed with ductal carcinoma in situ of the breast by stereotactic vacuum-assisted biopsy. Breast Cancer 2014, 23, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Gentilini, O.; Botteri, E.; Dadda, P.; Sangalli, C.; Boccardo, C.; Peradze, N.; Ghisini, R.; Galimberti, V.; Veronesi, P.; Luini, A.; et al. Physical function of the upper limb after breast cancer surgery. Results from the SOUND (Sentinel node vs. Observation after axillary Ultra-souND) trial. Eur. J. Surg. Oncol. (EJSO) 2016, 42, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Kim, M.J.; Moon, H.J.; Kim, E.-K.; Yoon, J.H. False Negative Results of Preoperative Axillary Ultrasound in Patients with Invasive Breast Cancer: Correlations with Clinicopathologic Findings. Ultrasound Med. Biol. 2012, 38, 1881–1886. [Google Scholar] [CrossRef]
- van Seijen, M.; Lips, E.H.; Thompson, A.M.; Nik-Zainal, S.; Futreal, A.; Hwang, E.S.; Verschuur, E.; Lane, J.; Jonkers, J.; Rea, D.W.; et al. Ductal carcinoma in situ: To treat or not to treat, that is the question. Br. J. Cancer 2019, 121, 285–292. [Google Scholar] [CrossRef]
- Francis, A.; Thomas, J.; Fallowfield, L.; Wallis, M.; Bartlett, J.M.; Brookes, C.; Roberts, T.; Pirrie, S.; Gaunt, C.; Young, J.; et al. Addressing overtreatment of screen detected DCIS; the LORIS trial. Eur. J. Cancer 2015, 51, 2296–2303. [Google Scholar] [CrossRef]
- Hwang, E.S.; Hyslop, T.; Lynch, T.; Frank, E.; Pinto, D.; Basila, D.; Collyar, D.; Bennett, A.; Kaplan, C.; Rosenberg, S.; et al. The COMET (Comparison of Operative versus Monitoring and Endocrine Therapy) trial: A phase III randomised controlled clinical trial for low-risk ductal carcinoma in situ (DCIS). BMJ Open 2019, 9, e026797. [Google Scholar] [CrossRef]
- Elshof, L.E.; Tryfonidis, K.; Slaets, L.; van Leeuwen-Stok, A.E.; Skinner, V.P.; Dif, N.; Pijnappel, R.M.; Bijker, N.; Rutgers, E.J.; Wesseling, J. Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ—The LORD study. Eur. J. Cancer 2015, 51, 1497–1510. [Google Scholar] [CrossRef]
- Morgan, J.; Potter, S.; Sharma, N.; McIntosh, S.; Coles, C.; Dodwell, D.; Elder, K.; Gaunt, C.; Lyburn, I.; Paramasivan, S.; et al. The SMALL Trial: A Big Change for Small Breast Cancers. Clin. Oncol. 2019, 31, 659–663. [Google Scholar] [CrossRef]
- McIntosh, S.; Coles, C.; Conefrey, C.; Dodwell, D.; Elder, K.; Foster, J.; Gaunt, C.; Kirkham, A.; Lyburn, I.; Morgan, J.; et al. Abstract OT1-06-02: SMALL—Open surgery versus minimally invasive vacuum-assisted excision for small screen detected breast cancer: A phase 3 randomised trial. Cancer Res. 2022, 82, OT1-06. [Google Scholar] [CrossRef]
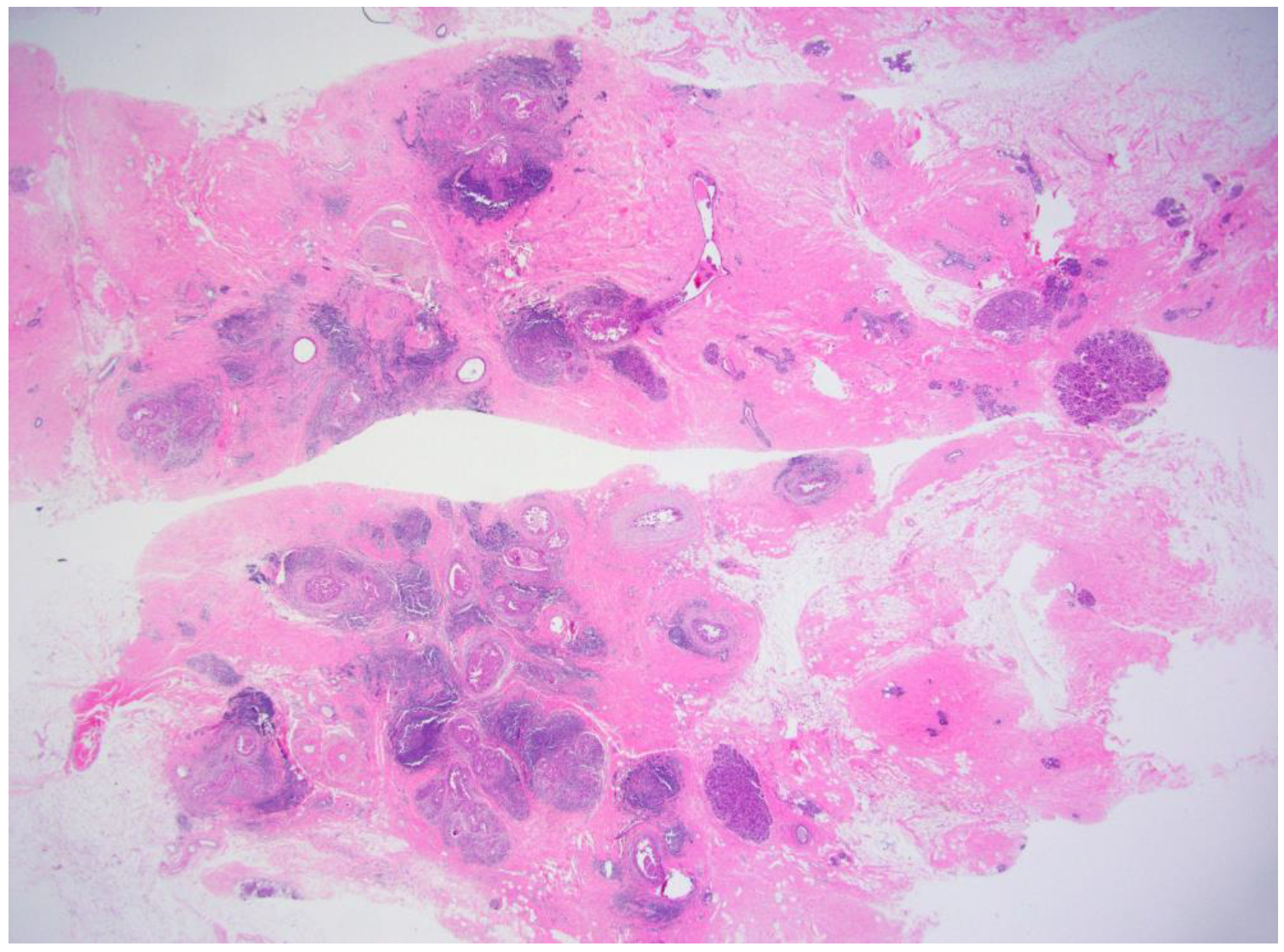
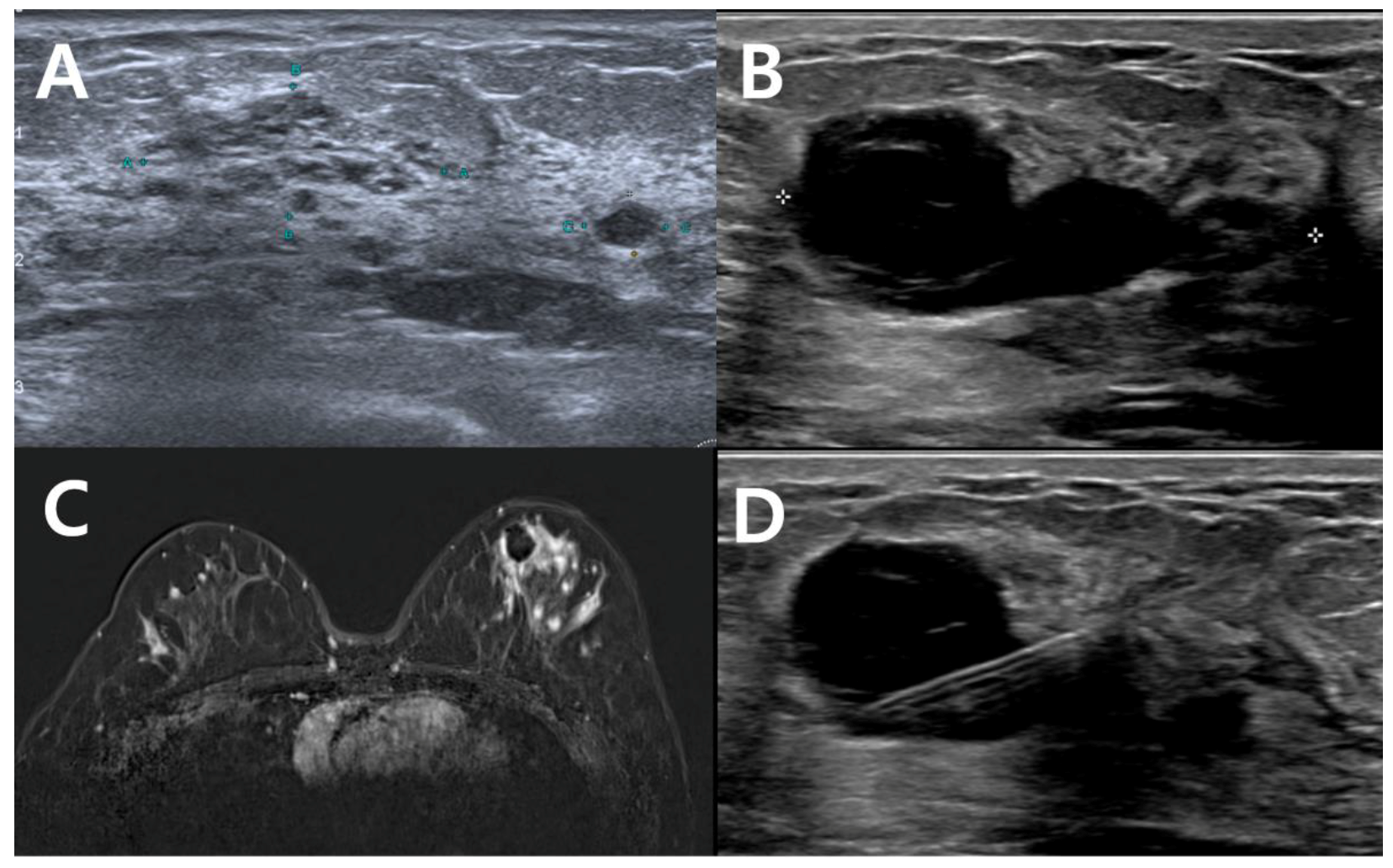
| No Residual Tumor (n = 9) | Residual Tumor on Excision (n = 40) | p | |
|---|---|---|---|
| Age (years) | 49.44 ± 17.01 | 51.20 ± 10.67 | 0.773 |
| Menopausal status | 1.000 | ||
| Premenopausal | 4 (44.4%) | 19 (47.5%) | |
| Postmenopausal | 5 (55.6%) | 21 (52.5%) | |
| BMI (kg/m2) | 24.18 ± 4.22 | 23.45 ± 3.23 | 0.631 |
| Tumor size on US * | 1.42 ± 0.79 | 1.54 ± 0.80 | 0.698 |
| BI-RADS category † | 0.781 | ||
| 3 | 1 (33.3%) | 6 (54.5%) | |
| 4A | 1 (33.3%) | 3 (27.3%) | |
| 4B | 1 (33.3%) | 2 (18.2%) | |
| Initial biopsy method | 0.725 | ||
| FNA | 2 (22.2%) | 5 (12.5%) | |
| CNB | 3 (33.3%) | 13 (32.5%) | |
| Upfront VABB | 4 (44.4%) | 22 (55.0%) | |
| Surgery | 0.569 | ||
| BCS | 9 (100%) | 35 (87.5%) | |
| TM | 0 | 5 (12.5%) | |
| Histology | 0.128 | ||
| DCIS | 2 (22.2%) | 20 (50.0%) | |
| Microinvasive carcinoma | 2 (22.2%) | 2 (5.0%) | |
| IDC | 5 (55.6%) | 18 (45.0%) | |
| HR status | 0.364 | ||
| Negative | 3 (33.3%) | 7 (17.5%) | |
| Positive | 6 (66.7%) | 33 (82.5%) | |
| HER2 status ‡ | 0.326 | ||
| Negative | 7 (77.8%) | 20 (51.3%) | |
| Equivocal | 1 (11.1%) | 13 (33.3%) | |
| Positive | 1 (11.1%) | 6 (15.4%) |
| Mammography (n = 26) | Ultrasonography (n = 47) | MRI (n = 49) | |
|---|---|---|---|
| Sensitivity | 5/23 (21.7%) | 22/38 (57.9%) | 24/40 (60.0%) |
| Specificity | 3/3 (100%) | 6/9 (66.7%) | 6/9 (66.7%) |
| PPV | 5/5 (100%) | 22/25 (88.0%) | 24/27 (88.9%) |
| NPV | 3/22 (13.6%) | 6/22 (27.2%) | 6/22 (27.2%) |
| Accuracy | 8/26 (30.8%) | 28/47 (59.6%) | 30/49 (61.2%) |
| Pathologic Tumor Size | No Residual Tumor (n = 9) | Residual Tumor on Excision (n = 40) |
|---|---|---|
| ≤0.5 cm | 6 | 4 |
| 0.6~1 cm | 0 | 12 |
| 1.1~2 cm | 0 | 5 |
| No data | 3 | 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.H.; Ahn, S.E.; Kim, S.; Kwon, M.J.; Suh, Y.J.; Kim, D. Complete Surgical Excision Is Necessary following Vacuum-Assisted Biopsy for Breast Cancer. Curr. Oncol. 2022, 29, 9357-9364. https://doi.org/10.3390/curroncol29120734
Park JH, Ahn SE, Kim S, Kwon MJ, Suh YJ, Kim D. Complete Surgical Excision Is Necessary following Vacuum-Assisted Biopsy for Breast Cancer. Current Oncology. 2022; 29(12):9357-9364. https://doi.org/10.3390/curroncol29120734
Chicago/Turabian StylePark, Jung Ho, So Eun Ahn, Sanghwa Kim, Mi Jung Kwon, Yong Joon Suh, and Doyil Kim. 2022. "Complete Surgical Excision Is Necessary following Vacuum-Assisted Biopsy for Breast Cancer" Current Oncology 29, no. 12: 9357-9364. https://doi.org/10.3390/curroncol29120734
APA StylePark, J. H., Ahn, S. E., Kim, S., Kwon, M. J., Suh, Y. J., & Kim, D. (2022). Complete Surgical Excision Is Necessary following Vacuum-Assisted Biopsy for Breast Cancer. Current Oncology, 29(12), 9357-9364. https://doi.org/10.3390/curroncol29120734






