Association between Temporal Muscle Thickness and Overall Survival in Non-Small Cell Lung Cancer Patients with Brain Metastasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Temporal Muscle Thickness
2.3. Clinical Variates
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
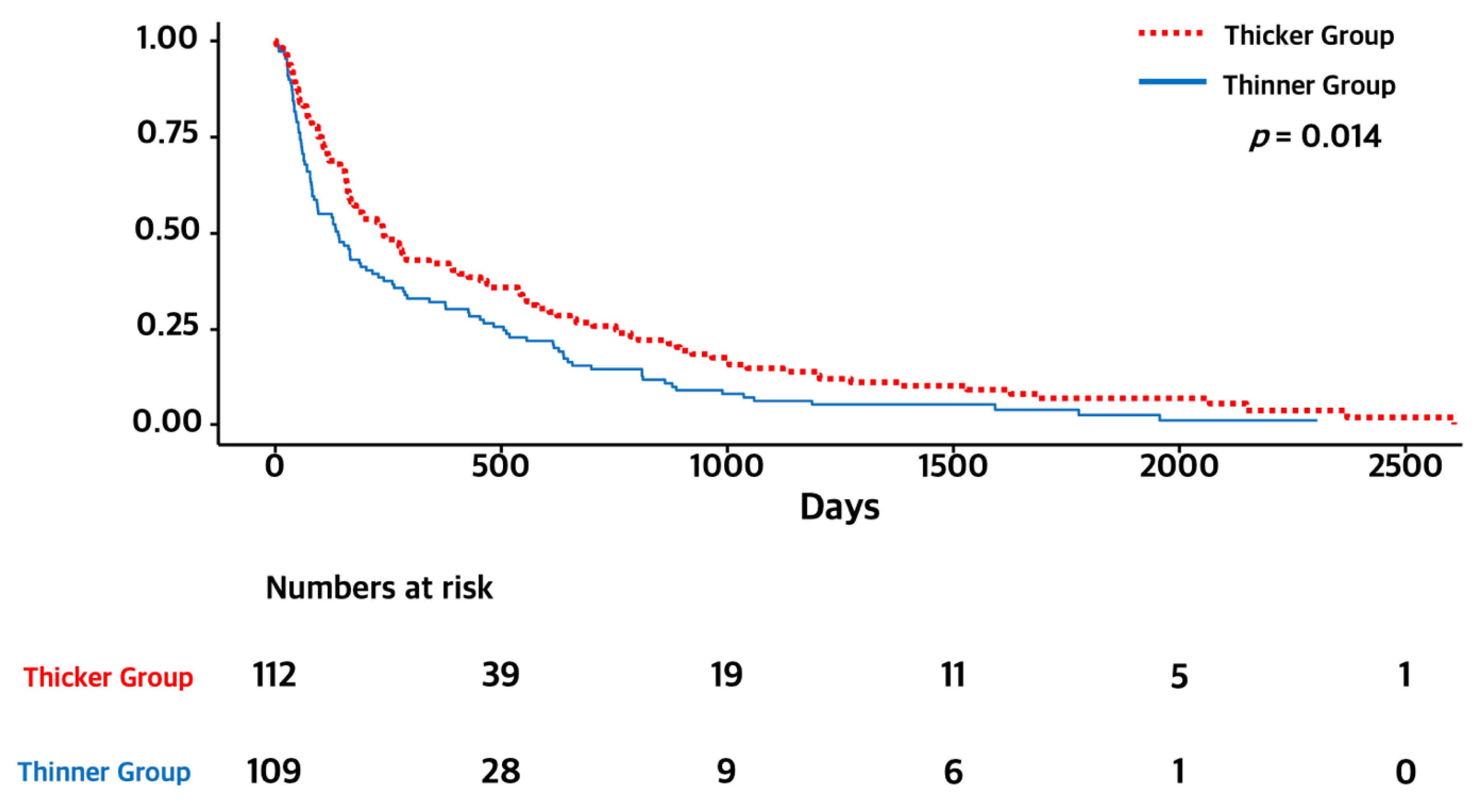
3.2. Temporal Muscle Thickness and Overall Survival
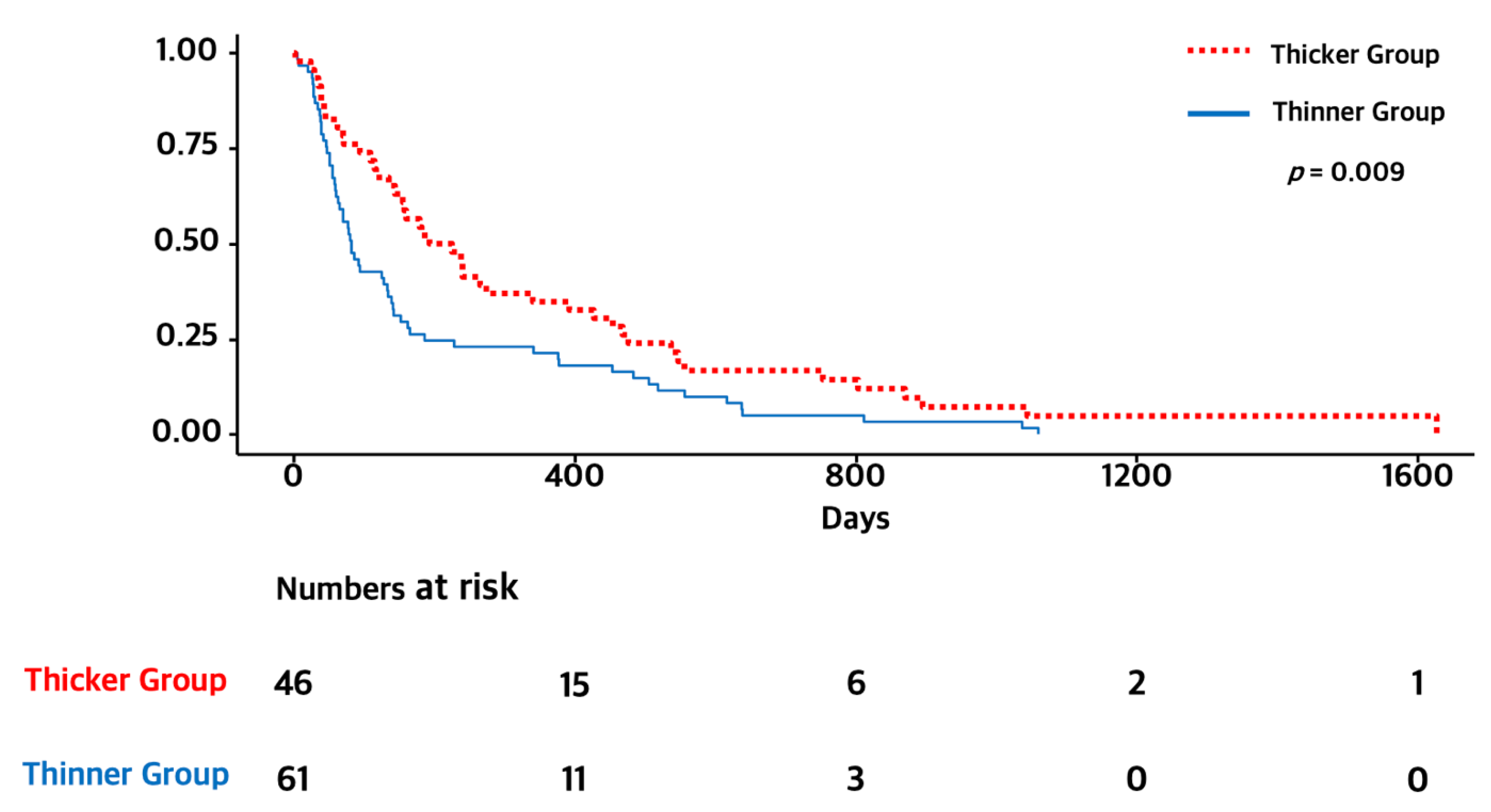
3.3. Subgroup Analysis of Elderly Patients (≥65 Years)
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Lamba, N.; Wen, P.Y.; Aizer, A.A. Epidemiology of brain metastases and leptomeningeal disease. Neuro-Oncology 2021, 23, 1447–1456. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Yang, T.J.; Beal, K.; Pan, H.; Brown, P.D.; Bangdiwala, A.; Shanley, R.; Yeh, N.; Gaspar, L.E.; Braunstein, S.; et al. Estimating Survival in Patients with Lung Cancer and Brain Metastases: An Update of the Graded Prognostic Assessment for Lung Cancer Using Molecular Markers (Lung-molGPA). JAMA Oncol. 2017, 3, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Ubachs, J.; Ziemons, J.; Minis-Rutten, I.J.G.; Kruitwagen, R.; Kleijnen, J.; Lambrechts, S.; Olde Damink, S.W.M.; Rensen, S.S.; Van Gorp, T. Sarcopenia and ovarian cancer survival: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Dunne, R.F.; Loh, K.P.; Williams, G.R.; Jatoi, A.; Mustian, K.M.; Mohile, S.G. Cachexia and Sarcopenia in Older Adults with Cancer: A Comprehensive Review. Cancers 2019, 11, 1861. [Google Scholar] [CrossRef] [PubMed]
- Findlay, M.; White, K.; Stapleton, N.; Bauer, J. Is sarcopenia a predictor of prognosis for patients undergoing radiotherapy for head and neck cancer? A meta-analysis. Clin. Nutr. 2021, 40, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chi, J.; Liu, Y.; Fan, L.; Hu, K. Impact of preoperative sarcopenia on postoperative complications and prognosis of gastric cancer resection: A meta-analysis of cohort studies. Arch. Gerontol. Geriatr. 2022, 98, 104534. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Ranganathan, K.; Terjimanian, M.; Lisiecki, J.; Rinkinen, J.; Mukkamala, A.; Brownley, C.; Buchman, S.R.; Wang, S.C.; Levi, B. Temporalis muscle morphomics: The psoas of the craniofacial skeleton. J. Surg. Res. 2014, 186, 246–252. [Google Scholar] [CrossRef]
- Leitner, J.; Pelster, S.; Schöpf, V.; Berghoff, A.S.; Woitek, R.; Asenbaum, U.; Nenning, K.H.; Widhalm, G.; Kiesel, B.; Gatterbauer, B.; et al. High correlation of temporal muscle thickness with lumbar skeletal muscle cross-sectional area in patients with brain metastases. PLoS ONE 2018, 13, e0207849. [Google Scholar] [CrossRef]
- An, G.; Ahn, S.; Park, J.S.; Jeun, S.S.; Hong, Y.K. Association between temporal muscle thickness and clinical outcomes in patients with newly diagnosed glioblastoma. J. Cancer Res. Clin. Oncol. 2021, 147, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Muglia, R.; Simonelli, M.; Pessina, F.; Morenghi, E.; Navarria, P.; Persico, P.; Lorenzi, E.; Dipasquale, A.; Grimaldi, M.; Scorsetti, M.; et al. Prognostic relevance of temporal muscle thickness as a marker of sarcopenia in patients with glioblastoma at diagnosis. Eur. Radiol. 2021, 31, 4079–4086. [Google Scholar] [CrossRef] [PubMed]
- Pasqualetti, F.; Gabelloni, M.; Gonnelli, A.; Faggioni, L.; Cantarella, M.; Montrone, S.; Gadducci, G.; Giannini, N.; Montemurro, N.; Mattioni, R.; et al. Impact of temporalis muscle thickness in elderly patients with newly diagnosed glioblastoma treated with radio or radio-chemotherapy. Radiol. Med. 2022, 127, 919–924. [Google Scholar] [CrossRef]
- Furtner, J.; Berghoff, A.S.; Albtoush, O.M.; Woitek, R.; Asenbaum, U.; Prayer, D.; Widhalm, G.; Gatterbauer, B.; Dieckmann, K.; Birner, P.; et al. Survival prediction using temporal muscle thickness measurements on cranial magnetic resonance images in patients with newly diagnosed brain metastases. Eur. Radiol. 2017, 27, 3167–3173. [Google Scholar] [CrossRef]
- Furtner, J.; Berghoff, A.S.; Schöpf, V.; Reumann, R.; Pascher, B.; Woitek, R.; Asenbaum, U.; Pelster, S.; Leitner, J.; Widhalm, G.; et al. Temporal muscle thickness is an independent prognostic marker in melanoma patients with newly diagnosed brain metastases. J. Neurooncol. 2018, 140, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Furtner, J.; Genbrugge, E.; Gorlia, T.; Bendszus, M.; Nowosielski, M.; Golfinopoulos, V.; Weller, M.; van den Bent, M.J.; Wick, W.; Preusser, M. Temporal muscle thickness is an independent prognostic marker in patients with progressive glioblastoma: Translational imaging analysis of the EORTC 26101 trial. Neuro-Oncology 2019, 21, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Furtner, J.; Weller, M.; Weber, M.; Gorlia, T.; Nabors, B.; Reardon, D.A.; Tonn, J.C.; Stupp, R.; Preusser, M. Temporal Muscle Thickness as a Prognostic Marker in Patients with Newly Diagnosed Glioblastoma: Translational Imaging Analysis of the CENTRIC EORTC 26071-22072 and CORE Trials. Clin. Cancer Res. 2022, 28, 129–136. [Google Scholar] [CrossRef]
- Ilic, I.; Faron, A.; Heimann, M.; Potthoff, A.L.; Schäfer, N.; Bode, C.; Borger, V.; Eichhorn, L.; Giordano, F.A.; Güresir, E.; et al. Combined Assessment of Preoperative Frailty and Sarcopenia Allows the Prediction of Overall Survival in Patients with Lung Cancer (NSCLC) and Surgically Treated Brain Metastasis. Cancers 2021, 13, 3353. [Google Scholar] [CrossRef]
- Cho, A.; Hennenberg, J.; Untersteiner, H.; Hirschmann, D.; Gatterbauer, B.; Zöchbauer-Müller, S.; Hochmair, M.J.; Preusser, M.; Rössler, K.; Dorfer, C.; et al. Influence of temporal muscle thickness on the outcome of radiosurgically treated patients with brain metastases from non-small cell lung cancer. J. Neurosurg. 2022; 1–7, online ahead of print. [Google Scholar] [CrossRef]
- Gaspar, L.; Scott, C.; Rotman, M.; Asbell, S.; Phillips, T.; Wasserman, T.; McKenna, W.G.; Byhardt, R. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 745–751. [Google Scholar] [CrossRef]
- Venur, V.A.; Ahluwalia, M.S. Prognostic scores for brain metastasis patients: Use in clinical practice and trial design. Chin. Clin. Oncol. 2015, 4, 18. [Google Scholar]
- Sperduto, P.W.; Berkey, B.; Gaspar, L.E.; Mehta, M.; Curran, W. A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1,960 patients in the RTOG database. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Kased, N.; Roberge, D.; Xu, Z.; Shanley, R.; Luo, X.; Sneed, P.K.; Chao, S.T.; Weil, R.J.; Suh, J.; et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J. Clin. Oncol. 2012, 30, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Steindl, A.; Leitner, J.; Schwarz, M.; Nenning, K.H.; Asenbaum, U.; Mayer, S.; Woitek, R.; Weber, M.; Schöpf, V.; Berghoff, A.S.; et al. Sarcopenia in Neurological Patients: Standard Values for Temporal Muscle Thickness and Muscle Strength Evaluation. J. Clin. Med. 2020, 9, 1272. [Google Scholar] [CrossRef] [PubMed]
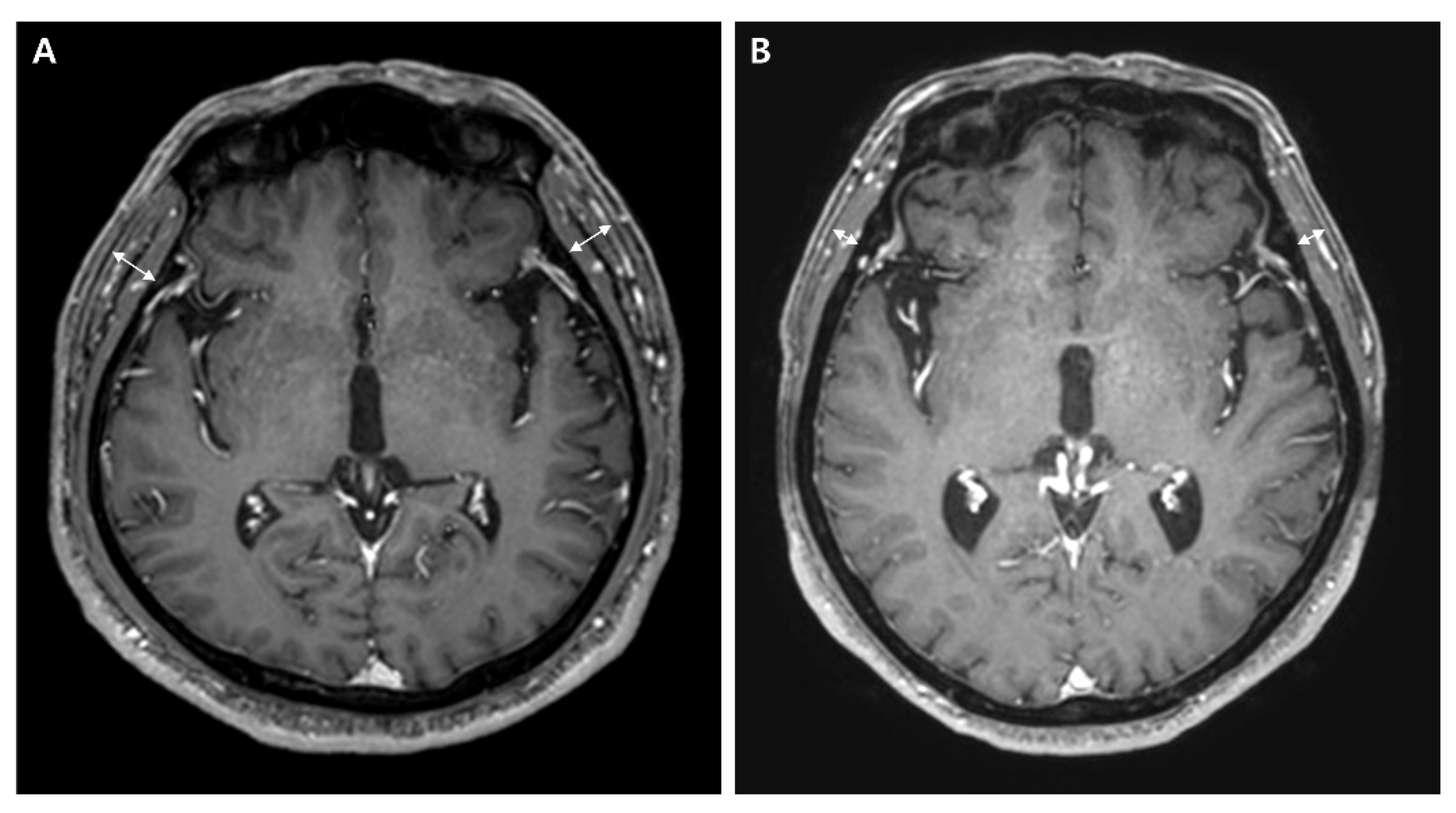

| Variates | Thicker TMT Group (n = 112) | Thinner TMT Group (n = 109) | p-Value |
|---|---|---|---|
| Male sex, n (%) | 67 (59.8) | 66 (60.6) | >0.999 |
| Age, years, n (range) | 61.7 (33–90) | 66.9 (36–90) | <0.001 |
| ≥65 years, n (%) | 46 (41.1) | 61 (56.0) | 0.038 |
| TMT, mm (range) | |||
| Male | 9.6 mm (7.7–14.8) | 6.0 mm (2.4–7.6) | <0.001 |
| Female | 8.7 mm (7.0–11.7) | 5.7 mm (2.4–6.9) | <0.001 |
| Pathological diagnosis, n (%) | 0.511 | ||
| Adenocarcinoma | 88 (78.6) | 78 (71.6) | |
| Squamous cell carcinoma | 13 (11.6) | 17 (15.6) | |
| Large cell carcinoma | 2 (1.8) | 1 (0.9) | |
| Sarcomatoid carcinoma | 1 (0.9) | 4 (3.7) | |
| Others | 8 (7.1) | 9 (8.3) | |
| Number of brain metastases, n (%) | 0.286 | ||
| 1 | 29 (25.9) | 35 (32.1) | |
| 2–4 | 36 (32.1) | 31 (28.4) | |
| 5 | 47 (42.0) | 43 (39.5) | |
| Extracranial metastases, n (%) | 55 (49.1) | 49 (45.0) | 0.629 |
| ECOG, n (%) | 0.358 | ||
| 0–1 | 65 (58.0) | 62 (56.9) | |
| 2 | 47 (42.0) | 47 (43.1) | |
| Radiotherapy, n (%) | 82 (73.2) | 72 (66.1) | 0.312 |
| Radiosurgery, n (%) | 15 (13.4) | 7 (6.4) | 0.132 |
| Surgical resection, n (%) | 12 (10.7) | 6 (5.5) | 0.242 |
| EGFR mutation, n (%) | 0.107 | ||
| Yes | 52 (46.4%) | 37 (33.9%) | |
| No | 43 (38.4%) | 46 (42.2%) | |
| Unknown | 17 (15.2%) | 26 (23.9%) | |
| ALK mutation, n (%) | 0.681 | ||
| Yes | 2 (1.8%) | 1 (0.9%) | |
| No | 58 (51.8%) | 52 (47.7%) | |
| Unknown | 108 (48.9%) | 56 (51.4%) |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variates | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value |
| Sex (male versus female) | 1.43 [1.08, 1.88] | 0.012 | 1.56 [1.18, 2.06] | 0.020 |
| Age (≥65 versus <65) | 1.98 [1.49, 2.63] | <0.001 | 2.09 [1.57, 2.79] | <0.001 |
| TMT (thicker versus <thinner) | 0.71 [0.54, 0.93] | 0.014 | 0.73 [0.55, 0.96] | 0.022 |
| Extracranial metastases (presence versus absence) | 0.87 [0.66, 1.14] | 0.300 | ||
| Number of brain metastases (single versus multiple) | 1.04 [0.77, 1.41] | 0.784 | ||
| ECOG (0 or 1versus ≥2) | 0.79 [0.60, 1.04] | 0.096 | 0.78 [0.59, 1.02] | 0.071 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.I.; Shin, J.Y.; Yang, S.H.; Kim, H.H.; Shim, B.Y.; Ahn, S. Association between Temporal Muscle Thickness and Overall Survival in Non-Small Cell Lung Cancer Patients with Brain Metastasis. Curr. Oncol. 2022, 29, 6463-6471. https://doi.org/10.3390/curroncol29090508
Kim YI, Shin JY, Yang SH, Kim HH, Shim BY, Ahn S. Association between Temporal Muscle Thickness and Overall Survival in Non-Small Cell Lung Cancer Patients with Brain Metastasis. Current Oncology. 2022; 29(9):6463-6471. https://doi.org/10.3390/curroncol29090508
Chicago/Turabian StyleKim, Young Il, Ja Young Shin, Seung Ho Yang, Hyun Ho Kim, Byoung Yong Shim, and Stephen Ahn. 2022. "Association between Temporal Muscle Thickness and Overall Survival in Non-Small Cell Lung Cancer Patients with Brain Metastasis" Current Oncology 29, no. 9: 6463-6471. https://doi.org/10.3390/curroncol29090508
APA StyleKim, Y. I., Shin, J. Y., Yang, S. H., Kim, H. H., Shim, B. Y., & Ahn, S. (2022). Association between Temporal Muscle Thickness and Overall Survival in Non-Small Cell Lung Cancer Patients with Brain Metastasis. Current Oncology, 29(9), 6463-6471. https://doi.org/10.3390/curroncol29090508





