Risk Factors and Prognostic Impact of Postoperative Complications in Patients with Advanced Gastric Cancer Receiving Neoadjuvant Chemotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Evaluation of POCs
2.3. Follow-Up and Study End Points
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Analysis of Risk Factors for POCs
3.3. Risk Factors for Different Categories of POCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.H.; Park, S.R.; Yang, H.K.; Chung, H.C.; Chung, I.J.; Kim, S.W.; Kim, H.H.; Choi, J.H.; Kim, H.K.; Yu, W.; et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1389–1396. [Google Scholar] [CrossRef]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27 (Suppl. S5), v38–v49. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Ricci, A.D.; Rizzo, A.; Rojas Llimpe, F.L.; Di Fabio, F.; de Biase, D.; Rihawi, K. Novel HER2-Directed Treatments in Advanced Gastric Carcinoma: AnotHER Paradigm Shift? Cancers 2021, 13, 1664. [Google Scholar] [CrossRef]
- Rihawi, K.; Ricci, A.D.; Rizzo, A.; Brocchi, S.; Marasco, G.; Pastore, L.V.; Llimpe, F.L.R.; Golfieri, R.; Renzulli, M. Tumor-Associated Macrophages and Inflammatory Microenvironment in Gastric Cancer: Novel Translational Implications. Int. J. Mol. Sci. 2021, 22, 3805. [Google Scholar] [CrossRef]
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Hofheinz, R.D.; Pauligk, C.; Kopp, H.G.; Haag, G.M.; Luley, K.B.; Meiler, J.; Homann, N.; Lorenzen, S.; Schmalenberg, H.; et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): Results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016, 17, 1697–1708. [Google Scholar]
- Saito, T.; Kurokawa, Y.; Miyazaki, Y.; Makino, T.; Takahashi, T.; Yamasaki, M.; Nakajima, K.; Takiguchi, S.; Mori, M.; Doki, Y. Which is a more reliable indicator of survival after gastric cancer surgery: Postoperative complication occurrence or C-reactive protein elevation? J. Surg. Oncol. 2015, 112, 894–899. [Google Scholar] [CrossRef]
- Kubota, T.; Hiki, N.; Sano, T.; Nomura, S.; Nunobe, S.; Kumagai, K.; Aikou, S.; Watanabe, R.; Kosuga, T.; Yamaguchi, T. Prognostic significance of complications after curative surgery for gastric cancer. Ann. Surg. Oncol. 2014, 21, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Han, W.H.; Oh, Y.J.; Eom, B.W.; Yoon, H.M.; Kim, Y.W.; Ryu, K.W. Prognostic impact of infectious complications after curative gastric cancer surgery. Eur. J. Surg. Oncol. 2020, 46, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar]
- Kang, Y.K.; Yook, J.H.; Park, Y.K.; Lee, J.S.; Kim, J.S.; Kim, J.Y.; Ryu, M.H.; Rha, S.Y.; Chung, I.J.; Kim, I.H.; et al. PRODIGY: A Phase III Study of Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 Versus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer. J. Clin. Oncol. 2021, 39, 2903–2913. [Google Scholar] [CrossRef]
- Liu, L.; Wang, C.; Li, F.; Zhang, X.; Cheng, X.; Lin, S.; Liu, Y.; Yang, C.; Li, W. The safety and efficacy of laparoscopic gastrectomy for patients with locally advanced gastric cancer following neoadjuvant chemotherapy. Sci. Rep. 2022, 12, 10384. [Google Scholar] [CrossRef]
- Li, S.S.; Udelsman, B.V.; Parikh, A.; Klempner, S.J.; Clark, J.W.; Roeland, E.J.; Wo, J.Y.; Hong, T.S.; Mullen, J.T. Impact of Postoperative Complication and Completion of Multimodality Therapy on Survival in Patients Undergoing Gastrectomy for Advanced Gastric Cancer. J. Am. Coll. Surg. 2020, 230, 912–924. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Tsujimoto, H.; Ono, S.; Shinomiya, N.; Miyazaki, H.; Hiraki, S.; Takahata, R.; Yoshida, K.; Saitoh, D.; Yamori, T.; et al. Abdominal Infection Suppresses the Number and Activity of Intrahepatic Natural Killer Cells and Promotes Tumor Growth in a Murine Liver Metastasis Model. Ann. Surg. Oncol. 2016, 23 (Suppl. S2), S257–S265. [Google Scholar] [CrossRef]
- Gowing, S.D.; Cool-Lartigue, J.J.; Spicer, J.D.; Seely, A.J.E.; Ferri, L.E. Toll-like receptors: Exploring their potential connection with post-operative infectious complications and cancer recurrence. Clin. Exp. Metastasis 2020, 37, 225–239. [Google Scholar] [CrossRef]
- Eto, K.; Hiki, N.; Kumagai, K.; Shoji, Y.; Tsuda, Y.; Kano, Y.; Yasufuku, I.; Okumura, Y.; Tsujiura, M.; Ida, S.; et al. Prophylactic effect of neoadjuvant chemotherapy in gastric cancer patients with postoperative complications. Gastric Cancer 2018, 21, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, N.; Zhou, H.; Wang, T.; Mao, Q.; Zhang, X.; Zhao, D. Effects of Neoadjuvant Chemotherapy Toxicity and Postoperative Complications on Short-Term and Long-Term Outcomes after Curative Resection of Gastric Cancer. J. Gastrointest. Surg. 2020, 24, 1278–1289. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hu, J.; Huang, C.; Ying, M.; Peng, X.; Wei, H.; Jiang, Z.; Du, X.; Liu, Z.; Liu, H.; et al. The impact of age and comorbidity on postoperative complications in patients with advanced gastric cancer after laparoscopic D2 gastrectomy: Results from the Chinese laparoscropic gastrointestinal surgery study (CLASS) group. Eur. J. Surg. Oncol. 2013, 39, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tian, Z.; Zeng, J.; Zhou, W.; Wu, K.; Shen, W. Effect of neoadjuvant treatment combined with radical gastrectomy on postoperative complications and prognosis of gastric cancer patients. Scand. J. Gastroenterol. 2021, 56, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.S.; Jeong, S.H.; Son, Y.G.; Lee, H.J.; Im, S.A.; Bang, Y.J.; Kim, H.H.; Yang, H.K. Effect of neoadjuvant chemotherapy on postoperative morbidity and mortality in patients with locally advanced gastric cancer. Br. J. Surg. 2014, 101, 1560–1565. [Google Scholar] [CrossRef]
- Dias, A.R.; Pereira, M.A.; Oliveira, R.J.; Ramos, M.F.; Szor, D.J.; Ribeiro, U.; Zilberstein, B.; Cecconello, I. Multivisceral resection vs. standard gastrectomy for gastric adenocarcinoma. J. Surg. Oncol. 2020, 121, 840–847. [Google Scholar] [CrossRef]
- Procter, L.D.; Davenport, D.L.; Bernard, A.C.; Zwischenberger, J.B. General surgical operative duration is associated with increased risk-adjusted infectious complication rates and length of hospital stay. J. Am. Coll. Surg. 2010, 210, 60–65.e52. [Google Scholar] [CrossRef]
- Manilich, E.; Vogel, J.D.; Kiran, R.P.; Church, J.M.; Seyidova-Khoshknabi, D.; Remzi, F.H. Key factors associated with postoperative complications in patients undergoing colorectal surgery. Dis. Colon Rectum 2013, 56, 64–71. [Google Scholar] [CrossRef]
- Ball, C.G.; Pitt, H.A.; Kilbane, M.E.; Dixon, E.; Sutherland, F.R.; Lillemoe, K.D. Peri-operative blood transfusion and operative time are quality indicators for pancreatoduodenectomy. HPB 2010, 12, 465–471. [Google Scholar] [CrossRef]
- Molina, J.C.; Al-Hinai, A.; Gosseling-Tardif, A.; Bouchard, P.; Spicer, J.; Mulder, D.; Mueller, C.L.; Ferri, L.E. Multivisceral Resection for Locally Advanced Gastric and Gastroesophageal Junction Cancers-11-Year Experience at a High-Volume North American Center. J. Gastrointest. Surg. 2019, 23, 43–50. [Google Scholar] [CrossRef]
- Saunders, J.H.; Yanni, F.; Dorrington, M.S.; Bowman, C.R.; Vohra, R.S.; Parsons, S.L. Impact of postoperative complications on disease recurrence and long-term survival following oesophagogastric cancer resection. Br. J. Surg. 2020, 107, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, C.; Chen, S.; Zhang, X.; Wang, F.; Shi, T.; Li, Q.; Lin, L. Association of body mass index with severity and mortality of COVID-19 pneumonia: A two-center, retrospective cohort study from Wuhan, China. Aging 2021, 13, 7767–7780. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Fan, J.; Yu, C.; Guo, Y.; Bian, Z.; Wei, Y.; Yang, L.; Chen, Y.; Du, H.; Chang, L.; et al. Body-mass index and long-term risk of sepsis-related mortality: A population-based cohort study of 0.5 million Chinese adults. Crit. Care 2020, 24, 534. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, A.; Toyokawa, T.; Miki, Y.; Yoshii, M.; Tamura, T.; Sakurai, K.; Kubo, N.; Tanaka, H.; Lee, S.; Muguruma, K.; et al. Preoperative C-reactive protein to albumin ratio predicts anastomotic leakage after esophagectomy for thoracic esophageal cancer: A single-center retrospective cohort study. BMC Surg. 2021, 21, 348. [Google Scholar] [CrossRef]
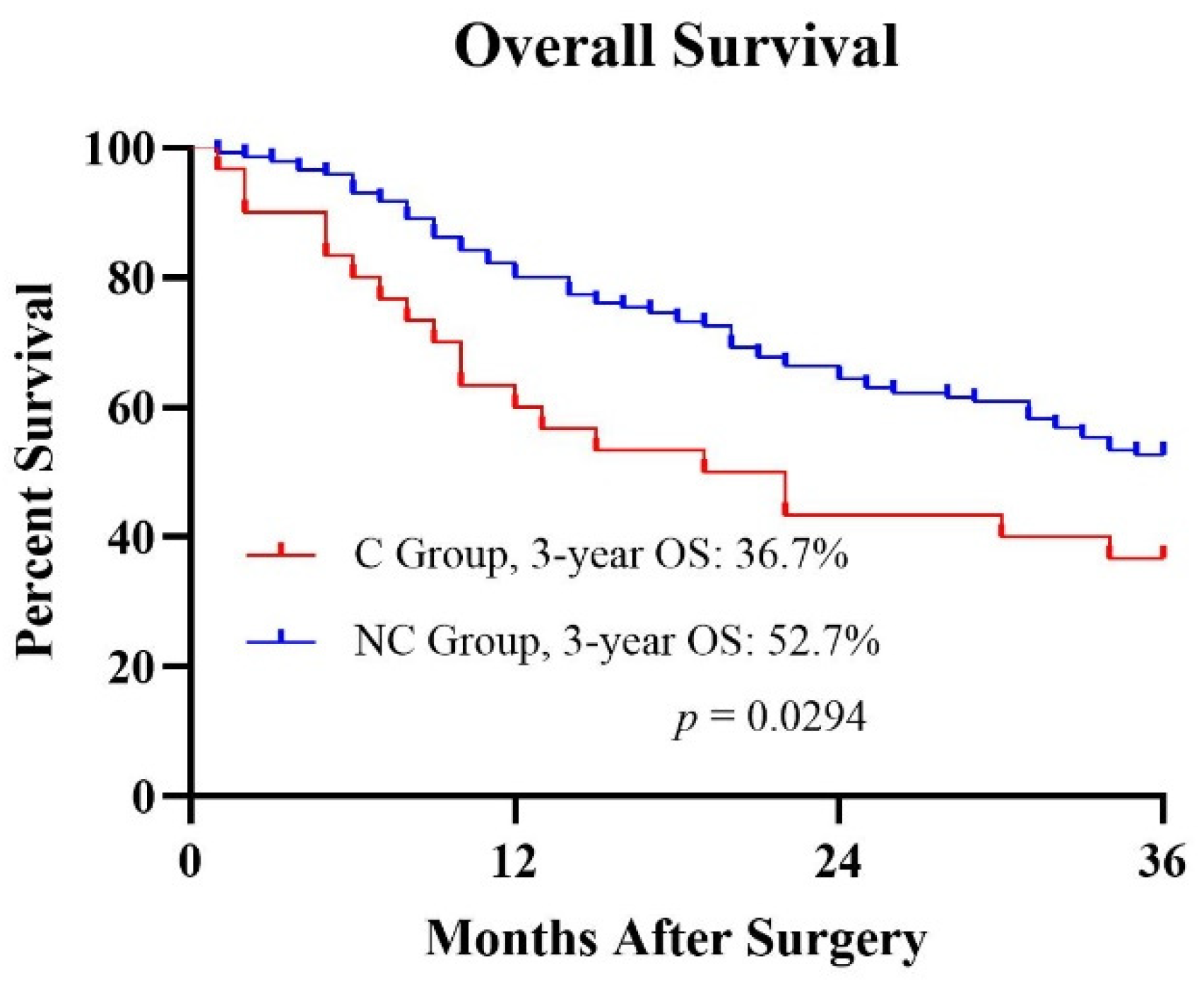
| Characteristics | No. of Patients | Complication (%) |
|---|---|---|
| Gender | ||
| Male | 112 | 22 (19.6) |
| Female | 64 | 8 (12.5) |
| Age (years) | ||
| <65 | 133 | 15 (11.3) |
| ≥65 | 43 | 15 (34.9) |
| Body mass index (kg/m2) | ||
| <25 | 153 | 21 (13.7) |
| ≥25 | 23 | 9 (39.1) |
| Extent of gastrectomy | ||
| Subtotal | 53 | 2 (3.8) |
| Total | 123 | 28 (22.8) |
| ypStage | ||
| 0–II | 71 | 7 (9.9) |
| III–IV | 105 | 23 (21.9) |
| ypT category | ||
| T0–T2 | 40 | 3 (7.5) |
| T3–T4 | 136 | 27 (19.9) |
| ypN category | ||
| N0–N1 | 93 | 14 (15.1) |
| N2–N3 | 83 | 16 (19.3) |
| Multivisceral resection | ||
| Yes | 47 | 16 (34.0) |
| No | 129 | 14 (10.9) |
| Operation time (hours) | ||
| <5 | 74 | 5 (6.8) |
| ≥5 | 102 | 25 (24.5) |
| Preoperative hemoglobin (g/L) | ||
| <120 | 102 | 16 (15.7) |
| ≥120 | 74 | 14 (18.9) |
| Preoperative neutrophil (109/L) | ||
| <1.5 | 34 | 8 (23.5) |
| ≥1.5 | 142 | 22 (15.5) |
| Preoperative platelet (109/L) | ||
| <100 | 38 | 7 (18.4) |
| ≥100 | 138 | 23 (16.7) |
| Preoperative albumin (g/L) | ||
| <40 | 57 | 16 (28.1) |
| ≥40 | 119 | 14 (11.8) |
| Postoperative complications | ||
| Yes | 30 | - |
| No | 146 | - |
| Characteristics | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Gender | 0.229 | |||
| Male | 1.711(0.713–4.106) | |||
| Female | 1.000 | |||
| Age (years) | 0.001 | 0.003 | ||
| <65 | 1.000 | 1.000 | ||
| ≥65 | 4.214(1.845–9.624) | 4.920(1.694–14.293) | ||
| Body mass index (kg/m2) | 0.004 | 0.005 | ||
| <25 | 1.000 | 1.000 | ||
| ≥25 | 4.041(1.554–10.507) | 5.907(1.700–20.529) | ||
| Extent of gastrectomy | 0.007 | 0.063 | ||
| Subtotal | 1.000 | 1.000 | ||
| Total | 7.516(1.721–32.830) | 4.586(0.923–22.779) | ||
| ypStage | 0.042 | 0.560 | ||
| 0–II | 1.000 | 1.000 | ||
| III–IV | 2.564(1.035–6.352) | 1.434(0.427–4.817) | ||
| ypT category | 0.080 | 0.259 | ||
| T0–T2 | 1.000 | 1.000 | ||
| T3–T4 | 3.055(0.876–10.660) | 2.664(0.485–14.620) | ||
| ypN category | 0.458 | |||
| N0–N1 | 1.000 | |||
| N2–N3 | 1.348(0.613–2.962) | |||
| Multivisceral resection | 0.001 | 0.013 | ||
| Yes | 4.240(1.868–9.623) | 3.703(1.320–10.393) | ||
| No | 1.000 | 1.000 | ||
| Operation time (hours) | 0.004 | 0.018 | ||
| <5 | 1.000 | 1.000 | ||
| ≥5 | 4.481(1.626–12.347) | 4.583(1.298–16.186) | ||
| Preoperative hemoglobin (g/L) | 0.547 | |||
| <120 | 0.797(0.362–1.756) | |||
| ≥120 | 1.000 | |||
| Preoperative neutrophil (109/L) | 0.267 | |||
| <1.5 | 1.678(0.673–4.184) | |||
| ≥1.5 | 1.000 | |||
| Preoperative platelet (109/L) | 0.799 | |||
| <100 | 1.129(0.444–2.874) | |||
| ≥100 | 1.000 | |||
| Preoperative albumin (g/L) | 0.009 | 0.086 | ||
| <40 | 2.927(1.311–6.533) | 2.501(0.878–7.128) | ||
| ≥40 | 1.000 | 1.000 | ||
| Characteristics | Number of Cases | Grade I–II | Grade III–IV | p Value |
|---|---|---|---|---|
| Gender | 1.000 | |||
| Male | 22 | 10 | 12 | |
| Female | 8 | 4 | 4 | |
| Age (years) | 0.715 | |||
| <65 | 15 | 8 | 7 | |
| ≥65 | 15 | 6 | 9 | |
| Body mass index (kg/m2) | 0.440 | |||
| <25 | 21 | 11 | 10 | |
| ≥25 | 9 | 3 | 6 | |
| Extent of gastrectomy | 1.000 | |||
| Subtotal | 2 | 1 | 1 | |
| Total | 28 | 13 | 15 | |
| ypStage | 1.000 | |||
| 0–II | 7 | 3 | 4 | |
| III–IV | 23 | 11 | 12 | |
| ypT category | 0.586 | |||
| T0–T2 | 3 | 2 | 1 | |
| T3–T4 | 27 | 12 | 15 | |
| ypN category | 0.299 | |||
| N0–N1 | 14 | 5 | 9 | |
| N2–N3 | 16 | 9 | 7 | |
| Multivisceral resection | 0.026 | |||
| Yes | 16 | 4 | 12 | |
| No | 14 | 10 | 4 | |
| Operation time (hours) | 1.000 | |||
| <5 | 5 | 2 | 3 | |
| ≥5 | 25 | 12 | 13 | |
| Preoperative hemoglobin (g/L) | 0.464 | |||
| <120 | 16 | 6 | 10 | |
| ≥120 | 14 | 8 | 6 | |
| Preoperative neutrophil (109/L) | 1.000 | |||
| <1.5 | 8 | 4 | 4 | |
| ≥1.5 | 22 | 10 | 12 | |
| Preoperative platelet (109/L) | 1.000 | |||
| <100 | 7 | 3 | 4 | |
| ≥100 | 23 | 11 | 12 | |
| Preoperative albumin (g/L) | 0.464 | |||
| <40 | 16 | 6 | 10 | |
| ≥40 | 14 | 8 | 6 |
| Characteristics | Number of Cases | Non-Inflammation | Inflammation | p Value |
|---|---|---|---|---|
| Gender | 1.000 | |||
| Male | 22 | 10 | 12 | |
| Female | 8 | 4 | 4 | |
| Age (years) | 0.715 | |||
| <65 | 15 | 8 | 7 | |
| ≥65 | 15 | 6 | 9 | |
| Body mass index (kg/m2) | 0.017 | |||
| <25 | 21 | 13 | 8 | |
| ≥25 | 9 | 1 | 8 | |
| Extent of gastrectomy | 1.000 | |||
| Subtotal | 2 | 1 | 1 | |
| Total | 28 | 13 | 15 | |
| ypStage | 0.675 | |||
| 0–II | 7 | 4 | 3 | |
| III–IV | 23 | 10 | 13 | |
| ypT category | 1.000 | |||
| T0–T2 | 3 | 1 | 2 | |
| T3–T4 | 27 | 13 | 14 | |
| ypN category | 0.730 | |||
| N0–N1 | 14 | 6 | 8 | |
| N2–N3 | 16 | 8 | 8 | |
| Multivisceral resection | 1.000 | |||
| Yes | 16 | 7 | 9 | |
| No | 14 | 7 | 7 | |
| Operation time (hours) | 1.000 | |||
| <5 | 5 | 2 | 3 | |
| ≥5 | 25 | 12 | 13 | |
| Preoperative hemoglobin (g/L) | 0.299 | |||
| <120 | 16 | 9 | 7 | |
| ≥120 | 14 | 5 | 9 | |
| Preoperative neutrophil (109/L) | 0.417 | |||
| <1.5 | 8 | 5 | 3 | |
| ≥1.5 | 22 | 9 | 13 | |
| Preoperative platelet (109/L) | 0.675 | |||
| <100 | 7 | 4 | 3 | |
| ≥100 | 23 | 10 | 13 | |
| Preoperative albumin (g/L) | 0.464 | |||
| <40 | 16 | 6 | 10 | |
| ≥40 | 14 | 8 | 6 |
| Characteristics | Number of Cases | Non-Leakage | Leakage | p Value |
|---|---|---|---|---|
| Gender | 0.698 | |||
| Male | 22 | 13 | 9 | |
| Female | 8 | 4 | 4 | |
| Age (years) | 1.000 | |||
| <65 | 15 | 9 | 6 | |
| ≥65 | 15 | 8 | 7 | |
| Body mass index (kg/m2) | 0.443 | |||
| <25 | 21 | 13 | 8 | |
| ≥25 | 9 | 4 | 5 | |
| Extent of gastrectomy | 1.000 | |||
| Subtotal | 2 | 1 | 1 | |
| Total | 28 | 16 | 12 | |
| ypStage | 0.190 | |||
| 0–II | 7 | 2 | 5 | |
| III–IV | 23 | 15 | 8 | |
| ypT category | 0.070 | |||
| T0–T2 | 3 | 0 | 3 | |
| T3–T4 | 27 | 17 | 10 | |
| ypN category | 0.713 | |||
| N0–N1 | 14 | 7 | 7 | |
| N2–N3 | 16 | 10 | 6 | |
| Multivisceral resection | 0.484 | |||
| Yes | 16 | 8 | 8 | |
| No | 14 | 9 | 5 | |
| Operation time (hours) | 0.628 | |||
| <5 | 5 | 2 | 3 | |
| ≥5 | 25 | 15 | 10 | |
| Preoperative hemoglobin (g/L) | 0.484 | |||
| <120 | 16 | 8 | 8 | |
| ≥120 | 14 | 9 | 5 | |
| Preoperative neutrophil (109/L) | 0.407 | |||
| <1.5 | 8 | 6 | 2 | |
| ≥1.5 | 22 | 11 | 11 | |
| Preoperative platelet (109/L) | 0.675 | |||
| <100 | 7 | 4 | 3 | |
| ≥100 | 23 | 13 | 10 | |
| Preoperative albumin (g/L) | 0.464 | |||
| <40 | 16 | 6 | 10 | |
| ≥40 | 14 | 11 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Xu, L.; Yin, S.; Jiang, J.; Hong, C.; He, Y.; Zhang, C. Risk Factors and Prognostic Impact of Postoperative Complications in Patients with Advanced Gastric Cancer Receiving Neoadjuvant Chemotherapy. Curr. Oncol. 2022, 29, 6496-6507. https://doi.org/10.3390/curroncol29090511
Yu H, Xu L, Yin S, Jiang J, Hong C, He Y, Zhang C. Risk Factors and Prognostic Impact of Postoperative Complications in Patients with Advanced Gastric Cancer Receiving Neoadjuvant Chemotherapy. Current Oncology. 2022; 29(9):6496-6507. https://doi.org/10.3390/curroncol29090511
Chicago/Turabian StyleYu, Hong, Li Xu, Songcheng Yin, Jianlong Jiang, Chunhong Hong, Yulong He, and Changhua Zhang. 2022. "Risk Factors and Prognostic Impact of Postoperative Complications in Patients with Advanced Gastric Cancer Receiving Neoadjuvant Chemotherapy" Current Oncology 29, no. 9: 6496-6507. https://doi.org/10.3390/curroncol29090511
APA StyleYu, H., Xu, L., Yin, S., Jiang, J., Hong, C., He, Y., & Zhang, C. (2022). Risk Factors and Prognostic Impact of Postoperative Complications in Patients with Advanced Gastric Cancer Receiving Neoadjuvant Chemotherapy. Current Oncology, 29(9), 6496-6507. https://doi.org/10.3390/curroncol29090511




