Third- and Late Line Treatments of Metastatic Gastric Cancer: Still More to Be Done
Abstract
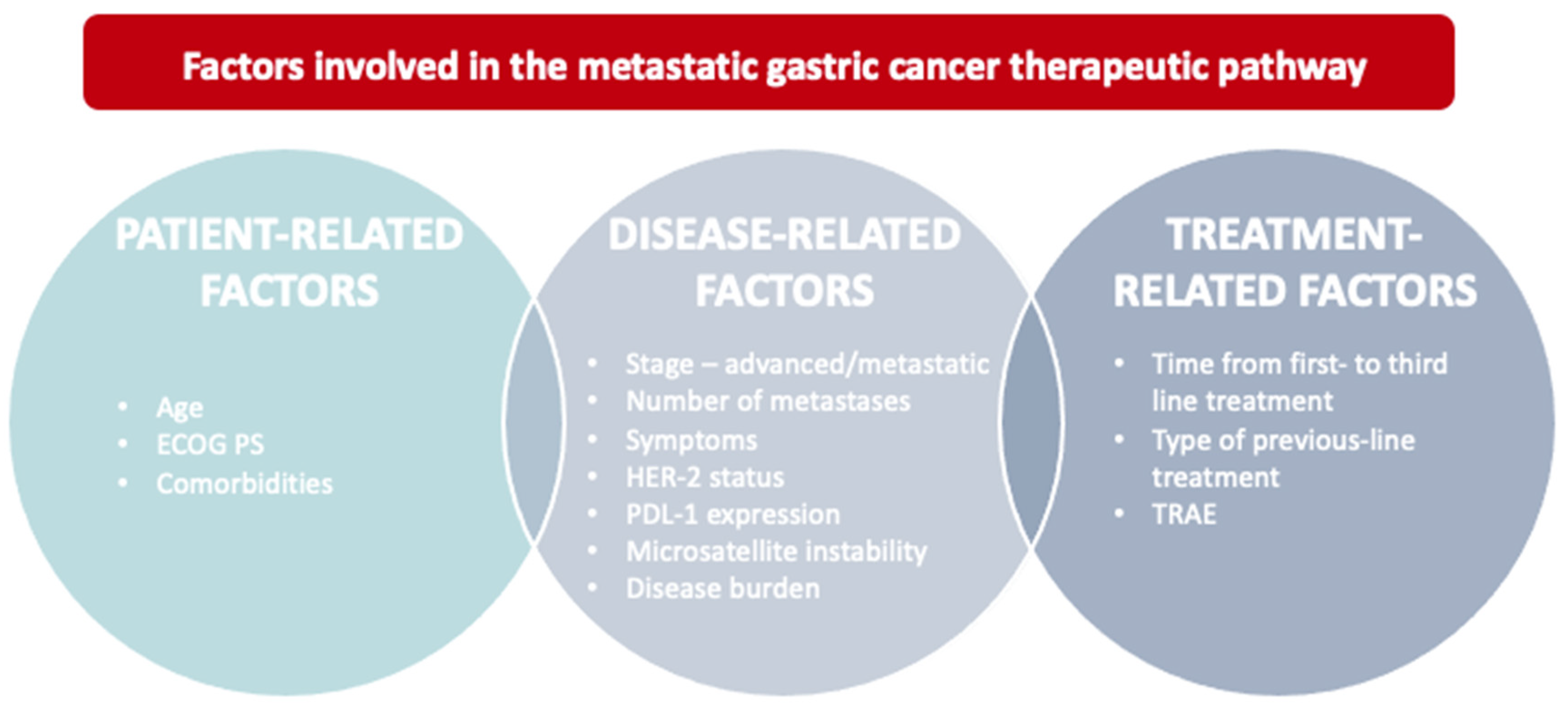
1. Introduction
2. Efficacy and Safety of Third Line Treatments for Metastatic Gastric Cancer
2.1. Chemotherapy
2.2. Immunotherapy
2.3. Targeted Therapy
| CHEMOTHERAPY | ||||
|---|---|---|---|---|
| STUDY | DESIGN | TREATMENT | ENDPOINTS | RESULTS |
| Kang et al. [20] | Multicentre, open label, randomised phase III trial on 202 adult patients with advanced GC who have failed at least two previous CT regimens | CT (irinotecan or docetaxel) + BSC vs. BSC alone | Primary: OS | CT + BSC vs. BSC ALONE |
| ||||
| Kang et al. [19] | Study conducted on 158 adult patients with m/rGC to evaluate the activity and safety of the combination CT of FOLFIRI regimen after failure of fluoropyrimidine, platinum, and taxane and to evaluate the prognostic factors for survival. | FOLFIRI 5-[fluorouracil (5-FU), leucovorin, and irinotecan] | PFS, OS |
|
| Roviello et al. [22] | Observational phase II study is to evaluate the efficacy and safety of the FOLFIRI regimen as a third-line CT for ramucirumab-pre-treated patients with metastatic gastric cancers | FOLFIRI 5-[fluorouracil (5-FU), leucovorin, and irinotecan] | Primary: Tumour response (CR, PR, SD, PD) Secondary: OS, PFS, safety and tumour response. | EFFICACYT
|
SAFETY
| ||||
| Shitara et al. [26] | Randomised, double-blind, multinational, placebo-controlled, phase III trial to assess the efficacy and safety of trifluridine/tipiracil in patients with mGC on 507 adult mGC patients who have failed at least two previous CT regimens | TAS-102 (trifluridine/tipiracil) + BSC vs. placebo + BSC. | Primary: OS Key secondary: PFS, safety and tolerability | TAS-102 + BSC vs. PLACEBO + BSC |
EFFICACY
| ||||
SAFETY
| ||||
| IMMUNOTHERAPY | ||||
| STUDY | DESIGN | TREATMENT | ENDPOINTS | RESULTS |
| Kang et al. [28] | Randomised, double-blind, placebo-controlled, phase III trial (ATTRACTION-02) to investigate the efficacy and safety of nivolumab, in 493 heavily pre-treated patients unselected for PD-L1 tumour expression. | Primary: OS Secondary: PFS, ORR, DCR, DOR, BOR, maximum percentage change from baseline in the sum of diameters of target lesions. | NIVOLUMAB vs. PLACEBO | |
| ||||
| Fuchs et al. [32] | Open-label, single-arm, multicohort, phase 2 study (KEYNOTE-059) on 259 adult patients with advances GC/GEJC | Pembrolizumab | Primary: ORR, safety Secondary: DOR (all pts and pts with PD-L1–positive tumours) | EFFICACY
|
SAFETY
| ||||
| Bang et al. [34] | Multicentre, international, randomised, open-label, phase III trial (JAVELIN Gastric 300) to demonstrate superiority of avelumab versus CT as a third-line in 371 adult patients with advanced GC/GEJC | Avelumab + BSC vs. physician’s choice of CT (paclitaxel/irinotecan) | Primary: OS. Secondary: PFS, ORR safety and tolerability | AVELUMAB vs. CT |
EFFICACY
| ||||
SAFETY
| ||||
| TARGETED THERAPY | ||||
| Pavlakis et al. [36] | Randomized, double blind phase II trial (INTEGRATE) on 152 adult patients randomly assigned at a 2-to-1 ratio and stratified by lines of prior (one or two) CT to assess the efficacy of regorafenib on advanced GC | Regorafenib vs. placebo | Primary: PFS Secondary: ORR (by RECIST criteria), CBS at 2 months, OS, AE | REGORAFENIB vs. PLACEBO |
EFFICACY
| ||||
SAFETY
| ||||
| Li et al. [35] | Phase II, randomized, double-blind, placebo-controlled trial aimed to assess the efficacy and safety of daily administration of apatinib as third-line or later treatment in 144 adult patients with mGC and to determine the tolerability of the once- or a twice-daily regimen. | Apatinib 850 mg o.d., apatinib 425 mg b.i.d. or placebo | Primary: PFS Secondary: DCR (including CR, PR, or SD); ORR (reduction in tumor size) and QoL | EFFICACY
|
| SAFETY AE grade 3 to 4: hypertension (8.51% and 10.86% of patients treated with apatinib 850 mg once daily and 425 mg twice daily, respectively). | ||||
| Li et al. [37] | Randomized double-blind, placebo-controlled, multicenter phase III trial on 273 adult patients with advanced or metastatic GC. | Apatinib vs. placebo | Primary: OS and PFS Secondary: ORR, DCR, QoL, and safety. | APATINIB vs. PLACEBO |
EFFICACY
| ||||
| SAFETY TRAE grade 3 to 4: nonhematologic adverse events were hand-foot syndrome, proteinuria, and hypertension. | ||||
| Liu et al. [38] | Retrospective cohort study using pooled data from two randomised double-blind, placebo-controlled clinical trials to investigate the relationship between adverse effects and antitumor efficacy of apatinib on 269 adult patients with mGC | Apatinib vs. placebo | Primary: OS Secondary: PFS, DCR, and ORR. Clinical outcomes were compared with and without AEs ¶ in the first 4 weeks | CLINICAL OUTCOMES WITHOUT AES (N = 119) |
| ||||
| CLINICAL OUTCOMES WITH AES (N = 150) | ||||
| ||||
| Kang et al. [39] | Multicenter, single arm open label phase II trial (ARQ 197)among 31 adult patients with mGC | Tivantinib | Primary: DCR Secondary: PFS, OS, and ORR. | DCR: 36.7 Median PFS: 43 days (95% CI: 29.0–92.0) ORR: 0% |
| AE (grade 3–4): 43.3% of patients | ||||
| Ohtsu et al. [40] | Double-blind phase III study (GRANITE-1) to compare the efficacy and safety of everolimus vs. BSC on 656 previously treated patients with advance GC | Everolimus + BSC vs. placebo vs. BSC | Primary: OS Secondary: PFS, ORR and safety | EVEROLIMUS + BSC vs. PLACEBO vs. BSC |
| Median OS: 5.4 vs. 4.3 months (HR 0.90; 95% CI, 0.75–1.08; p 0.124). Median PFS: 1.7 vs. 1.4 months (HR 0.66; 95% CI, 0.56 to 0.78) | ||||
| AE (at least 1): 99.1% vs. 96.7% pts AEs leading to discontinuation: 21.5% vs. 15.8% pts | ||||
| Peng et al. [43] | Single-arm, open-labelled, phase II trial assessing the efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic GC/GEJA | RC48 | Primary: ORR (CR or PR). Secondary: PFS, OS, DOR, TTP, DCR (CR, PR, or SD), and safety. | EFFICACY
|
SAFETY
| ||||
| Shitara et al. [41] | Open-label, randomized, phase 2 trial, we evaluated trastuzumab deruxtecan (T-DXd) as compared with chemotherapy in 187 adult patients with HER2-positive advanced gastric cancer | T-DXd vs. physician’s choice of CT | Primary: ORR Secondary: OS, DOR, PFS, confirmed response (persisting ≥4 months), and safety. | T-DXd vs. CT |
EFFICACY
| ||||
SAFETY
| ||||
3. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- The Global Cancer Observatory (GCO); International Agency for Research on Cancer (IARC). Stomach—Globocan. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/7-Stomach-fact-sheet.pdf (accessed on 1 June 2022).
- Associazione Italiana di Oncologia Medica (AIOM). Linee Guida Neoplasie Dello Stomaco e Della Giunzione Esofago-Gastrica. Edizione 2020; AIOM: Milano, Italy, 2020. [Google Scholar]
- Ishaq, S.; Nunn, L. Helicobacter pylori and gastric cancer: A state of the art review. Gastroenterol. Hepatol. Bed Bench 2015, 8 (Suppl. S1), S6–S14. [Google Scholar]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric Cancer: Descriptive Epidemiology, Risk Factors, Screening, and Prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Moehler, M. Late-line treatment in metastatic gastric cancer: Today and tomorrow. Ther. Adv. Med Oncol. 2019, 11, 1758835919867522. [Google Scholar] [CrossRef]
- Balakrishnan, M.; George, R.; Sharma, A.; Graham, D.Y. Changing Trends in Stomach Cancer Throughout the World. Curr. Gastroenterol. Rep. 2017, 19, 36. [Google Scholar] [CrossRef]
- Kamiya, S.; Rouvelas, I.; Lindblad, M.; Nilsson, M. Current trends in gastric cancer treatment in Europe. J. Cancer Metastasis Treat. 2018, 4, 35. [Google Scholar] [CrossRef]
- Fontana, E.; Smyth, E.C. Novel targets in the treatment of advanced gastric cancer: A perspective review. Ther. Adv. Med Oncol. 2016, 8, 113–125. [Google Scholar] [CrossRef]
- Digklia, A.; Wagner, A.D. Advanced gastric cancer: Current treatment landscape and future perspectives. World J. Gastroenterol. 2016, 22, 2403–2414. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Nam, C.M.; Kim, S.-G.; Mun, J.E.; Rha, S.Y.; Chung, H.C. Comparative efficacy and tolerability of third-line treatments for advanced gastric cancer: A systematic review with Bayesian network meta-analysis. Eur. J. Cancer 2021, 144, 49–60. [Google Scholar] [CrossRef]
- Wagner, A.D.; Syn, N.L.X.; Moehler, M.; Grothe, W.; Yong, W.P.; Tai, B.C.; Ho, J.; Unverzagt, S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2017, 8, CD004064. [Google Scholar] [CrossRef]
- Attia, H.; Smyth, E. Evolving therapies in advanced oesophago-gastric cancers and the increasing role of immunotherapy. Expert Rev. Anticancer Ther. 2021, 21, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; ToGA Trial Investigators; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697, Erratum in Lancet 2010, 376, 1302. [Google Scholar] [CrossRef]
- Camejo, N.; Castillo, C.; Alonso, R.; Correa, F.; Rivero, E.; Mezquita, C.; Rosich, A.; Dellacasa, F.; Silveira, L.; Delgado, L. Effectiveness of Trastuzumab for Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer in a Real-Life Setting: One Decade of Experience Under National Treatment Coverage Regulations. JCO Glob. Oncol. 2020, 6, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-L.; Lam, K.-O.; So, T.-H.; Lee, V.H.-F.; Kwong, L.-W.D. Third-line systemic treatment in advanced/metastatic gastric cancer: A comprehensive review. Ther. Adv. Med. Oncol. 2019, 11, 1758835919859990. [Google Scholar] [CrossRef]
- Gelsomino, F.; Spallanzani, A.; De Vita, F.; Di Bartolomeo, M.; Gavazzi, C.; Rimassa, L.; Cascinu, S. Il trattamento di prima e seconda linea nel carcinoma gastrico. Rec. Prog. Med. 2017, 108, 120–127. [Google Scholar]
- Schade, S.; Koenig, U.; Mekolli, A.; Gaedcke, J.; Neesse, A.; Reinecke, J.; Brunner, M.; Hosseini, A.S.A.; Kitz, J.; Stroebel, P.; et al. Cure Is Possible: Extensively Metastatic HER2-Positive Gastric Carcinoma with 5 years of Complete Remission after Therapy with the FLOT Regimen and Trastuzumab. Case Rep. Gastroenterol. 2022, 16, 80–88. [Google Scholar] [CrossRef]
- Kang, E.J.; Im, S.-A.; Oh, D.-Y.; Han, S.-W.; Kim, J.-S.; Choi, I.S.; Kim, Y.J.; Kim, J.H.; Kim, T.-Y.; Lee, J.S.; et al. Irinotecan combined with 5-fluorouracil and leucovorin third-line chemotherapy after failure of fluoropyrimidine, platinum, and taxane in gastric cancer: Treatment outcomes and a prognostic model to predict survival. Gastric Cancer 2013, 16, 581–589. [Google Scholar] [CrossRef][Green Version]
- Kang, J.H.; Lee, S.I.; Lim, D.H.; Park, K.-W.; Oh, S.Y.; Kwon, H.C.; Hwang, I.G.; Lee, S.C.; Nam, E.; Shin, D.B. Salvage chemotherapy for pretreated gastric cancer: A randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J. Clin. Oncol. 2012, 30, 1513–1518. [Google Scholar] [CrossRef]
- Rizzo, A.; Mollica, V.; Ricci, A.D.; Maggio, I.; Massucci, M.; Rojas Limpe, F.L.; Fabio, F.D.; Ardizzoni, A. Third- and later-line treatment in advanced or metastatic gastric cancer: A systematic review and meta-analysis. Future Oncol. 2020, 16, 4409–4418. [Google Scholar] [CrossRef]
- Roviello, G.; Petrioli, R.; Rosellini, P.; Multari, A.G.; Conca, R.; Paganini, G.; Chiriacò, G.; Aieta, M. The influence of prior ramucirumab treatment on the clinical activity of FOLFIRI as third-line therapy in patients with metastatic gastric Cancer. Investig. New Drugs 2019, 37, 524–530. [Google Scholar] [CrossRef]
- Lorenzen, S.; Knorrenschild, J.R.; Pauligk, C.; Hegewisch-Becker, S.; Seraphin, J.; Thuss-Patience, P.; Kopp, H.G.; Dechow, T.; Vogel, A.; Luley, K.B.; et al. Phase III randomized, double-blind study of paclitaxel with and without everolimus in patients with advanced gastric or esophagogastric junction carcinoma who have progressed after therapy with a fluoropyrimidine/platinum-containing regimen (RADPAC). Int. J. Cancer 2020, 147, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Fancelli, S.; Gatta Michelet, M.R.; Aprile, G.; Nobili, S.; Roviello, F.; Cianchi, F.; Mini, E.; Lavacchi, D. TAS-102 in gastric cancer: Development and perspectives of a new biochemically modulated fluroropyrimidine drug combination. Crit. Rev. Oncol. Hematol. 2020, 152, 102987. [Google Scholar] [CrossRef] [PubMed]
- Alsina, M.; Tabernero, J.; Diez, M. Chemorefractory Gastric Cancer: The Evolving Terrain of Third-Line Therapy and Beyond. Cancers 2022, 14, 1408. [Google Scholar] [CrossRef]
- Shitara, K.; Doi, T.; Dvorkin, M.; Mansoor, W.; Arkenau, H.-T.; Prokharau, A.; Alsina, M.; Ghidini, M.; Faustino, C.; Gorbunova, V.; et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018, 19, 1437–1448, Erratum in Lancet Oncol. 2018, 19, e668. [Google Scholar] [CrossRef] [PubMed]
- Puhr, H.C.; Ilhan-Mutlu, A. Innovative strategies in metastatic gastric cancer: A short review. Memo 2022, 15, 29–34. [Google Scholar] [CrossRef]
- Kang, Y.-K.; Boku, N.; Satoh, T.; Ryu, M.-H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.-S.; Muro, K.; Kang, W.K.; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 2461–2471. [Google Scholar] [CrossRef]
- Sano, A.; Sohda, M.; Nakazawa, N.; Ubukata, Y.; Kuriyama, K.; Kimura, A.; Kogure, N.; Hosaka, H.; Naganuma, A.; Sekiguchi, M.; et al. Clinical features as potential prognostic factors in patients treated with nivolumab for highly pretreated metastatic gastric cancer: A multicenter retrospective study. BMC Cancer 2022, 22, 22. [Google Scholar] [CrossRef]
- Boku, N.; Satoh, T.; Ryu, M.-H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.-S.; Muro, K.; Kang, W.K.; Yeh, K.-H.; et al. Nivolumab in previously treated advanced gastric cancer (ATTRACTION-2): 3-year update and outcome of treatment beyond progression with nivolumab. Gastric Cancer 2021, 24, 946–958. [Google Scholar] [CrossRef]
- Högner, A. Thuss-Patience P. Immune Checkpoint Inhibition in Oesophago-Gastric Carcinoma. Pharmaceuticals 2021, 14, 151. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Doi, T.; Jang, R.W. Safety and Efficacy of Pembrolizumab Monotherapy in Patients with Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018, 4, e180013, Erratum in JAMA Oncol. 2019, 5, 579. [Google Scholar] [CrossRef]
- Keytruda (Pembrolizumab) [Package Insert]. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125514s014lbl.pdf (accessed on 9 May 2022).
- Bang, Y.-J.; Ruiz, E.; Van Cutsem, E.; Lee, K.-W.; Wyrwicz, L.; Schenker, M.; Alsina, M.; Ryu, M.-H.; Chung, H.-C.; Evesque, L.; et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: Primary analysis of JAVELIN Gastric 300. Ann. Oncol. 2018, 29, 2052–2060. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, S.; Xu, J.; Guo, W.; Xiong, J.; Bai, Y.; Sun, G.; Yang, Y.; Wang, L.; Xu, N.; et al. Apatinib for Chemotherapy-Refractory Advanced Metastatic Gastric Cancer: Results from a Randomized, Placebo-Controlled, Parallel-Arm, Phase II Trial. J. Clin. Oncol. 2013, 31, 3219–3225. [Google Scholar] [CrossRef]
- Pavlakis, N.; Sjoquist, K.M.; Martin, A.J.; Tsobanis, E.; Yip, S.; Kang, Y.-K.; Bang, Y.-J.; Alcindor, T.; O’Callaghan, C.J.; Burnell, M.J.; et al. Regorafenib for the Treatment of Advanced Gastric Cancer (INTEGRATE): A Multinational Placebo-Controlled Phase II Trial. J. Clin. Oncol. 2016, 34, 2728–2735. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, S.; Xu, J.; Xiong, J.; Wu, C.; Bai, Y.; Liu, W.; Tong, J.; Liu, Y.; Xu, R.; et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients with Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J. Clin. Oncol. 2016, 34, 1448–1454. [Google Scholar] [CrossRef]
- Liu, X.; Qin, S.; Wang, Z.; Xu, J.; Xiong, J.; Bai, Y.; Wang, Z.; Xinyang, L.; Sun, G.; Wang, L.; et al. Early presence of anti-angiogenesis-related adverse events as a potential biomarker of antitumor efficacy in metastatic gastric cancer patients treated with apatinib: A cohort study. J. Hematol. Oncol. 2017, 10, 153, Erratum in J. Hematol. Oncol. 2018, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-K.; Muro, K.; Ryu, M.-H.; Yasui, H.; Nishina, T.; Ryoo, B.-Y.; Kamiya, Y.; Akinaga, S.; Boku, N. A phase II trial of a selective c-Met inhibitor tivantinib (ARQ 197) monotherapy as a second- or third-line therapy in the patients with metastatic gastric cancer. Investig. New Drugs 2014, 32, 355–361. [Google Scholar] [CrossRef]
- Ohtsu, A.; Ajani, J.A.; Bai, Y.X.; Bang, Y.-J.; Chung, H.C.; Pan, H.M.; Sahmoud, T.; Shen, L.; Yeh, K.H.; Chin, K.; et al. Everolimus for previously treated advanced gastric cancer: Results of the randomized, double-blind, phase III GRANITE-1 study. J. Clin. Oncol. 2013, 31, 3935–3943. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.-J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.-C.; Kawakami, H.; Yabusaki, H.; Destiny-Gastric01 Investigators; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Bang, Y.J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.C.C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2–positive advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma: Final overall survival (OS) results from a randomized, multicenter, open-label, phase 2 study (DESTINY-Gastric01). J. Clin. Oncol. 2022, 40 (Suppl. S4), 242, 2021 ASCO Annual Meeting. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, T.; Wei, J.; Wang, A.; He, Y.; Yang, L.; Zhang, X.; Fan, N.; Luo, S.; Li, Z.; et al. Efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: A single-arm phase II study. Cancer Commun. 2021, 41, 1173–1182. [Google Scholar] [CrossRef]
- Chan, W.-L.; Yuen, K.-K.; Siu, S.W.-K.; Lam, K.-O.; Kwong, D.L.-W. Third-line systemic treatment versus best supportive care for advanced/metastatic gastric cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2017, 116, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.T.; Kim, K.; Lee, H.; Kozarewa, I.; Mortimer, P.G.S.; Odegaard, J.I.; Harrington, E.A.; Lee, H.; Lee, T.; et al. Tumor Genomic Profiling Guides Patients with Metastatic Gastric Cancer to Targeted Treatment: The VIKTORY Umbrella Trial. Cancer Discov. 2019, 9, 1388–1405. [Google Scholar] [CrossRef]
- Catenacci, D.V.T.; Moya, S.; Lomnicki, S.; Chase, L.M.; Peterson, B.F.; Reizine, N.; Alpert, L.; Setia, N.; Xiao, S.-Y.; Hart, J.; et al. Personalized Antibodies for Gastroesophageal Adenocarcinoma (PANGEA): A Phase II Study Evaluating an Individualized Treatment Strategy for Metastatic Disease. Cancer Discov. 2021, 11, 308–325. [Google Scholar] [CrossRef] [PubMed]
- Mulazzani, G.E.; Corti, F.; Della Valle, S.; Di Bartolomeo, M. Nutritional Support Indications in Gastroesophageal Cancer Patients: From Perioperative to Palliative Systemic Therapy. A Comprehensive Review of the Last Decade. Nutrients 2021, 13, 2766. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mare, M.; Memeo, L.; Colarossi, C.; Giuffrida, D. Third- and Late Line Treatments of Metastatic Gastric Cancer: Still More to Be Done. Curr. Oncol. 2022, 29, 6433-6444. https://doi.org/10.3390/curroncol29090506
Mare M, Memeo L, Colarossi C, Giuffrida D. Third- and Late Line Treatments of Metastatic Gastric Cancer: Still More to Be Done. Current Oncology. 2022; 29(9):6433-6444. https://doi.org/10.3390/curroncol29090506
Chicago/Turabian StyleMare, Marzia, Lorenzo Memeo, Cristina Colarossi, and Dario Giuffrida. 2022. "Third- and Late Line Treatments of Metastatic Gastric Cancer: Still More to Be Done" Current Oncology 29, no. 9: 6433-6444. https://doi.org/10.3390/curroncol29090506
APA StyleMare, M., Memeo, L., Colarossi, C., & Giuffrida, D. (2022). Third- and Late Line Treatments of Metastatic Gastric Cancer: Still More to Be Done. Current Oncology, 29(9), 6433-6444. https://doi.org/10.3390/curroncol29090506





