A Review of Current and Emerging Therapies for Advanced Hepatocellular Carcinoma
Abstract
:1. Introduction
2. First-Line Treatment
2.1. Sorafenib
2.2. Lenvatinib
2.3. Atezolizumab and Bevacizumab
2.4. Durvalumab and Tremelimumab
3. Second-Line Treatments
3.1. Nivolumab
3.2. Nivolumab + Ipilimumab
3.3. Pembrolizumab
3.4. Ramucirumab
3.5. Regorafenib
3.6. Cabozantinib
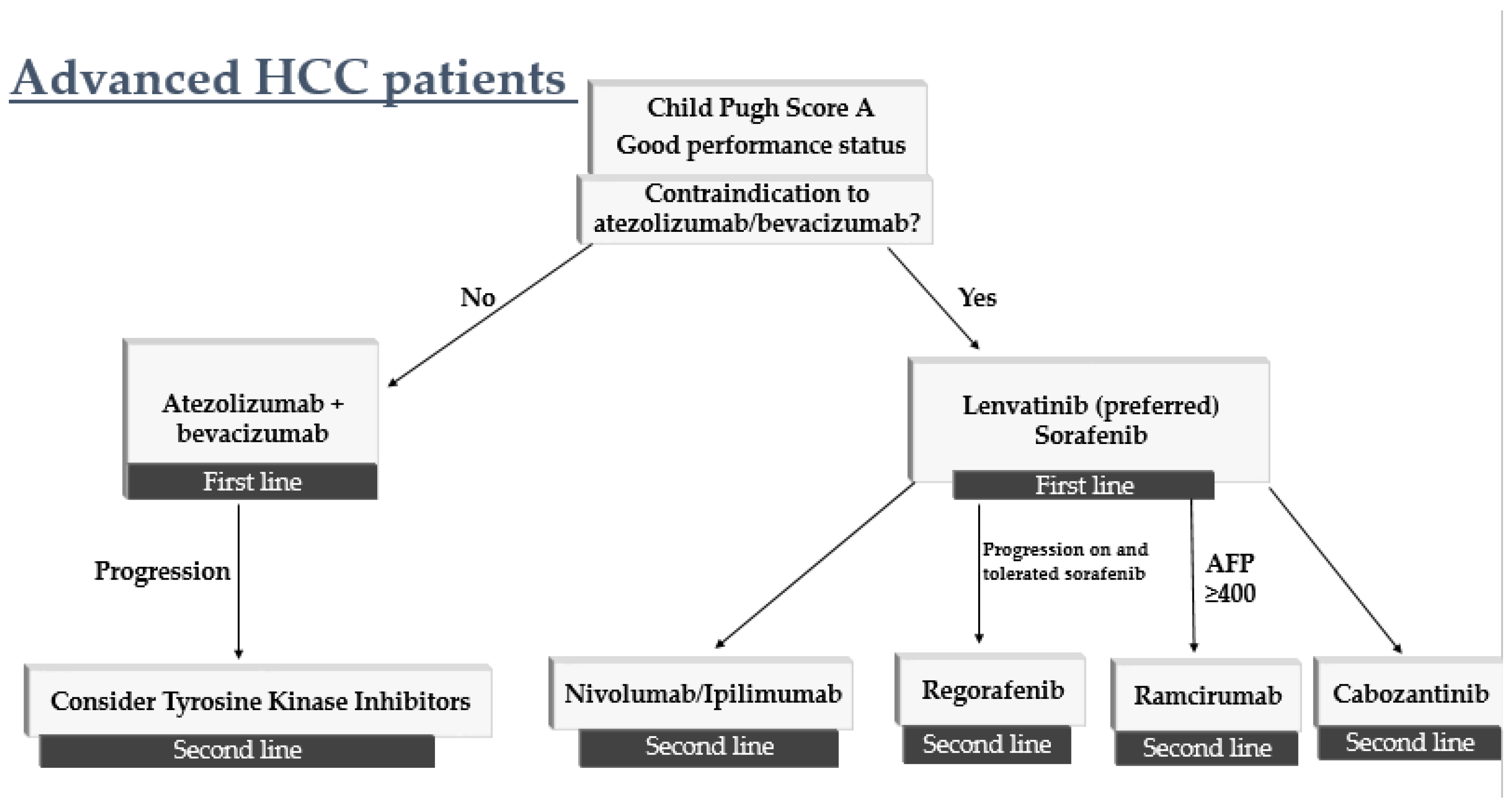
4. How to Sequence Therapy
5. Advanced HCC in Patients with Child–Pugh Class B and C
6. Ongoing and Future Clinical Trials for HCC
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ryerson, A.B.; Eheman, C.R.; Altekruse, S.F.; Ward, J.W.; Jemal, A.; Sherman, R.L.; Henley, S.J.; Holtzman, D.; Lake, A.; Noone, A.-M.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, Featuring the Increasing Incidence of Liver Cancer: Report on Status of Cancer, 1975–2012. Cancer 2016, 122, 1312–1337. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Liver Cancer Collaboration; Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases: Marrero et Al. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Islami, F.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Ward, E.M.; Jemal, A. Disparities in Liver Cancer Occurrence in the United States by Race/Ethnicity and State: Liver Cancer in the United States. CA Cancer J. Clin. 2017, 67, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Melkonian, S.C.; Jim, M.A.; Reilley, B.; Erdrich, J.; Berkowitz, Z.; Wiggins, C.L.; Haverkamp, D.; White, M.C. Incidence of Primary Liver Cancer in American Indians and Alaska Natives, US, 1999–2009. Cancer Causes Control 2018, 29, 833–844. [Google Scholar] [CrossRef]
- Balogh, J.; Victor, D.; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, M.; Monsour, H. Hepatocellular Carcinoma: A Review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef]
- Frenette, C.T.; Isaacson, A.J.; Bargellini, I.; Saab, S.; Singal, A.G. A Practical Guideline for Hepatocellular Carcinoma Screening in Patients at Risk. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 302–310. [Google Scholar] [CrossRef]
- Mazzanti, R. Hepatocellular Carcinoma: Where Are We? World J. Exp. Med. 2016, 6, 21. [Google Scholar] [CrossRef]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Harris, W.P.; et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 4317–4345. [Google Scholar] [CrossRef]
- Calvisi, D.F.; Ladu, S.; Gorden, A.; Farina, M.; Conner, E.A.; Lee, J.; Factor, V.M.; Thorgeirsson, S.S. Ubiquitous Activation of Ras and Jak/Stat Pathways in Human HCC. Gastroenterology 2006, 130, 1117–1128. [Google Scholar] [CrossRef]
- Shimamura, T.; Saito, S.; Morita, K.; Kitamura, T.; Morimoto, M.; Kiba, T.; Numata, K.; Tanaka, K.; Sekihara, H. Detection of Vascular Endothelial Growth Factor and Its Receptor Expression in Human Hepatocellular Carcinoma Biopsy Specimens. J. Gastroenterol. Hepatol. 2000, 15, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Meadows, K.N.; Bryant, P.; Pumiglia, K. Vascular Endothelial Growth Factor Induction of the Angiogenic Phenotype Requires Ras Activation. J. Biol. Chem. 2001, 276, 49289–49298. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 Exhibits Broad Spectrum Oral Antitumor Activity and Targets the RAF/MEK/ERK Pathway and Receptor Tyrosine Kinases Involved in Tumor Progression and Angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Schwartz, L.; Ricci, S.; Amadori, D.; Santoro, A.; Figer, A.; De Greve, J.; Douillard, J.-Y.; Lathia, C.; Schwartz, B.; et al. Phase II Study of Sorafenib in Patients with Advanced Hepatocellular Carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 4293–4300. [Google Scholar] [CrossRef]
- Yeo, W.; Mok, T.S.; Zee, B.; Leung, T.W.T.; Lai, P.B.S.; Lau, W.Y.; Koh, J.; Mo, F.K.F.; Yu, S.C.H.; Chan, A.T.; et al. A Randomized Phase III Study of Doxorubicin Versus Cisplatin/Interferon α-2b/Doxorubicin/Fluorouracil (PIAF) Combination Chemotherapy for Unresectable Hepatocellular Carcinoma. JNCI J. Natl. Cancer Inst. 2005, 97, 1532–1538. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S.; et al. Efficacy and Safety of Sorafenib in Patients in the Asia-Pacific Region with Advanced Hepatocellular Carcinoma: A Phase III Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Bruix, J.; Cheng, A.-L.; Meinhardt, G.; Nakajima, K.; De Sanctis, Y.; Llovet, J. Prognostic Factors and Predictors of Sorafenib Benefit in Patients with Hepatocellular Carcinoma: Analysis of Two Phase III Studies. J. Hepatol. 2017, 67, 999–1008. [Google Scholar] [CrossRef]
- Bruix, J.; Raoul, J.-L.; Sherman, M.; Mazzaferro, V.; Bolondi, L.; Craxi, A.; Galle, P.R.; Santoro, A.; Beaugrand, M.; Sangiovanni, A.; et al. Efficacy and Safety of Sorafenib in Patients with Advanced Hepatocellular Carcinoma: Subanalyses of a Phase III Trial. J. Hepatol. 2012, 57, 821–829. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Guan, Z.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Yang, T.-S.; Tak, W.Y.; Pan, H.; Yu, S.; et al. Efficacy and Safety of Sorafenib in Patients with Advanced Hepatocellular Carcinoma According to Baseline Status: Subset Analyses of the Phase III Sorafenib Asia–Pacific Trial. Eur. J. Cancer 2012, 48, 1452–1465. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus Sorafenib in First-Line Treatment of Patients with Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 Non-Inferiority Trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Huynh, J.; Cho, M.T.; Kim, E.J.-H.; Ren, M.; Robbins, C.; Amaya-Chanaga, C.; Vogel, A. Post Hoc Analysis in Patients (Pts) with Unresectable Hepatocellular Carcinoma (UHCC) Who Progressed to Child-Pugh B (CPB) Liver Function in the Phase III REFLECT Study of Lenvatinib (LEN). J. Clin. Oncol. 2021, 39 (Suppl. 3), 298. [Google Scholar] [CrossRef]
- Alsina, A.; Kudo, M.; Vogel, A.; Cheng, A.-L.; Tak, W.Y.; Ryoo, B.-Y.; Evans, T.R.J.; López López, C.; Daniele, B.; Misir, S.; et al. Effects of Subsequent Systemic Anticancer Medication Following First-Line Lenvatinib: A Post Hoc Responder Analysis from the Phase 3 REFLECT Study in Unresectable Hepatocellular Carcinoma. Liver Cancer 2020, 9, 93–104. [Google Scholar] [CrossRef]
- Alsina, A.; Kudo, M.; Vogel, A.; Cheng, A.-L.; Tak, W.Y.; Ryoo, B.-Y.; Evans, T.R.J.; Lopéz Lopéz, C.; Daniele, B.; Blanc, J.-F.; et al. Subsequent Anticancer Procedures Following First-Line Lenvatinib (LEN): A Post Hoc Analysis from the Phase III REFLECT Study in Unresectable Hepatocellular Carcinoma (UHCC). J. Clin. Oncol. 2020, 38 (Suppl. 4), 520. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and Its Ligands in Tolerance and Immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wang, X.-Y.; Qiu, S.-J.; Yamato, I.; Sho, M.; Nakajima, Y.; Zhou, J.; Li, B.-Z.; Shi, Y.-H.; Xiao, Y.-S.; et al. Overexpression of PD-L1 Significantly Associates with Tumor Aggressiveness and Postoperative Recurrence in Human Hepatocellular Carcinoma. Clin. Cancer Res. 2009, 15, 971–979. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive Correlates of Response to the Anti-PD-L1 Antibody MPDL3280A in Cancer Patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef]
- Finn, R.S.; Bentley, G.; Britten, C.D.; Amado, R.; Busuttil, R.W. Targeting Vascular Endothelial Growth Factor with the Monoclonal Antibody Bevacizumab Inhibits Human Hepatocellular Carcinoma Cells Growing in an Orthotopic Mouse Model. Liver Int. Off. J. Int. Assoc. Study Liver 2009, 29, 284–290. [Google Scholar] [CrossRef]
- Lee, M.S.; Ryoo, B.-Y.; Hsu, C.-H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.P.; Spahn, J.; Liu, B.; Abdullah, H.; et al. GO30140 investigators. Atezolizumab with or without Bevacizumab in Unresectable Hepatocellular Carcinoma (GO30140): An Open-Label, Multicentre, Phase 1b Study. Lancet Oncol. 2020, 21, 808–820. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.V.; Merle, P.; et al. IMbrave150: Updated Overall Survival (OS) Data from a Global, Randomized, Open-Label Phase III Study of Atezolizumab (Atezo) + Bevacizumab (Bev) versus Sorafenib (Sor) in Patients (Pts) with Unresectable Hepatocellular Carcinoma (HCC). J. Clin. Oncol. 2021, 39 (Suppl. 3), 267. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Segal, N.H.; Jaeger, D.; Lee, K.-H.; Marshall, J.; Antonia, S.J.; Butler, M.; Sanborn, R.E.; Nemunaitis, J.J.; Carlson, C.A.; et al. Safety and Clinical Activity of Durvalumab Monotherapy in Patients with Hepatocellular Carcinoma (HCC). J. Clin. Oncol. 2017, 35 (Suppl. 15), 4071. [Google Scholar] [CrossRef]
- Sangro, B.; Gomez-Martin, C.; de la Mata, M.; Iñarrairaegui, M.; Garralda, E.; Barrera, P.; Riezu-Boj, J.I.; Larrea, E.; Alfaro, C.; Sarobe, P.; et al. A Clinical Trial of CTLA-4 Blockade with Tremelimumab in Patients with Hepatocellular Carcinoma and Chronic Hepatitis C. J. Hepatol. 2013, 59, 81–88. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Chan, S.L.; Kudo, M.; Lau, G.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Dao, T.V.; De Toni, E.N.; et al. Phase 3 Randomized, Open-Label, Multicenter Study of Tremelimumab (T) and Durvalumab (D) as First-Line Therapy in Patients (Pts) with Unresectable Hepatocellular Carcinoma (UHCC): HIMALAYA. J. Clin. Oncol. 2022, 40 (Suppl. 4), 379. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in Patients with Advanced Hepatocellular Carcinoma (CheckMate 040): An Open-Label, Non-Comparative, Phase 1/2 Dose Escalation and Expansion Trial. Lancet Lond. Engl. 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.-W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus Sorafenib in Advanced Hepatocellular Carcinoma (CheckMate 459): A Randomised, Multicentre, Open-Label, Phase 3 Trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib (KEYNOTE-224): A Non-Randomised, Open-Label Phase 2 Trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.-K.; Yen, C.-J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after Sorafenib in Patients with Advanced Hepatocellular Carcinoma and Increased α-Fetoprotein Concentrations (REACH-2): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Zhu, A.X.; Park, J.O.; Ryoo, B.-Y.; Yen, C.-J.; Poon, R.; Pastorelli, D.; Blanc, J.-F.; Chung, H.C.; Baron, A.D.; Pfiffer, T.E.F.; et al. Ramucirumab versus Placebo as Second-Line Treatment in Patients with Advanced Hepatocellular Carcinoma Following First-Line Therapy with Sorafenib (REACH): A Randomised, Double-Blind, Multicentre, Phase 3 Trial. Lancet Oncol. 2015, 16, 859–870. [Google Scholar] [CrossRef]
- Choucair, K.; Kamran, S.; Saeed, A. Clinical Evaluation of Ramucirumab for the Treatment of Hepatocellular Carcinoma (HCC): Place in Therapy. OncoTargets Ther. 2021, 14, 5521–5532. [Google Scholar] [CrossRef] [PubMed]
- Frenette, C.T. The Role of Regorafenib in Hepatocellular Carcinoma. Gastroenterol. Hepatol. 2017, 13, 122–124. [Google Scholar]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.-H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for Patients with Hepatocellular Carcinoma Who Progressed on Sorafenib Treatment (RESORCE): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Lond. Engl. 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Finn, R.S.; Merle, P.; Granito, A.; Huang, Y.-H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Gerolami, R.; Caparello, C.; et al. Outcomes of Sequential Treatment with Sorafenib Followed by Regorafenib for HCC: Additional Analyses from the Phase III RESORCE Trial. J. Hepatol. 2018, 69, 353–358. [Google Scholar] [CrossRef]
- Yakes, F.M.; Chen, J.; Tan, J.; Yamaguchi, K.; Shi, Y.; Yu, P.; Qian, F.; Chu, F.; Bentzien, F.; Cancilla, B.; et al. Cabozantinib (XL184), a Novel MET and VEGFR2 Inhibitor, Simultaneously Suppresses Metastasis, Angiogenesis, and Tumor Growth. Mol. Cancer Ther. 2011, 10, 2298–2308. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Kelley, R.K.; Meyer, T.; Rimassa, L.; Merle, P.; Park, J.-W.; Yau, T.; Chan, S.L.; Blanc, J.-F.; Tam, V.C.; Tran, A.; et al. Serum Alpha-Fetoprotein Levels and Clinical Outcomes in the Phase III CELESTIAL Study of Cabozantinib versus Placebo in Patients with Advanced Hepatocellular Carcinoma. Clin. Cancer Res. 2020, 26, 4795–4804. [Google Scholar] [CrossRef]
- Lencioni, R.; Kudo, M.; Ye, S.-L.; Bronowicki, J.-P.; Chen, X.-P.; Dagher, L.; Furuse, J.; Geschwind, J.F.; Guevara, L.L.; Papandreou, C.; et al. GIDEON (Global Investigation of Therapeutic DE Cisions in Hepatocellular Carcinoma and Of Its Treatment with SorafeNib): Second Interim Analysis. Int. J. Clin. Pract. 2014, 68, 609–617. [Google Scholar] [CrossRef]
- Giannini, E.G.; Farinati, F.; Ciccarese, F.; Pecorelli, A.; Rapaccini, G.L.; Di Marco, M.; Benvegnù, L.; Caturelli, E.; Zoli, M.; Borzio, F.; et al. Prognosis of Untreated Hepatocellular Carcinoma. Hepatology 2015, 61, 184–190. [Google Scholar] [CrossRef]
- Exposito, M.J.; Akce, M.; Alvarez, J.; Assenat, E.; Balart, L.; Baron, A.; Decaens, T.; Heurgue-Berlot, A.; Martin, A.; Paik, S.; et al. Abstract No. 526 CheckMate-9DX: Phase 3, Randomized, Double-Blind Study of Adjuvant Nivolumab vs. Placebo for Patients with Hepatocellular Carcinoma (HCC) at High Risk of Recurrence after Curative Resection or Ablation. J. Vasc. Interv. Radiol. 2019, 30, S227–S228. [Google Scholar] [CrossRef] [Green Version]
- Knox, J.; Cheng, A.; Cleary, S.; Galle, P.; Kokudo, N.; Lencioni, R.; Park, J.; Zhou, J.; Mann, H.; Morgan, S.; et al. A Phase 3 Study of Durvalumab with or without Bevacizumab as Adjuvant Therapy in Patients with Hepatocellular Carcinoma at High Risk of Recurrence after Curative Hepatic Resection or Ablation: EMERALD-2. Ann. Oncol. 2019, 30, iv59–iv60. [Google Scholar] [CrossRef]
- Zhu, A.; Kudo, M.; Vogel, A.; Yau, T.; Zhou, J.; Kim, E.; Malhotra, U.; Siegel, A.B.; Cheng, A.-L. Abstract CT284: Phase 3 KEYNOTE-937: Adjuvant Pembrolizumab versus Placebo in Patients with Hepatocellular Carcinoma and Complete Radiologic Response after Surgical Resection or Local Ablation. Cancer Res. 2020, 80 (Suppl. 16), CT284. [Google Scholar] [CrossRef]
- Hack, S.P.; Spahn, J.; Chen, M.; Cheng, A.-L.; Kaseb, A.; Kudo, M.; Lee, H.C.; Yopp, A.; Chow, P.; Qin, S. IMbrave 050: A Phase III Trial of Atezolizumab plus Bevacizumab in High-Risk Hepatocellular Carcinoma after Curative Resection or Ablation. Future Oncol. 2020, 16, 975–989. [Google Scholar] [CrossRef]
- Sangro, B.; Kudo, M.; Qin, S.; Ren, Z.; Chan, S.; Joseph, E.; Arai, Y.; Mann, H.; Morgan, S.; Cohen, G.; et al. P-347 A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study of Transarterial Chemoembolization Combined with Durvalumab or Durvalumab plus Bevacizumab Therapy in Patients with Locoregional Hepatocellular Carcinoma: EMERALD-1. Ann. Oncol. 2020, 31, S202–S203. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Llovet, J.M.; Vogel, A.; Madoff, D.C.; Finn, R.S.; Ogasawara, S.; Ren, Z.; Mody, K.; Li, J.J.; Siegel, A.B.; et al. LEAP-012 Trial in Progress: Transarterial Chemoembolization (TACE) with or without Lenvatinib plus Pembrolizumab for Intermediate-Stage Hepatocellular Carcinoma (HCC). J. Clin. Oncol. 2022, 40 (Suppl. 4), TPS494. [Google Scholar] [CrossRef]
- Sangro, B.; Harding, J.J.; Johnson, M.; Palmer, D.H.; Edeline, J.; Abou-Alfa, G.K.; Cheng, A.-L.; Decaens, T.; El-Khoueiry, A.B.; Finn, R.S.; et al. A Phase III, Double-Blind, Randomized Study of Nivolumab (NIVO) and Ipilimumab (IPI), Nivo Monotherapy or Placebo plus Transarterial Chemoembolization (TACE) in Patients with Intermediate-Stage Hepatocellular Carcinoma (HCC). J. Clin. Oncol. 2021, 39 (Suppl. 3), TPS349. [Google Scholar] [CrossRef]
- Qin, S.; Finn, R.S.; Kudo, M.; Meyer, T.; Vogel, A.; Ducreux, M.; Macarulla, T.M.; Tomasello, G.; Boisserie, F.; Hou, J.; et al. RATIONALE 301 Study: Tislelizumab versus Sorafenib as First-Line Treatment for Unresectable Hepatocellular Carcinoma. Future Oncol. 2019, 15, 1811–1822. [Google Scholar] [CrossRef]
- A Randomized, Multi-Center, Phase 3 Study of Nivolumab in Combination With Ipilimumab Compared to Sorafenib or Lenvatinib as First-Line Treatment in Participants With Advanced Hepatocellular Carcinoma. Available online: https://www.orpha.net/consor/cgi-bin/ResearchTrials_Networks.php?lng=EN&data_id=140940&title=CheckMate%209DW:%20A%20Randomized,%20Multi-center,%20Phase%203%20Study%20of%20Nivolumab%20in%20Combination%20With%20Ipili-mumab%20Compared%20to%20Sorafenib%20or%20Lenvatinib%20as%20First-Line%20Treatment%20in%20Participants%20With%20Advanced%20Hepatocellular%20Carcinoma&search=Disease_Search_Simple (accessed on 15 August 2022).
- Llovet, J.M.; Kudo, M.; Cheng, A.-L.; Finn, R.S.; Galle, P.R.; Kaneko, S.; Meyer, T.; Qin, S.; Dutcus, C.E.; Chen, E.; et al. Lenvatinib (Len) plus Pembrolizumab (Pembro) for the First-Line Treatment of Patients (Pts) with Advanced Hepatocellular Carcinoma (HCC): Phase 3 LEAP-002 Study. J. Clin. Oncol. 2019, 37 (Suppl. 15), TPS4152. [Google Scholar] [CrossRef]
- Kelley, R.K.; Oliver, J.W.; Hazra, S.; Benzaghou, F.; Yau, T.; Cheng, A.-L.; Rimassa, L. Cabozantinib in Combination with Atezolizumab versus Sorafenib in Treatment-Naive Advanced Hepatocellular Carcinoma: COSMIC-312 Phase III Study Design. Future Oncol. 2020, 16, 1525–1536. [Google Scholar] [CrossRef]
- Guo, J.; Tang, Q. Recent Updates on Chimeric Antigen Receptor T Cell Therapy for Hepatocellular Carcinoma. Cancer Gene Ther. 2021, 28, 1075–1087. [Google Scholar] [CrossRef]
- Dal Bo, M.; De Mattia, E.; Baboci, L.; Mezzalira, S.; Cecchin, E.; Assaraf, Y.G.; Toffoli, G. New Insights into the Pharmacological, Immunological, and CAR-T-Cell Approaches in the Treatment of Hepatocellular Carcinoma. Drug Resist. Updat. 2020, 51, 100702. [Google Scholar] [CrossRef]
| Approved Advanced HCC Systemic Therapies | ||||||
|---|---|---|---|---|---|---|
| Line of Therapy | Drug | Trial | Total Number (N) | Patient Characteristics | Overall Survival (Months) | Progression Free Survival (Months) |
| First Line | Atezolizumab + bevacizumab vs. sorafenib | IMBrave150: Phase III | 336 vs. 165 | Unresectable, CTP-A, ECOG 0-1, no prior systemic therapy | 67.2% (95% CI, 61.3–73.1) vs. 54.6% (45.2–64) at 12 months | 6.8 (95% CI, 5.7–8.3) vs. 4.3 (95% CI, 4.0–5.6) |
| First Line | Lenvatinib vs. Sorafenib | REFLECT: Phase III | 478 vs. 476 | Unresectable without invasion of main portal vein or biliary tree, CTP-A, ECOG 0-1, no prior systemic therapy | 13·6 months (95% CI, 12.1–14.9) vs. 12.3 months (10.4–13.9) NON INFERIOR | 7.4 (95% CI, 6.9–8.8) vs. 3.7 months (95% CI, 3.6 to 4·6 months) ORR: 24.1% vs. 9.2% |
| First Line | Sorafenib vs. placebo | SHARP: Phase III | 299 vs. 303 | Advanced, CTP-A, ECOG 0-1-2 | 10.7 months (95% CI, 9.4–13.3) vs. 7.9 (95% CI, 6.8–9.1) | PFS not assessed Time to radiologic progression: 5.5 (4.1–6.9) vs. 2.8 (2.7–3.9) |
| Second Line | Pembrolizumab vs. Placebo [OS and PFS not reached] | KEYNOTE-240: Phase III | 278 vs. 135 | Progression or intolerance to sorafenib, advanced, CTP-A, ECOG 0-1 | 13.9 months (95% CI, 8.3—4.1) vs. 10.6 months (8.3–13.5) | 3.0 (95% CI, 2.8–4.1) vs. 2.8 (95% CI, 2.5–4.1) |
| Second Line | Ramacirumab vs. Placebo | REACH-2: Phase III | 197 vs. 95 | BCLC-B or C, CTP-A, ECOG 0-1, AFP >399, previous sorafenib use (first use of biomarker) | 8.5 months (95% CI, 7.0–10.6) vs. 7.3 months (5.4–9.1) | 2.8 (95% CI, 2.8–4.1) vs. 1.6 (95% CI, 1.5–2.7) |
| Second Line | Pembrolizumab | KEYNOTE224: Phase II | 104 | Intolerant of or previously treated with Sorafenib, Advanced, CTP-A, ECOG 0-1 | OS not studied Objective Response: 17%; 95% CI, 11–26 | PFF Not studied |
| Second Line | Cabozantinib vs. placebo | CELESTIAL: Phase III | 470 vs. 237 | Advanced HCC that had received at least one, but up to two lines of previous systemic therapy, including sorafenib, CTP-A, ECOC 0-1 | 10.2 months (95% CI, 9.1–12.0) vs. 8.0 (95% CI, 6.4 to 9.4) | 5.2 (95% CI, 4.0–5.5) vs. 1.9 (95% CI, 1.9–1.9) |
| Second Line | 1.Nivolumab 2. Nivolumab plus ipilimumab | CheckMate040: Phase II | (1)262 (2)148 | Advanced, CTP-A or B7, ECOG 0-1, with or without HBV and HCV, previous sorafenib allowed | (1) 9 month OS: 74% (95% CI, 67–79) ORR 20% (95% CI, 15–26) (2) OS 22.8 months (95% CI 9.4–NA) ORR 32% (95% CI 20–47) | |
| Second Line | Regorafenib vs. placebo | RESORCE:Phase III | 379 vs. 194 | Advanced, Progression on sorafenib, CTP-A, ECOG 0-1 | 10.6 months (95% CI, 9.1–12.1) vs. 7.8 (95% CI, 6.3–8.8) | 3.1 (95% CI, 2.8–4.2) vs. 1.5 (1.4–1.6) |
| Trial Identifier | Phase | Setting | Child–Pugh | Treatment Arms | Primary Endpoint |
|---|---|---|---|---|---|
| Checkmate-9DX NCT03383458 | 3 | Adjuvant | Child–Pugh A | Nivolumab vs. placebo | Recurrence-free survival |
| EMERALD-2 NCT03847428 | 3 | Adjuvant | Child–Pugh A | Durvalumab + Bevacizumab Durvalumab + Placebo Placebo + Placebo | Recurrence-free survival |
| KEYNOTE-937 NCT03867084 | 3 | Adjuvant | Child–Pugh A | Pembrolizumab vs. Placebo | Recurrence-free survival |
| Imbrave 050 BCT04102098 | 3 | Adjuvant | Child–Pugh A | Atezolizumab + Bevacizumab vs. Active surveillance | Recurrence-free survival |
| EMERALD-1 NCT03778957 | 3 | Intermediate | Child–Pugh A—B7 | TACE + Durvalumab + placebo vs. Durvalumab + Bevacizumab + TACE vs. Placebo + TACE | Progression free survival |
| LEAP-012 NCT04246177 | 3 | Intermediate | Child–Pugh A | Lenvatinib + Pembrolizumab + TACE vs. Oral placebo + IV placebo + TACE | Progression free survival |
| Checkmate-74W NCT04340193 | 3 | Intermediate | Child–Pugh A | Nivolumab + Ipilimumab + TACE vs. Nivolumab + Placebo + TACE vs. Placebo + Placebo + TACE | Time to TACE Progression |
| RATIONALE-301 NCT03412773 | 3 | Advanced | Child–Pugh A | Tislelizumab vs. Sorafenib | Overall survival |
| Checkmate 9DW NCT04039607 | 3 | Advanced | Child–Pugh A | Nivolumab + Ipilimumab vs. Sorafenib/Lenvatinib | Overall survival |
| LEAP-002 NCT03713593 | 3 | Advanced | Child–Pugh A | Lenvatinib + Pembrolizumab vs. Lenvatinib + Placebo | Progression free survival Overall survival |
| COSMIC312 NCT03755791 | 3 | Advanced | Child–Pugh A | Cabozantinib + Atezolizumab vs. Sorafenib vs. Cabozantinib | Progression free survival Overall survival |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Zahid, S.; Noginskiy, I.; Pak, T.; Usta, S.; Barsoum, M.; Khan, U. A Review of Current and Emerging Therapies for Advanced Hepatocellular Carcinoma. Curr. Oncol. 2022, 29, 6445-6462. https://doi.org/10.3390/curroncol29090507
Singh A, Zahid S, Noginskiy I, Pak T, Usta S, Barsoum M, Khan U. A Review of Current and Emerging Therapies for Advanced Hepatocellular Carcinoma. Current Oncology. 2022; 29(9):6445-6462. https://doi.org/10.3390/curroncol29090507
Chicago/Turabian StyleSingh, Angelica, Sofia Zahid, Ilya Noginskiy, Timothy Pak, Soeb Usta, Marina Barsoum, and Uqba Khan. 2022. "A Review of Current and Emerging Therapies for Advanced Hepatocellular Carcinoma" Current Oncology 29, no. 9: 6445-6462. https://doi.org/10.3390/curroncol29090507
APA StyleSingh, A., Zahid, S., Noginskiy, I., Pak, T., Usta, S., Barsoum, M., & Khan, U. (2022). A Review of Current and Emerging Therapies for Advanced Hepatocellular Carcinoma. Current Oncology, 29(9), 6445-6462. https://doi.org/10.3390/curroncol29090507





