Immunogenic Cell Death Role in Urothelial Cancer Therapy
Abstract
1. Introduction
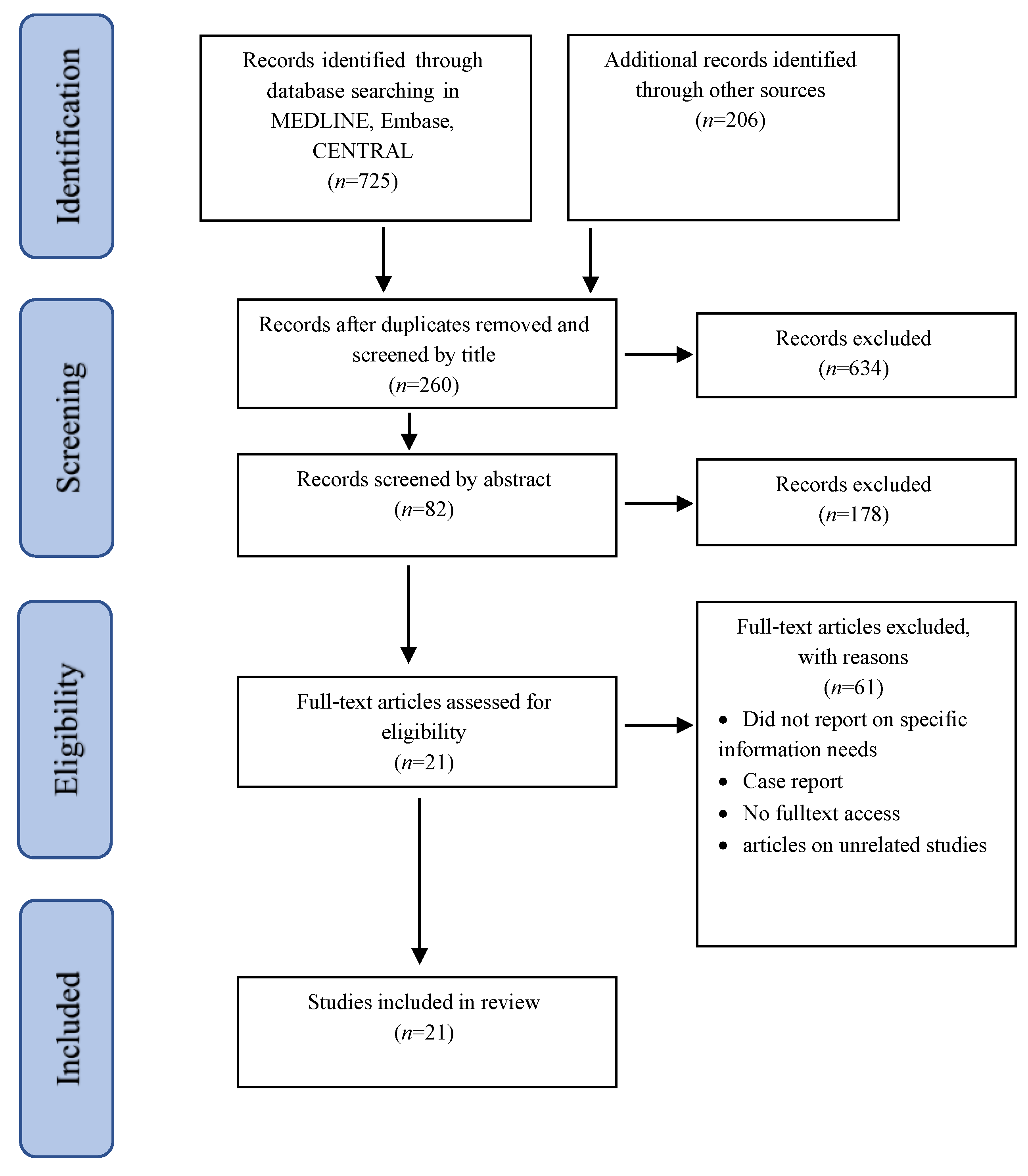
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Information Sources
2.3. Data Extraction
3. Results
4. Outcome
4.1. Oncolytic Viruses
4.2. Anticancer Vaccination Effect
4.3. Photodynamic Therapy (PDT)
4.4. Inhibitory DAMPs (iDAMPs)
4.5. Radiotherapy, Chemotherapy and Combination Therapy
4.6. New Therapeutic Agents
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A. Search Strategies
- Medline (ovid), Central (ovid)
- ((Antigen exposure).mp or (antigen presentation).mp or (immune system activation).mp or (immun* activation).mp or (immun* necrosis).mp or (immunogenic cell death).mp or ICD.mp) AND (exp Urinary Bladder Neoplasms or (Urinary Bladder Neoplasm*).mp or (bladder neoplasm*).mp or (bladder tumor*).mp or (bladder tumour*).mp or (bladder cancer*).mp or (Urinary Bladder cancer*).mp or (Urinary Bladder malignant tumor*).mp or Exp Carcinoma, Transitional Cell or Exp Ureteral Neoplasms or (upper tract urothelial cancer).mp).
- Embase
- ((‘Antigen exposure’:ti,ab or ‘antigen presentation’:ti,ab or ‘immune system activation’:ti,ab or ‘immun* activation’:ti,ab or ‘immun* necrosis’:ti,ab or ‘immunogenic cell death’:ti,ab or ICD:ti,ab) AND (‘bladder tumor’/exp or ‘Urinary Bladder Neoplasm*’:ti,ab or ‘bladder neoplasm*’:ti,ab or ‘bladder tumor*’:ti,ab or ‘bladder tumour*’:ti,ab or ‘bladder cancer*’:ti,ab or ‘Urinary Bladder cancer*’:ti,ab or ‘Urinary Bladder malignant tumor*’:ti,ab or ‘transitional cell carcinoma’/exp or ‘ureter tumor’/exp or ‘upper tract urothelial cancer’:ti,ab)) AND [embase]/lim.
References
- Reed, J.C. Drug Insight: Cancer Therapy Strategies Based on Restoration of Endogenous Cell Death Mechanisms. Nat. Clin. Pract. Oncol. 2006, 3, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Ferguson, T.; Zitvogel, L.; Kroemer, G. Immunogenic and Tolerogenic Cell Death. Nat. Rev. Immunol. 2009, 9, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Mishchenko, T.; Mitroshina, E.; Balalaeva, I.; Krysko, O.; Vedunova, M.; Krysko, D.v. An Emerging Role for Nanomaterials in Increasing Immunogenicity of Cancer Cell Death. Biochim. Biophys. Acta (BBA) Rev. Cancer 2019, 1871, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ren, M.; Feng, F.; Chen, K.; Ju, X. Treatment of Colon Cancer with Liver X Receptor Agonists Induces Immunogenic Cell Death. Mol. Carcinog. 2018, 57, 903–910. [Google Scholar] [CrossRef]
- Jin, M.Z.; Wang, X.P. Immunogenic Cell Death-Based Cancer Vaccines. Front. Immunol. 2021, 12, 697964. [Google Scholar] [CrossRef]
- Showalter, A.; Limaye, A.; Oyer, J.L.; Igarashi, R.; Kittipatarin, C.; Copik, A.J.; Khaled, A.R. Cytokines in Immunogenic Cell Death: Applications for Cancer Immunotherapy. Cytokine 2017, 97, 123–132. [Google Scholar] [CrossRef]
- Shakfa, N.; Siemens, D.R.; Koti, M. Revisiting Immunogenic Cell Death to Improve Treatment Response in Cancer. In Biological Mechanisms and the Advancing Approaches to Overcoming Cancer Drug Resistance; Elsevier: Amsterdam, The Netherlands, 2021; pp. 65–90. [Google Scholar] [CrossRef]
- Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G.M.; Apetoh, L.; Perfettini, J.L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N.; et al. Calreticulin Exposure Dictates the Immunogenicity of Cancer Cell Death. Nat. Med. 2007, 13, 54–61. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Apetoh, L.; Tesniere, A.; Aymeric, L.; Ma, Y.; Ortiz, C.; Vermaelen, K.; Panaretakis, T.; Mignot, G.; Ullrich, E.; et al. Activation of the NLRP3 Inflammasome in Dendritic Cells Induces IL-1β–Dependent Adaptive Immunity against Tumors. Nat. Med. 2009, 15, 1170–1178. [Google Scholar] [CrossRef]
- Wang, Q.; Ju, X.; Wang, J.; Fan, Y.; Ren, M.; Zhang, H. Immunogenic Cell Death in Anticancer Chemotherapy and Its Impact on Clinical Studies. Cancer Lett. 2018, 438, 17–23. [Google Scholar] [CrossRef]
- Chen, C.W. Comment on ‘Long Noncoding RNA UCA1 Promotes Glutamine-Driven Anaplerosis of Bladder Cancer by Interacting with HnRNP I/L to Upregulate GPT2 Expression’ by Chen et al.’”. Transl. Oncol. 2022, 18, 101372. [Google Scholar] [CrossRef]
- Narii, N.; Sobue, T.; Zha, L.; Kitamura, T.; Sawada, N.; Iwasaki, M.; Inoue, M.; Yamaji, T.; Tsugane, S. Vegetable and Fruit Intake and the Risk of Bladder Cancer: Japan Public Health Center-Based Prospective Study. Br. J. Cancer 2022, 126, 1647–1658. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, B.A.; de Giorgi, U.; Ciardiello, D.; Schinzari, G.; Cisternino, A.; Tortora, G.; Maiello, E. Immune-Checkpoint Inhibitors in Advanced Bladder Cancer: Seize the Day. Biomedicines 2022, 10, 411. [Google Scholar] [CrossRef] [PubMed]
- Patelli, G.; Zeppellini, A.; Spina, F.; Righetti, E.; Stabile, S.; Amatu, A.; Tosi, F.; Ghezzi, S.; Siena, S.; Sartore-Bianchi, A. The Evolving Panorama of HER2-Targeted Treatments in Metastatic Urothelial Cancer: A Systematic Review and Future Perspectives. Cancer Treat. Rev. 2022, 104, 102351. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; West, A.; Hayes, J.; Teoh, J.; Decaestecker, K.; Vasdev, N. Methods of Sentinel Lymph Node Detection and Management in Urinary Bladder Cancer—A Narrative Review. Curr. Oncol. 2022, 29, 1335–1348. [Google Scholar] [CrossRef]
- Tran, L.; Xiao, J.F.; Agarwal, N.; Duex, J.E.; Theodorescu, D. Advances in Bladder Cancer Biology and Therapy. Nat. Cancer 2020, 21, 104–121. [Google Scholar] [CrossRef]
- Lenis, A.T.; Lec, P.M.; Chamie, K. Bladder Cancer a Review. J. Am. Med. Assoc. 2020, 324, 1980–1991. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non–Muscle-Invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2021, 81, 75–94. [Google Scholar] [CrossRef]
- Rouanne, M.; Roumiguié, M.; Houédé, N.; Masson-Lecomte, A.; Colin, P.; Pignot, G.; Larré, S.; Xylinas, E.; Rouprêt, M.; Neuzillet, Y. Development of Immunotherapy in Bladder Cancer: Present and Future on Targeting PD(L)1 and CTLA-4 Pathways. World J. Urol. 2018, 36, 1727–1740. [Google Scholar] [CrossRef]
- Labi, V.; Erlacher, M. How Cell Death Shapes Cancer. Cell Death Dis. 2015, 6, e1675. [Google Scholar] [CrossRef]
- Wiman, K.G.; Zhivotovsky, B. Understanding Cell Cycle and Cell Death Regulation Provides Novel Weapons against Human Diseases. J. Intern. Med. 2017, 281, 483–495. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. Methodol. Theory Pract. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Kuusisto, A.; Santavirta, J.; Saranto, K.; Korhonen, P.; Haavisto, E. Advance Care Planning for Patients with Cancer in Palliative Care: A Scoping Review from a Professional Perspective. J. Clin. Nurs. 2020, 29, 2069–2082. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, H.L.; Levac, D.; O’Brien, K.K.; Straus, S.; Tricco, A.C.; Perrier, L.; Kastner, M.; Moher, D. Scoping Reviews: Time for Clarity in Definition, Methods, and Reporting. J. Clin. Epidemiol. 2014, 67, 1291–1294. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.C.; Khalil, H. Chapter 11: Scoping reviews (2020 version). In JBI Manual for Evidence Synthesis; JBI: Adelaide, South Australia, 2020. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Gilardini Montani, M.S.; D’Eliseo, D.; Cirone, M.; di Renzo, L.; Faggioni, A.; Santoni, A.; Velotti, F. Capsaicin-Mediated Apoptosis of Human Bladder Cancer Cells Activates Dendritic Cells via CD91. Nutrition 2015, 31, 578–581. [Google Scholar] [CrossRef]
- Annels, N.E.; Arif, M.; Simpson, G.R.; Denyer, M.; Moller-Levet, C.; Mansfield, D.; Butler, R.; Shafren, D.; Au, G.; Knowles, M.; et al. Oncolytic Immunotherapy for Bladder Cancer Using Coxsackie A21 Virus. Mol. Ther. Oncolytics 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Zeng, X.; Cui, Z.; Chen, J.; Tang, F.; Hu, Z.; Luo, D.; Zhou, J.; Liu, L.; Qiu, W.; Ye, Y.; et al. Hypofractionated Radiation Induced the Immunogenic Death of Bladder Cancer Cells Leading to the Immune Sensitization of Dendritic Cells. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Fukushima, H.; Yoshida, S.; Kijima, T.; Nakamura, Y.; Fukuda, S.; Uehara, S.; Yasuda, Y.; Tanaka, H.; Yokoyama, M.; Matsuoka, Y.; et al. Combination of Cisplatin and Irradiation Induces Immunogenic Cell Death and Potentiates Postirradiation Anti–PD-1 Treatment Efficacy in Urothelial Carcinoma. Int. J. Mol. Sci. 2021, 22, 535. [Google Scholar] [CrossRef]
- D’Eliseo, D.; Manzi, L.; Velotti, F. Capsaicin as an Inducer of Damage-Associated Molecular Patterns (DAMPs) of Immunogenic Cell Death (ICD) in Human Bladder Cancer Cells. Cell Stress Chaperones 2013, 18, 801–808. [Google Scholar] [CrossRef]
- Molinari, R.; D’Eliseo, D.; Manzi, L.; Zolla, L.; Velotti, F.; Merendino, N. The N3-Polyunsaturated Fatty Acid Docosahexaenoic Acid Induces Immunogenic Cell Death in Human Cancer Cell Lines via Pre-Apoptotic Calreticulin Exposure. Cancer Immunol. Immunother. 2011, 60, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Lichtenegger, E.; Koll, F.; Haas, H.; Mantwill, K.; Janssen, K.P.; Laschinger, M.; Gschwend, J.; Steiger, K.; Black, P.C.; Moskalev, I.; et al. The Oncolytic Adenovirus XVir-N-31 as a Novel Therapy in Muscle-Invasive Bladder Cancer. Hum. Gene Ther. 2019, 30, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Hojeij, R.; Domingos-Pereira, S.; Nkosi, M.; Gharbi, D.; Derré, L.; Schiller, J.; Jichlinski, P.; Nardelli-Haefliger, D. Immunogenic Human Papillomavirus Pseudovirus-Mediated Suicide-Gene Therapy for Bladder Cancer. Int. J. Mol. Sci. 2016, 17, 1125. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Su, B.; Mo, L.; Zhao, C.; Zhao, Z.; Li, H.; Hu, Z.; Li, J. Norcantharidin Induces Immunogenic Cell Death of Bladder Cancer Cells through Promoting Autophagy in Acidic Culture. Int. J. Mol. Sci. 2022, 23, 3944. [Google Scholar] [CrossRef]
- Liljenfeldt, L.; Gkirtzimanaki, K.; Vyrla, D.; Svensson, E.; Loskog, A.S.; Eliopoulos, A.G. Enhanced Therapeutic Anti-Tumor Immunity Induced by Co-Administration of 5-Fluorouracil and Adenovirus Expressing CD40 Ligand. Cancer Immunol. Immunother. 2014, 63, 273–282. [Google Scholar] [CrossRef]
- Garg, A.D.; Elsen, S.; Krysko, D.v.; Vandenabeele, P.; de Witte, P.; Agostinis, P. Resistance to Anticancer Vaccination Effect Is Controlled by a Cancer Cell-Autonomous Phenotype That Disrupts Immunogenic Phagocytic Removal. Oncotarget 2015, 6, 26841–26860. [Google Scholar] [CrossRef]
- Hayashi, K.; Nikolos, F.; Lee, Y.C.; Jain, A.; Tsouko, E.; Gao, H.; Kasabyan, A.; Leung, H.E.; Osipov, A.; Jung, S.Y.; et al. Tipping the Immunostimulatory and Inhibitory DAMP Balance to Harness Immunogenic Cell Death. Nat. Commun. 2020, 11, 6299. [Google Scholar] [CrossRef]
- Nikolos, F.; Hayashi, K.; Hoi, X.P.; Alonzo, M.E.; Mo, Q.; Kasabyan, A.; Furuya, H.; Trepel, J.; di Vizio, D.; Guarnerio, J.; et al. Cell Death-Induced Immunogenicity Enhances Chemoimmunotherapeutic Response by Converting Immune-Excluded into T-Cell Inflamed Bladder Tumors. Nat. Commun. 2022, 13, 1487. [Google Scholar] [CrossRef]
- Oresta, B.; Pozzi, C.; Braga, D.; Hurle, R.; Lazzeri, M.; Colombo, P.; Frego, N.; Erreni, M.; Faccani, C.; Elefante, G.; et al. Mitochondrial Metabolic Reprogramming Controls the Induction of Immunogenic Cell Death and Efficacy of Chemotherapy in Bladder Cancer. Sci. Transl. Med. 2021, 13, eaba6110. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Krysko, D.v; Verfaillie, T.; Kaczmarek, A.; Ferreira, G.B.; Marysael, T.; Rubio, N.; Firczuk, M.; Mathieu, C.; Roebroek, A.J.M.; et al. A Novel Pathway Combining Calreticulin Exposure and ATP Secretion in Immunogenic Cancer Cell Death. EMBO J. 2012, 31, 1062–1079. [Google Scholar] [CrossRef]
- Oseledchyk, A.; Ricca, J.M.; Gigoux, M.; Ko, B.; Redelman-Sidi, G.; Walther, T.; Liu, C.; Iyer, G.; Merghoub, T.; Wolchok, J.D.; et al. Lysis-Independent Potentiation of Immune Checkpoint Blockade by Oncolytic Virus. Oncotarget 2018, 9, 28702–28716. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garg, A.D.; Dudek, A.M.; Ferreira, G.B.; Verfaillie, T.; Vandenabeele, P.; Krysko, D.V.; Mathieu, C.; Agostinis, P. ROS-Induced Autophagy in Cancer Cells Assists in Evasion from Determinants of Immunogenic Cell Death. Autophagy 2013, 9, 1292–1307. [Google Scholar] [CrossRef] [PubMed]
- Rangsitratkul, C.; Lawson, C.; Bernier-Godon, F.; Niavarani, S.R.; Boudaud, M.; Rouleau, S.; Gladu-Corbin, A.O.; Surendran, A.; Ekindi-Ndongo, N.; Koti, M.; et al. Intravesical Immunotherapy with a GM-CSF Armed Oncolytic Vesicular Stomatitis Virus Improves Outcome in Bladder Cancer. Mol. Ther. Oncolytics 2022, 24, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Annels, N.E.; Mansfield, D.; Arif, M.; Ballesteros-Merino, C.; Simpson, G.R.; Denyer, M.; Sandhu, S.S.; Melcher, A.A.; Harrington, K.J.; Davies, B.; et al. Phase I Trial of an ICAM-1-Targeted Immunotherapeutic-Coxsackievirus A21 (CVA21) as an Oncolytic Agent Against Non Muscle-Invasive Bladder Cancer. Clin. Cancer Res. 2019, 25, 5818–5831. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Wang, H.; Hahn, N.M.; Twardowski, P.; Pal, S.K.; Albany, C.; Fleming, M.T.; Starodub, A.; Hauke, R.J.; Yu, M.; et al. Phase 2 Trial of Gemcitabine, Cisplatin, plus Ipilimumab in Patients with Metastatic Urothelial Cancer and Impact of DNA Damage Response Gene Mutations on Outcomes. Eur. Urol. 2018, 73, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Malmström, P.U.; Loskog, A.S.I.; Lindqvist, C.A.; Mangsbo, S.M.; Fransson, M.; Wanders, A.; Gårdmark, T.; Tötterman, T.H. AdCD40L Immunogene Therapy for Bladder Carcinoma—The First Phase I/IIa Trial. Clin. Cancer Res. 2010, 16, 3279–3287. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ramachandran, M.; Jin, C.; Quijano-Rubio, C.; Martikainen, M.; Yu, D.; Essand, M. Characterization of Virus-Mediated Immunogenic Cancer Cell Death and the Consequences for Oncolytic Virus-Based Immunotherapy of Cancer. Cell Death Dis. 2020, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Cayeux, S.; Lupton, S.D.; Dörken, B.; Blankenstein, T. The Thymidine Kinase/Ganciclovir-Mediated “Suicide” Effect Is Variable in Different Tumor Cells. Hum. Gene Ther. 1995, 6, 1525–1530. [Google Scholar] [CrossRef]
- Janeway, C.A.; Travers, P.J.; Walport, M.; Shlomchik, M.J. Immunobiology: The Immune System in Health and Disease, 6th ed.; Taylor & Francis Group: Abingdon, UK, 2005; 800p. [Google Scholar]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA A Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Hayashi, K.; Nikolos, F.; Chan, K.S. Inhibitory DAMPs in Immunogenic Cell Death and Its Clinical Implications. Cell Stress 2021, 5, 52–54. [Google Scholar] [CrossRef]
- Golden, E.B.; Apetoh, L. Radiotherapy and Immunogenic Cell Death. Semin. Radiat. Oncol. 2015, 25, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, T.L.; Olawale, F.; Olisah, C.; Adetunji, A.E.; Aremu, A.O. Capsaicin: A Two-Decade Systematic Review of Global Research Output and Recent Advances Against Human Cancer. Front. Oncol. 2022, 12, 908487. [Google Scholar] [CrossRef] [PubMed]
- Ti, D.; Yan, X.; Wei, J.; Wu, Z.; Wang, Y.; Han, W. Inducing Immunogenic Cell Death in Immuno-Oncological Therapies. Chin. J. Cancer Res. 2022, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh, Z.; Safarzadeh, E.; Safaei, S.; Baradaran, A.; Mohammadi, A.; Hajiasgharzadeh, K.; Derakhshani, A.; Argentiero, A.; Silvestris, N.; Baradaran, B. Current Approaches for Combination Therapy of Cancer: The Role of Immunogenic Cell Death. Cancers 2020, 12, 1047. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, C.; Shi, G.; Yue, D.; Shu, Y.; Hu, S.; Qi, Z.; Chen, Y.; Zhang, B.; Zhang, Y.; et al. High-Dose VitC plus Oncolytic Adenoviruses Enhance Immunogenic Tumor Cell Death and Reprogram Tumor Immune Microenvironment. Mol. Ther. 2022, 30, 644–661. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Xu, X.; Li, M.; Zhang, Y.; Lin, Q.; Gong, T.; Sun, X.; Zhang, Z.; Zhang, L. Multifunctional Size-Expandable Nanomedicines Enhance Tumor Accumulation and Penetration for Synergistic Chemo-Photothermal Therapy. ACS Appl. Mater. Interfaces 2021, 13, 46361–46374. [Google Scholar] [CrossRef]
- Sun, Y.; Feng, X.; Wan, C.; Lovell, J.F.; Jin, H.; Ding, J. Role of Nanoparticle-Mediated Immunogenic Cell Death in Cancer Immunotherapy. Asian J. Pharm. Sci. 2021, 16, 129–132. [Google Scholar] [CrossRef]
- Garg, A.D.; Agostinis, P. ER Stress, Autophagy and Immunogenic Cell Death in Photodynamic Therapy-Induced Anti-Cancer Immune Responses. Photochem. Photobiol. Sci. 2014, 13, 474–487. [Google Scholar] [CrossRef]
- De Nunzio, C.; Cicione, A.; Izquierdo, L.; Lombardo, R.; Tema, G.; Lotrecchiano, G.; Minervini, A.; Simone, G.; Cindolo, L.; D’Orta, C.; et al. Multicenter analysis of postoperative complications in octogenarians after radical cystectomy and ureterocutaneostomy: The role of the frailty index. Clin. Genitourin. Cancer. 2019, 17, 402–407. [Google Scholar] [CrossRef]
- Han, R.F.; Pan, J.G. Can Intravesical Bacillus Calmette-Guérin Reduce Recurrence in Patients with Superficial Bladder Cancer? A Meta-Analysis of Randomized Trials. Urology 2006, 67, 1216–1223. [Google Scholar] [CrossRef]
- Devanaboyina, S.C.; Lynch, S.M.; Ober, R.J.; Ram, S.; Kim, D.; Puig-Canto, A.; Breen, S.; Kasturirangan, S.; Fowler, S.; Peng, L.; et al. The Effect of PH Dependence of Antibody-Antigen Interactions on Subcellular Trafficking Dynamics. MAbs 2013, 5, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Encarnação, J.C.; Barta, P.; Fornstedt, T.; Andersson, K. Impact of Assay Temperature on Antibody Binding Characteristics in Living Cells: A Case Study. Biomed. Rep. 2017, 7, 400–406. [Google Scholar] [CrossRef] [PubMed]
| ICD Inducer | Tumor Type | Type of Cell Death | Sample Size | DAMPs | Other Outcome | Immune Response | References |
|---|---|---|---|---|---|---|---|
| Capsaicin | T24 and SD48 human BC cell line | Apoptosis | - | - | CD91 acted as DAMP (CRT and HSP90/70) receptor | DC activation shown by CD86 and CD83 upregulation | [28] |
| Coxsackievirus A21 (CVA21) | Multiple human BC cell lines and Orthotopic murine model | Apoptosis | - | CRT HMGB1 IFN | - | Vaccination effect * observed after injection with MB49 undergoing ICD from virus | [29] |
| Radiotherapy | Human BC cells BT-B | Apoptosis | - | CRT HMGB1 HSP70 | Upregulation of CD80, CD86, CCR5 and CCR7 on BC cells | DC activation | [30] |
| Chemoradiotherapy (Irradiation + Cisplatin) After anti-PD-1 treatment | Human UC + In vivo murine model and MB49 UC cell line | - | - | CRT HMGB1 | Increased cytotoxic T cells | Objective response rate and overall survival | [31] |
| Capsaicin | T24 and SD48 human BC cell lines | Apoptosis | - | CRT ATP HSP70/90 | - | Stimulate ICD | [32] |
| n3-polyunsaturated fatty acid docosahexaenoic acid | EJ Human BC cell lines | Apoptosis | - | CRT exposure | - | Stimulate ICD | [33] |
| YB-1-selective adenovirus Xvir-N-31 | Multiple human BC cell lines and Orthotopic murine model | - | - | HMGB1 HSP70 | - | Stimulate ICD | [34] |
| HPV non-replicant pseudovirions encoding for thymidine kinase (PsV-TK) in combination with Ganciclovir (GCV) | MB49 UC cell line and orthotopic murine model | Apoptosis | - | CRT HMGB1 IFN | Increased CD8+ T cells | Stimulate ICD | [35] |
| Norcantharidin (NCTD) | EJ and UMUC3 human BC cell lines, MB49 mouse cell lines and orthotopic murine model | Apoptosis | - | CRT | NCTD enhances autophagy, Increased CD4+ and CD8+ T cells | Promoted DC Maturation. NCTD reduced tumor growth. NCTD-induced ICD and increased survival | [36] |
| Recombinant adenovirus expressing CD40 ligand (Rad-CD40L) and 5-fluorouracil (5-FU) | MB49 UC cell line and Orthotopic murine model | Apoptosis | - | HMGB1 ATP | Rad-CD40L/5-FU combination treatment was more effective than each one alone | Stimulated ICD, Decreased tumor growth. Increased survival of the mice. | [37] |
| Hypericin-photodynamic (Hyp-PDT) or mitoxantrone (MTX) | AY27 cell lines and subcutaneous injection murine model | - | - | ATP HMGB1 | Decreased ecto-CRT level | None of vaccinated rats showed tumor rejection. | [38] |
| Gemcitabine | T24 human BC cell line, G69 murine BC cell line and subcutaneous injection murine model | - | - | ATP CRT HMGB1 HSP70/90 ANXA1 PDIA3 IFN | CD8+ T cell response after PGE2 blockade | Gemcitabine vaccination did not affect tumor volume and survival. Induction of DAMPs by gemcitabine was not sufficient to induce ICD | [39] |
| Gemcitabine-cisplatin chemotherapy, Celecoxib and anti-PD1 antibody | G69 and G7 murine BC cell line and intraperitoneally injection murine model | - | - | CRT HSP70 HMGB1 IFN | CD8+ response increased after PGE2 blockade | Prostaglandin E2 blockade enhanced immunity and sensitized tumor to anti-PD1 | [40] |
| Mitomycin C (MMC) | Multiple human UC cell lines, CT26 murine BC cell line and subcutaneous injection murine model | Apoptosis | - | HMGB1 CRT ATP | - | MMC induced ICD in short schedule treated cells. Cytoplasmic release of mitochondrial DNA, DC activation and induced ICD | [41] |
| Photodynamic therapy (PDT) | T24 human BC cell line, CT26 murine BC cell line and subcutaneous injection murine model | Apoptosis | CRT ATP HSP70/90 | - | ROS-based ER stress induced ICD, DC maturation. Adaptive immune system activation | [42] | |
| Newcastle Disease Virus (NDV), anti-PD-1 and anti CTLA4 monoclonal antibodies | Multiple human BC cell lines, MB49 UC cell line and flanks intradermally injection murine model | - | - | CRT IFN | - | Activation of innate immune pathway, induced ICD, increased immune infiltration plus delay of tumor growth and increased survival, upregulation of MHC I and II and PD-L1 | [43] |
| Hypericin-based Photodynamic therapy (PDT) | T24 human BC cell line | - | - | CRT | Autophagy induced | Induced ICD, DC maturation, | [44] |
| Vesicular stomatitis virus containing the human GM-CSF transgene (VSVd51-hGM-CSF) | MB49 murine cell lines, 5637 and UM-UC-3 human BC cell lines, Huan BC tissue and orthotopic murine model | Necrosis | - | ATP CRT HMGB1 IFN | Enhanced immunogenic gene expression in MB49 cells | Immune cell activation, induced ICD, DC activation, reduced tumor volume and improved mice survival | [45] |
| ICD Inducer | Tumor Type | Type of Cell Death | Sample Size | DAMPs | Other Outcome | Immune Response | Side Effect | References |
|---|---|---|---|---|---|---|---|---|
| Coxsackievirus A21 (CVA21) + Mitomycin C (MMC) | Phase I trial, Patients with NMIBC | Apoptosis Necrosis | 15 | HMGB1 CRT IFN | Virally induced cytokines (IL6, IL1a, IL1b, IL23 and TNFα), | Upregulating IFN inducible genes, including both immune checkpoint inhibitory genes (PD-L1 and LAG3) and Th1-associated chemokines, as well as the induction of the innate activator RIG-I, | No grade 2 or higher side effects. Urinary tract infection responsive to antibiotic in 6/15 patients | [46] |
| Gemcitabine and Cisplatin (GC) plus Ipilimumab | Phase II trial, Patients with metastatic UC | - | 36 | No significant increase in serum HMGB1 levels were observed after treatment with two cycles of GC | No significant changes in immune cell subsets after GC alone. After the addition of ipilimumab, there was a significant expansion of peripheral blood CD4+ and a numerical increase in peripheral blood CD8+ cells | Improvement in survival associated with a post-ipilimumab expansion of peripheral blood CD4+ cells | Grade 3 or higher side effects in 81% of patients. Most common grade 3 side effects were hematologic. Immune related diarrhea in 11% of patients | [47] |
| Adenoviral vectors expressing CD40 ligand (AdCD40L) | Phase I/II trial, Patients with metastatic UC | - | 8 | IFN-γ | - | Reduced the load of malignant cells. Boosted immune activation | No adverse effects ascribed to the vector | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadollahvandmiandoab, R.; Jalalizadeh, M.; Buosi, K.; Garcia-Perdomo, H.A.; Reis, L.O. Immunogenic Cell Death Role in Urothelial Cancer Therapy. Curr. Oncol. 2022, 29, 6700-6713. https://doi.org/10.3390/curroncol29090526
Yadollahvandmiandoab R, Jalalizadeh M, Buosi K, Garcia-Perdomo HA, Reis LO. Immunogenic Cell Death Role in Urothelial Cancer Therapy. Current Oncology. 2022; 29(9):6700-6713. https://doi.org/10.3390/curroncol29090526
Chicago/Turabian StyleYadollahvandmiandoab, Reza, Mehrsa Jalalizadeh, Keini Buosi, Herney Andrés Garcia-Perdomo, and Leonardo Oliveira Reis. 2022. "Immunogenic Cell Death Role in Urothelial Cancer Therapy" Current Oncology 29, no. 9: 6700-6713. https://doi.org/10.3390/curroncol29090526
APA StyleYadollahvandmiandoab, R., Jalalizadeh, M., Buosi, K., Garcia-Perdomo, H. A., & Reis, L. O. (2022). Immunogenic Cell Death Role in Urothelial Cancer Therapy. Current Oncology, 29(9), 6700-6713. https://doi.org/10.3390/curroncol29090526






