Patterns of First-Line Systemic Therapy Delivery and Outcomes in Advanced Epithelial Ovarian Cancer in Ontario
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Cohort Creation
2.3. Treatment Cohorts
2.4. Explanatory Variables
2.5. Outcome Variables
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Patterns of First-line Systemic Therapy
3.3. Acute Care Utilization during First-Line Treatment
3.4. Overall Survival
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Dataset | Description | Use |
|---|---|---|
| Activity Level Reporting (ALR) | This is the main database on systemic therapy for cancer care in Ontario, which became robust as of 2014 | Determine first-line regimen, hospital site, dates of treatment |
| Discharge Abstract Database (DAD) * | This captures demographic, clinical and administrative information on hospital admissions and discharges, including death. | Hospitalizations related to treatment toxicity during systemic therapy |
| ICES Physician Database (IPDB) | This contains information on physician specialty for those who provided this information | Determine physician type |
| National Ambulatory Care Reporting System (NACRS) * | This captures data for all hospital and community-based ambulatory care including emergency department visits and day surgery. | Determine ED visits and main diagnosis at ED related to toxicity during treatment |
| New Drug Funding Program (NDFP) | This contains all records of provincially funded drugs through Cancer Care Ontario | Determine receipt of bevacizumab |
| Ontario Cancer Registry (OCR) | This is the main registry for all cancer diagnoses in Ontario | Diagnosis and staging, histology and topography |
| Ontario Health Insurance Plan (OHIP) | This records all claims for physician reimbursement of inpatient and outpatient visits, consultations and procedures | Determine physician type, surgical type, referrals and consultations |
| Registered Persons Database (RPDB) | This data provides demographic information including health care card number, date of birth, sex and address | For baseline demographics including age and location |
| Same Day Surgery (SDS) | This records ambulatory visits for day surgeries | For ovarian cancer surgeries |
Appendix B
| Description | |
|---|---|
| C56 C560 C561 C569 | Malignant neoplasm of the ovary Malignant neoplasm of the ovary, unilateral Malignant neoplasm of the ovary, bilateral Malignant neoplasm of the ovary, unspecified unilateral/bilateral |
| C570 C5700 C5701 C5709 | Malignant neoplasm of fallopian tube Malignant neoplasm of fallopian tube, unilateral Malignant neoplasm of fallopian tube, bilateral Malignant neoplasm of fallopian tube, unspecified unilateral/bilateral |
| C48 C481 C482 C484 | Malignant neoplasm of peritoneum Malignant neoplasm of specified part of peritoneum Malignant neoplasm of peritoneum, unspecified Neoplasm of uncertain or unknown behaviour of peritoneum |
| ICD-O Code | Description |
|---|---|
| 80003 80013 80053 80103 80203 80503 81403 82553 82603 83103 83233 83403 83413 83423 83813 84403 84413 84503 84603 84613 89403 89503 89803 90143 | neoplasm, malignant tumor cells, malignant malignant tumor carcinoma NOS carcinoma, undifferentiated NOS papillary carcinoma NOS adenocarcinoma NOS adenocarcinoma of mixed subtypes papillary adenocarcinoma NOS clear cell carcinoma NOS mixed cell adenocarcinoma papillary carcinoma, follicular variant papillary microcarcinoma papillary carcinoma, oxyphilic cell endometrioid adenofibroma, malignant cystadenocarcinoma NOS serous cystadenocarcinoma NOS papillary cystadenocarcinoma NOS papillary serous cystadenocarcinoma serous surface papillary carcinoma mixed tumor, malignant NOS Mullerian mixed tumor carcinosarcoma NOS serous adenoarcinofibroma |
Appendix C
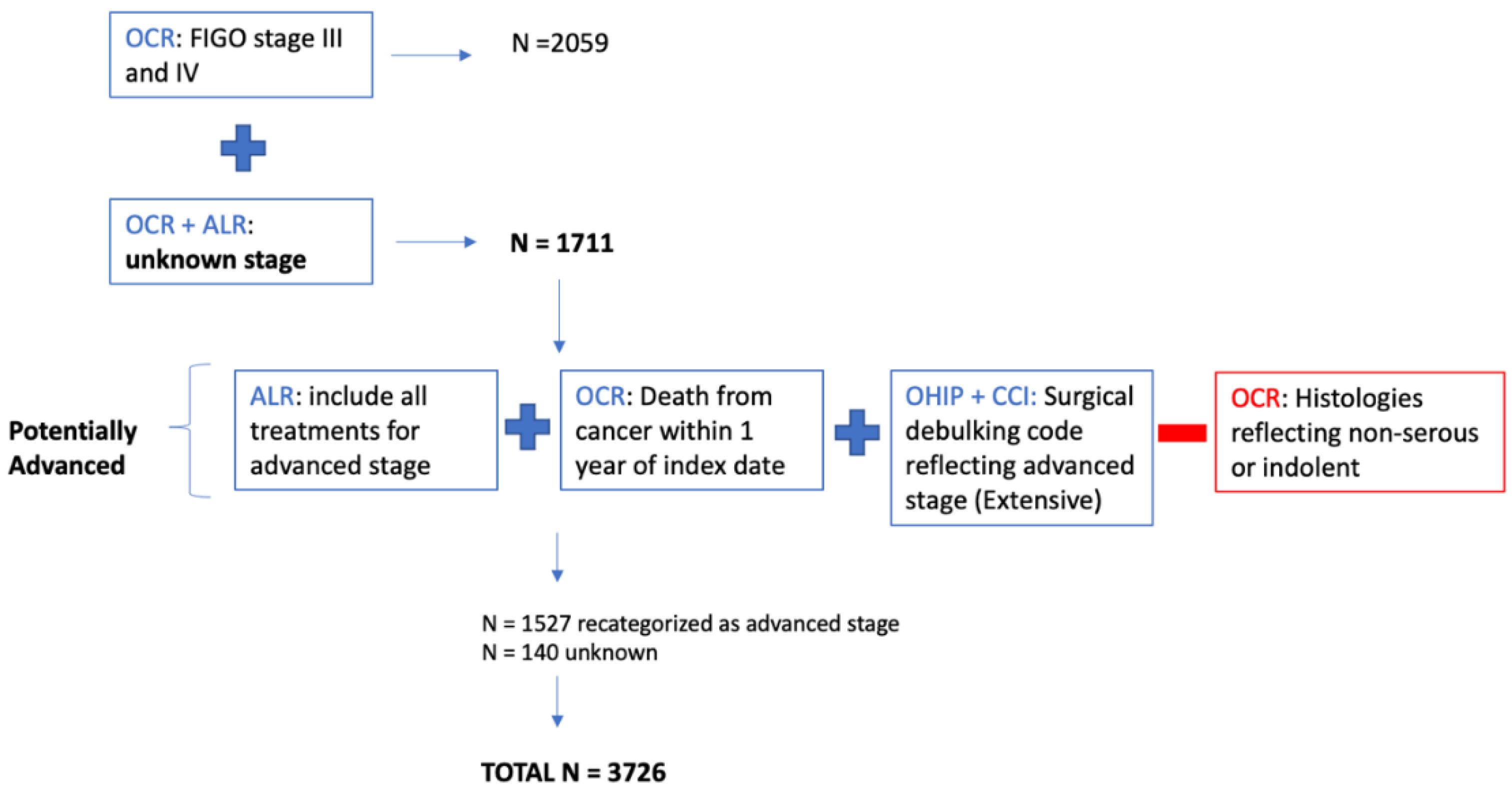
Appendix D
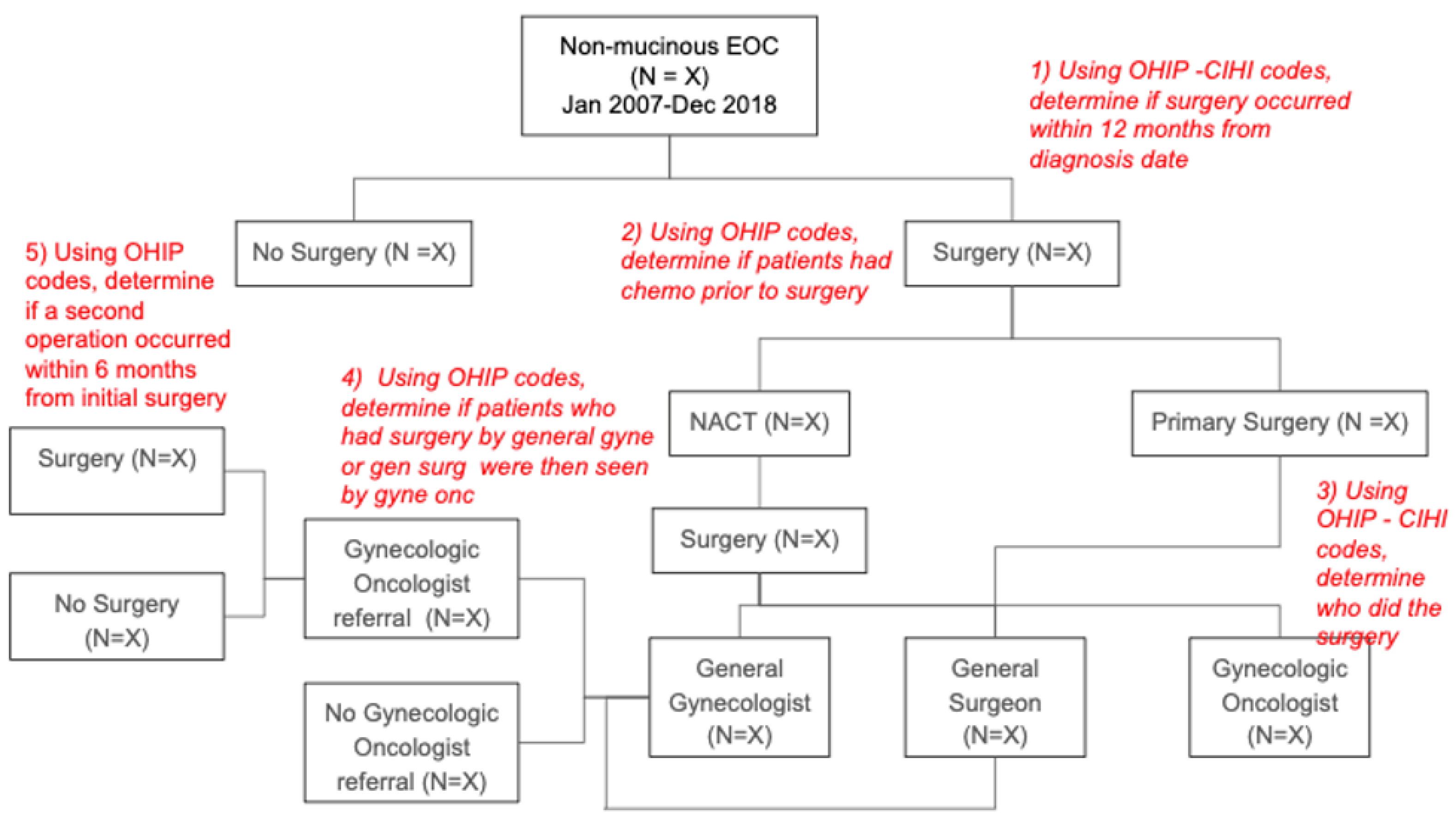
| CIHI-CCI | OHIP |
|---|---|
| Partial excision of uterus and surrounding structures: RM.87.DA-GX - Endoscopic (laparoscopic) approach. RM.87.CA-GX - Per orifice (transvaginal) approach. RM.87.LA-GX - Open approach Total excision of uterus and surrounding structures RM.89.AA - Using combined laparoscopic and vaginal approach RM.89.CA - Using vaginal approach. RM.89.DA - Using endoscopic (laparoscopic) approach. RM.89.LA - Using open approach. Radical excision of uterus and surrounding structures RM.91.AA - Using combined laparoscopic and vaginal approach. RM.91.CA - Using vaginal approach. RM.91.DA - Using endoscopic (laparoscopic) approach. RM.91.LA - Using abdominal approach (includes modified radical hysterectomy
| Debulking surgery
Extensive surgery for stage IV
|
Appendix E
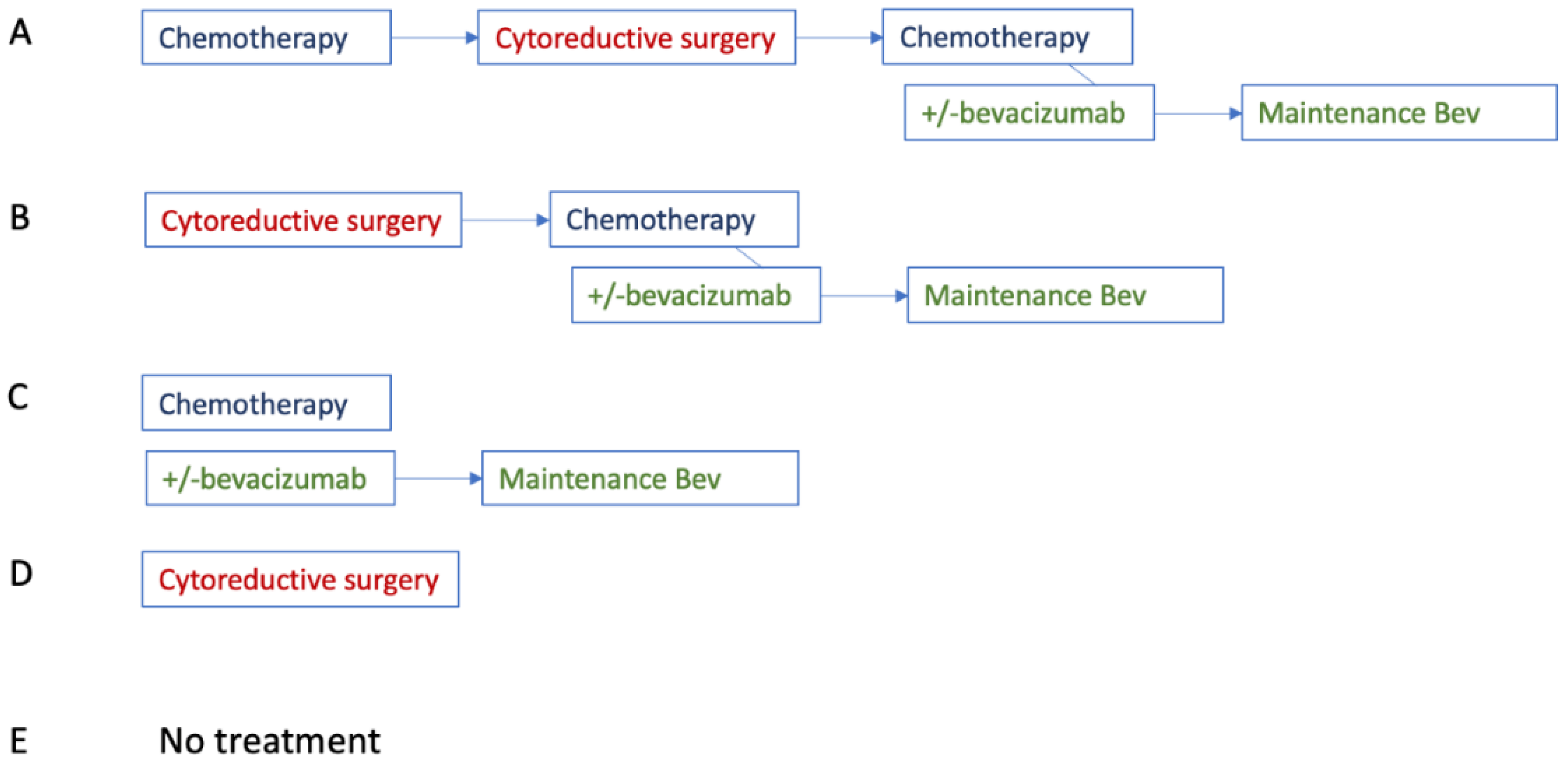
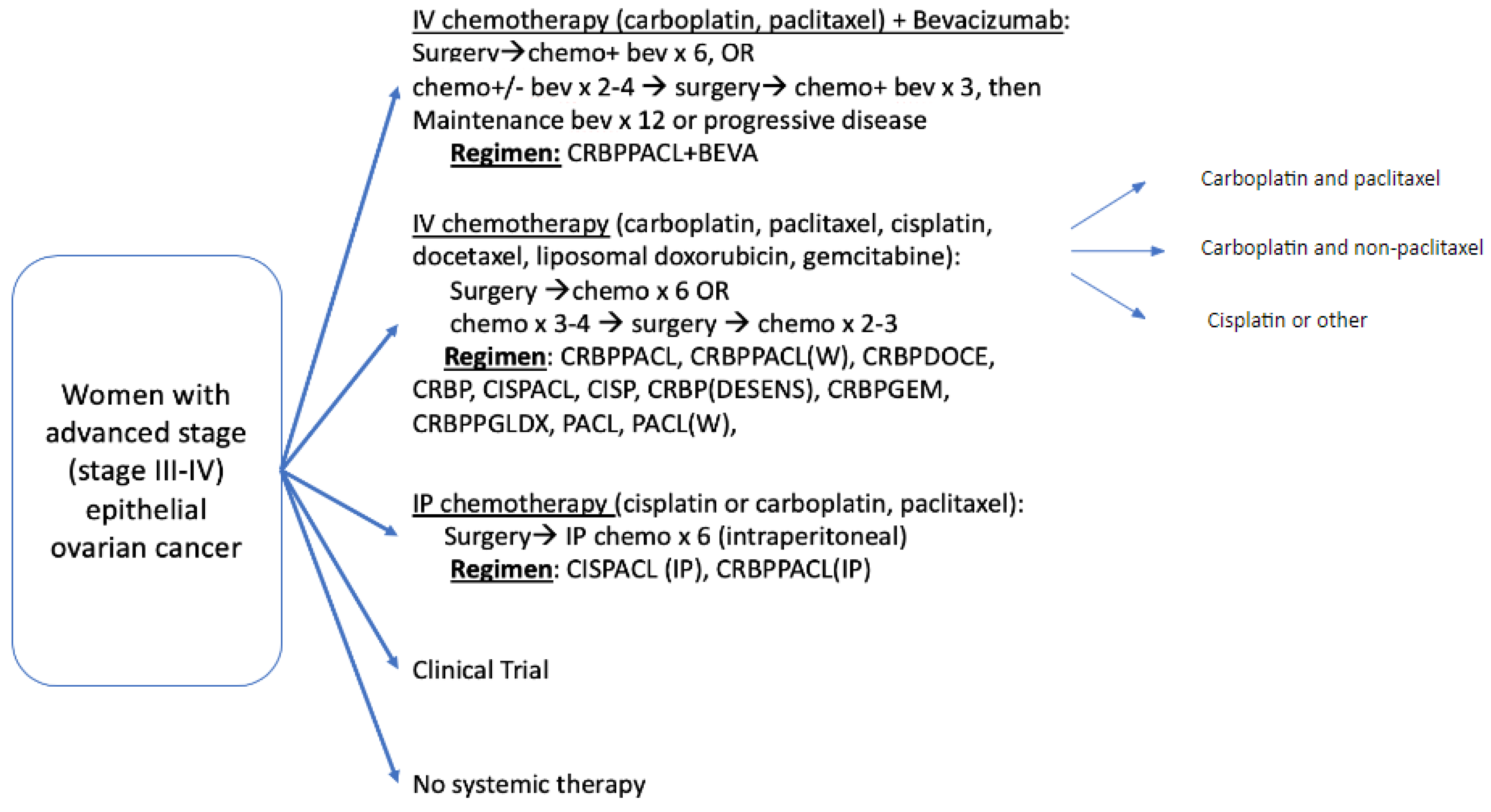
Appendix F
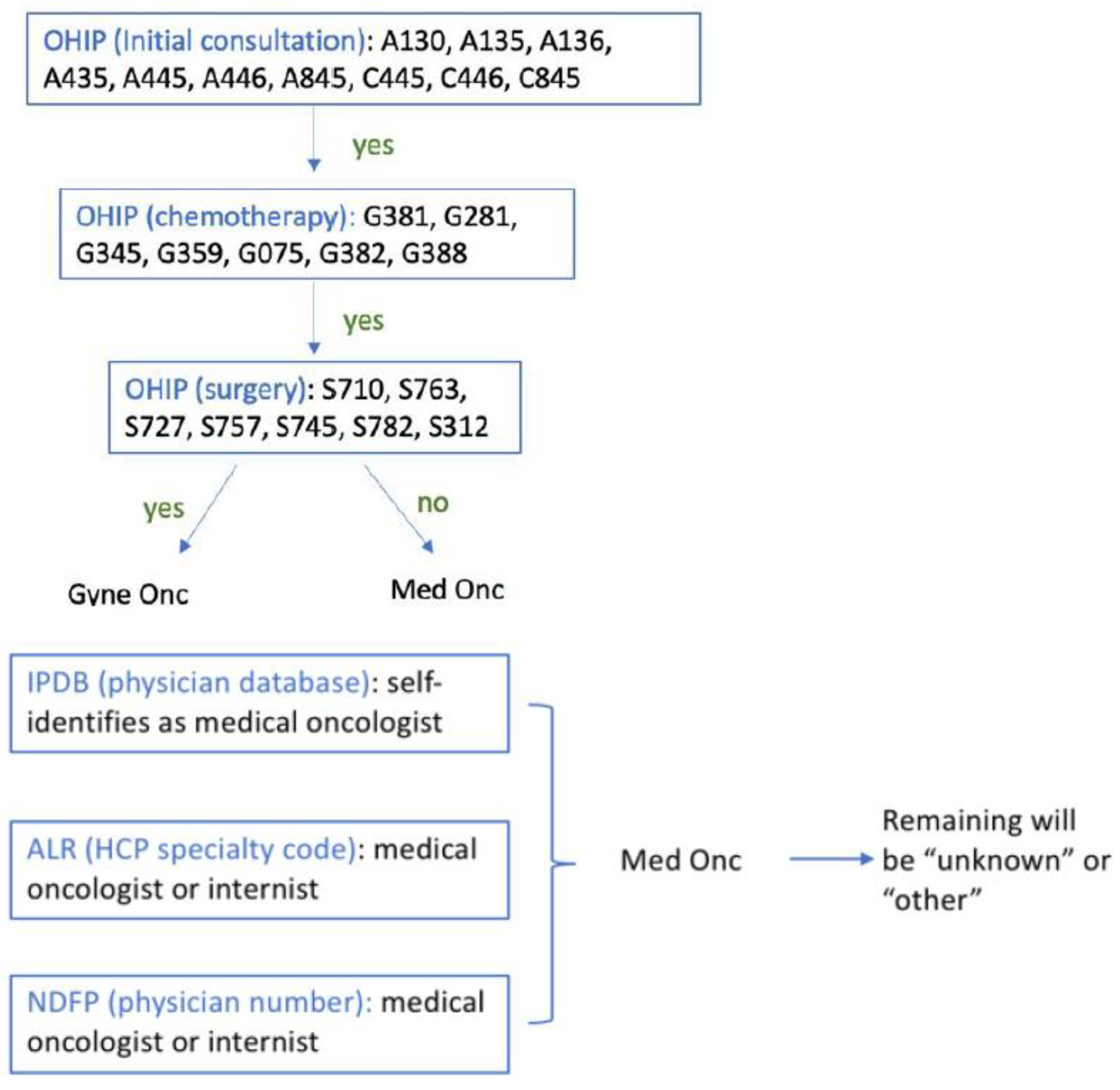
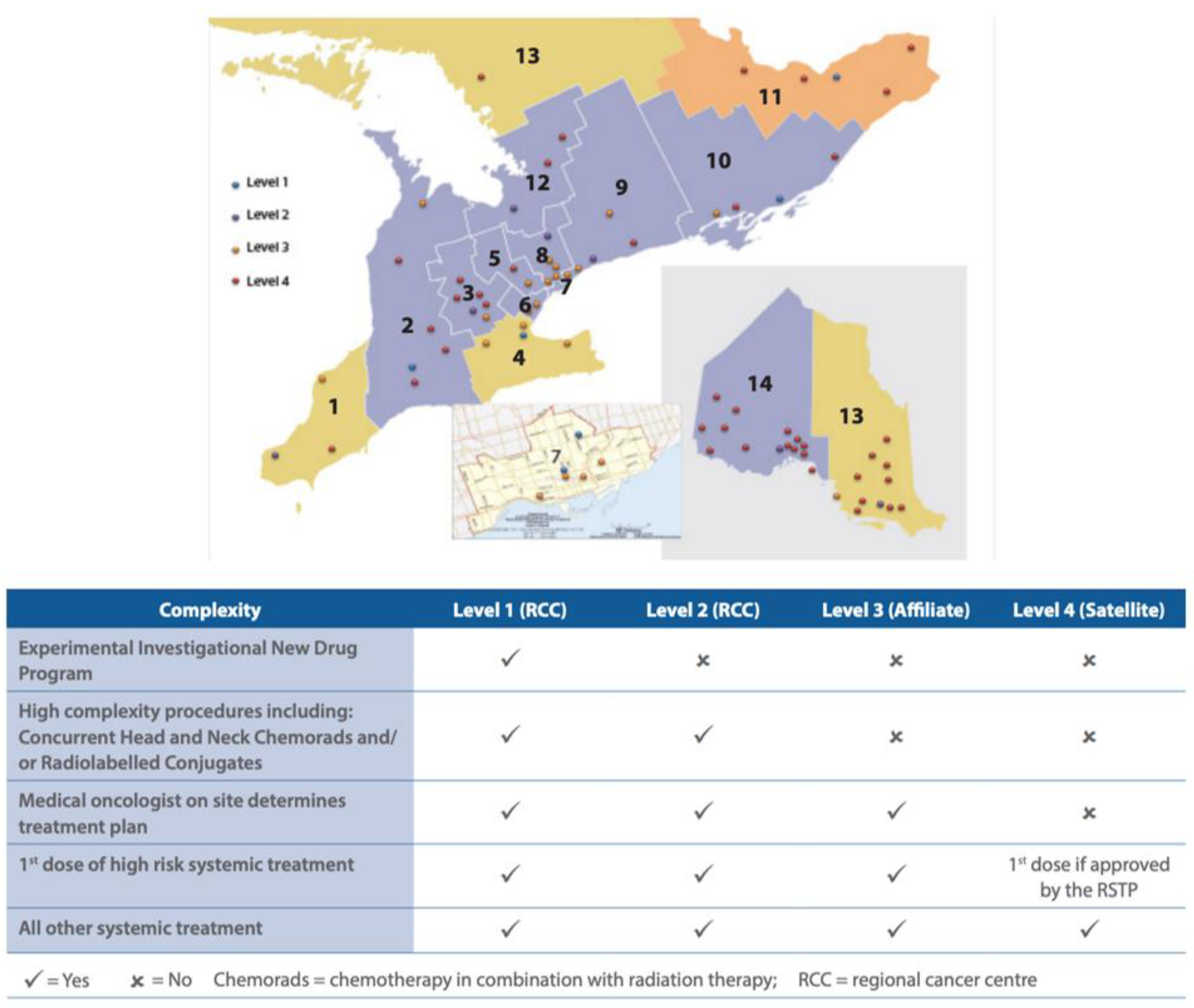
| Facility No. | Facility Legal Name | Facility Level |
|---|---|---|
| 981 | Chatham-Kent Health Alliance | 4 |
| 933 | Windsor Regional Hospital | 2 |
| 966 | Bluewater Health | 3 |
| 793 | St. Thomas Elgin General Hospital | 4 |
| 813 | Huron Perth Healthcare Alliance | 4 |
| 889 | Wingham & District Hospital | 4 |
| 890 | Woodstock General Hospital Trust | 4 |
| 936 | London Health Sciences Centre | 1 |
| 955 | Grey Bruce Health Services | 3 |
| 661 | Cambridge Memorial Hospital | 3 |
| 665 | Guelph General Hospital | 4 |
| 930 | Grand River Hospital | 2 |
| 963 | North Wellington Health Care Corporation | 4 |
| 718 | Joseph Brant Hospital | 3 |
| 942 | Hamilton Health Sciences Corporation | 1 |
| 962 | Niagara Health System | 2 |
| 970 | Brant Community Healthcare System | 3 |
| 916 | Headwaters Health Care Centre | 4 |
| 951 | William Osler Health System | 3 |
| 950 | Halton Healthcare Services Corporation | 3 |
| 975 | Trillium Health Partners | 2 |
| 976 | Sinai Health System | 3 |
| 980 | Unity Health Toronto | 3 |
| 947 | University Health Network | 1 |
| 858 | Toronto East Health Network | 3 |
| 953 | Sunnybrook Health Sciences Centre | 1 |
| 632 | North York General Hospital | 3 |
| 701 | Mackenzie Health | 3 |
| 736 | Southlake Regional Health Centre | 2 |
| 905 | Markham Stouffville Hospital Corporation | 3 |
| 941 | Humber River Hospital | 3 |
| 771 | Peterborough Regional Health Centre | 3 |
| 940 | Northumberland Hills Hospital | 3 |
| 952 | Lakeridge Health | 2 |
| 979 | Scarborough Health Network | 3 |
| 619 | Brockville General Hospital | 4 |
| 693 | Kingston General Hospital | 1 |
| 592 | Lennox and Addington County General Hospital | 4 |
| 928 | Perth and Smiths Falls District Hospital | 4 |
| 957 | Quinte Health Care | 3 |
| 763 | Pembroke Regional Hospital Inc. | 4 |
| 788 | Renfrew Victoria Hospital | 4 |
| 800 | Hopital General de Hawkesbury & District General Hospital Inc. | 4 |
| 882 | Winchester District Memorial Hospital | 4 |
| 967 | Cornwall Community Hospital | 4 |
| 958 | The Ottawa Hospital | 1 |
| 606 | Royal Victoria Regional Health Centre | 2 |
| 968 | Muskoka Algonquin Healthcare | 4 |
| 745 | Orillia Soldiers’ Memorial Hospital | 4 |
| 638 | The Lady Minto Hospital | 4 |
| 650 | St. Joseph’s General Hospital Elliot Lake | 4 |
| 687 | Sensenbrenner Hospital | 4 |
| 784 | Manitoulin Health Centre | 4 |
| 881 | Hopital General de Nipissing Ouest/The West Nipissing General Hospital | 4 |
| 888 | Temiskaming Hospital | 4 |
| 974 | North Bay Regional Health Centre | 4 |
| 681 | Hôpital Notre-Dame Hospital (Hearst) | 4 |
| 907 | Timmins and District Hospital | 4 |
| 696 | Kirkland and District Hospital | 4 |
| 931 | West Parry Sound Health Centre | 4 |
| 959 | Health Sciences North / Horizon Santé Nord | 2 |
| 965 | Sault Area Hospital | 3 |
| 600 | Atikokan General Hospital | 4 |
| 647 | Dryden Regional Health Centre | 4 |
| 662 | Geraldton District Hospital | 4 |
| 719 | Manitouwadge General Hospital | 4 |
| 977 | North of Superior Healthcare Group | 4 |
| 826 | Lake of the Woods District Hospital | 4 |
| 896 | The Red Lake Margaret Cochenour Memorial Hospital Corporation | 4 |
| 900 | Riverside Health Care Facilities Inc. | 4 |
| 935 | Thunder Bay Regional Health Sciences Centre | 2 |
| 964 | Sioux Lookout Meno-Ya-Win Health Centre | 4 |
Appendix G
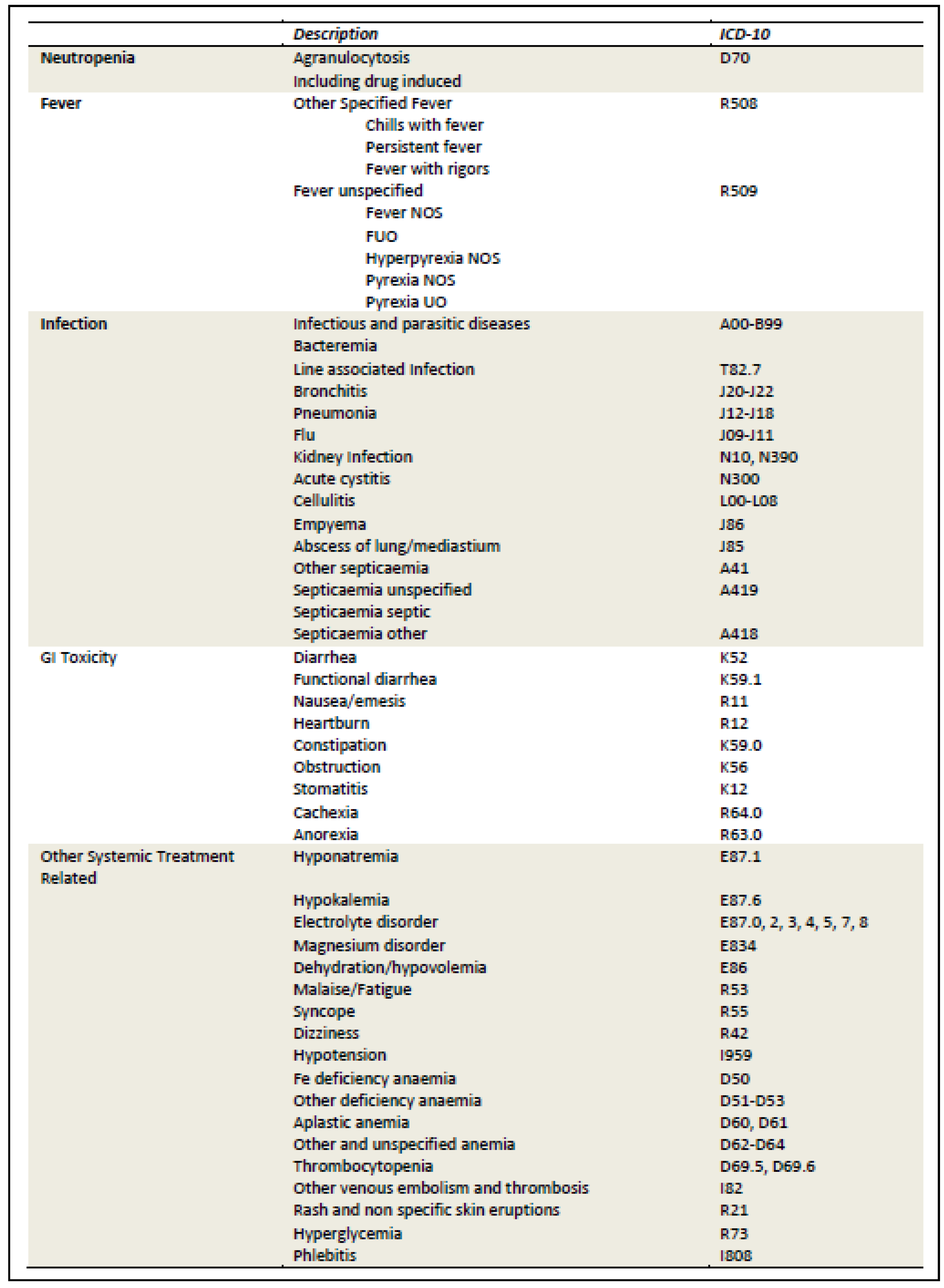
Appendix H
| Cohort D (Surgery Only) N = 290 | Cohort E (No Treatment) N = 598 | |
|---|---|---|
| Stage III or IV Missing | 266 (92%) 24 (9%) | 539 (90%) 59 (10%) |
| Age group Less than 70 70–79 80+ | 133 (45%) 90 (31%) 67 (23%) | 140 (23%) 116 (19%) 342 (57%) |
| Histology Serous carcinoma Neoplasm, carcinoma or adenocarcinoma | 138 (47%) 55 (19%) | 88 (15%) 461 (77%) |
| Death during follow-up | 212 (73%) | 556 (93%) |
Appendix I
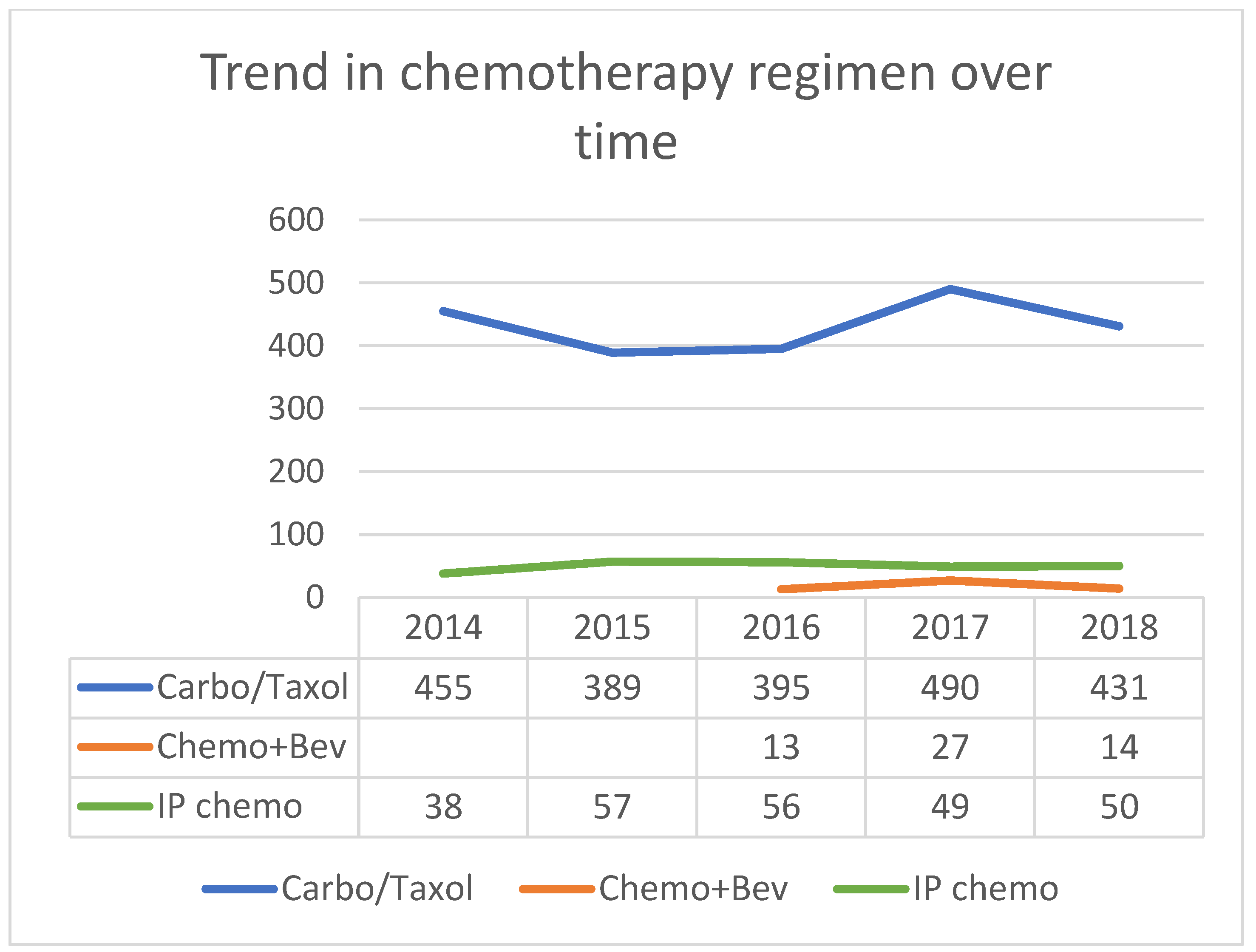
| Chemotherapy Regimen | Total N (%) | Providers | Facility Levels | ||
|---|---|---|---|---|---|
| Gyne Onc | Med Onc | Level 1 | Level 2–4 | ||
| IV carboplatin + Taxol | 2160 (76.1%) | 1286 | 808 | 1189 | 971 |
| IV carboplatin + non-Taxol | 341 (12%) | 178 | 133 | 204 | 137 |
| IP chemotherapy | 250 (8.8%) | 266 | 24 | 212 | 38 |
| IV chemotherapy + bev | 54 (1.9% *) | 17 | 37 | 16 | 38 |
| Other (including cisplatin and trial) | 33 (1.2%) | ||||
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef]
- Wright, A.A.; Bohlke, K.; Armstrong, D.K.; Bookman, M.A.; Cliby, W.A.; Coleman, R.L.; Dizon, D.S.; Kash, J.J.; Meyer, L.A.; Moore, K.N.; et al. Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 3460–3473. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Copeland, L.J.; Walker, J.L.; Burger, R.A. Intraperitoneal Cisplatin and Paclitaxel in Ovarian Cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A Phase 3 Trial of Bevacizumab in Ovarian Cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of Bevacizumab in the Primary Treatment of Ovarian Cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef]
- Aghajanian, C.; Blank, S.V.; Goff, B.A.; Judson, P.L.; Teneriello, M.G.; Husain, A.; Sovak, M.A.; Yi, J.; Nycum, L.R. OCEANS: A Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Chemotherapy With or Without Bevacizumab in Patients With Platinum-Sensitive Recurrent Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancer. J. Clin. Oncol. 2012, 30, 2039–2045. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Hilpert, F.; Weber, B.; Reuss, A.; Poveda, A.; Kristensen, G.; Sorio, R.; Vergote, I.; Witteveen, P.; Bamias, A.; et al. Bevacizumab Combined With Chemotherapy for Platinum-Resistant Recurrent Ovarian Cancer: The AURELIA Open-Label Randomized Phase III Trial. J. Clin. Oncol. 2014, 32, 1302–1308. [Google Scholar] [CrossRef]
- Liu, S.; Kasherman, L.; Fazelzad, R.; Wang, L.; Bouchard-Fortier, G.; Lheureux, S.; Krzyzanowska, M.K. The use of bevacizumab in the modern era of targeted therapy for ovarian cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2021, 161, 601–612. [Google Scholar] [CrossRef]
- pCODR EXPERT REVIEW COMMITTEE(pERC) FINAL RECOMMENDATION. Available online: https://www.cadth.ca/sites/default/files/pcodr/pcodr_bevacizumab_avastin_oc-fn_rec.pdf. (accessed on 12 December 2020).
- Reade, C.; Elit, L. Trends in Gynecologic Cancer Care in North America. Obstet. Gynecol. Clin. North Am. 2012, 39, 107–129. [Google Scholar] [CrossRef]
- Hillner, B.E.; Smith, T.; Desch, C.E. Hospital and Physician Volume or Specialization and Outcomes in Cancer Treatment: Importance in Quality of Cancer Care. J. Clin. Oncol. 2000, 18, 2327–2340. [Google Scholar] [CrossRef]
- Giede, K.C.; Kieser, K.; Dodge, J.; Rosen, B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol. Oncol. 2005, 99, 447–461. [Google Scholar] [CrossRef]
- Forbes, L.; Durocher-Allen, L.D.; Evidence-Based, K.V.I. Regional models of care for systemic treatment: Standards for the organization and delivery of systemic treatment. 2019. Available online: https://www.cancercareontario.ca/en/file/56426/download?token=cplZhXem (accessed on 10 February 2021).
- Vandenberg, T.; Coakley, N.; Nayler, J.; DeGrasse, C.; Green, E.; A Mackay, J.; McLennan, C.; Smith, A.; Wilcock, L.; E Trudeau, M. A Framework for the Organization and Delivery of Systemic Treatment. Curr. Oncol. 2009, 16, 4–15. [Google Scholar] [CrossRef][Green Version]
- Kagedan, D.J.; Abraham, L.; Goyert, N.; Li, Q.; Paszat, L.F.; Kiss, A.; Earle, C.C.; Mittmann, N.; Coburn, N.G. Beyond the dollar: Influence of sociodemographic marginalization on surgical resection, adjuvant therapy, and survival in patients with pancreatic cancer. Cancer 2016, 122, 3175–3182. [Google Scholar] [CrossRef]
- Enright, K.; Grunfeld, E.; Yun, L.; Moineddin, R.; Ghannam, M.; Dent, S.; Eisen, A.; Trudeau, M.; Kaizer, L.; Earle, C.; et al. Population-Based Assessment of Emergency Room Visits and Hospitalizations Among Women Receiving Adjuvant Chemotherapy for Early Breast Cancer. J. Oncol. Pr. 2015, 11, 126–132. [Google Scholar] [CrossRef]
- Fung-Kee-Fung, M.; Kennedy, E.B.; Biagi, J.; Colgan, T.; D’Souza, D.; Elit, L.M.; Hunter, A.; Irish, J.; McLeod, R.; Rosen, B. An Organizational Guideline for Gynecologic Oncology Services. Int. J. Gynecol. Cancer 2015, 25, 551–558. [Google Scholar] [CrossRef]
- Barber, E.L.; Dusetzina, S.B.; Stitzenberg, K.B.; Rossi, E.C.; Gehrig, P.A.; Boggess, J.F.; Garrett, J.M. Variation in neoadjuvant chemotherapy utilization for epithelial ovarian cancer at high volume hospitals in the United States and associated survival. Gynecol. Oncol. 2017, 145, 500–507. [Google Scholar] [CrossRef]
- Rauh-Hain, J.A.; Melamed, A.; Wright, A.; Gockley, A.; Clemmer, J.T.; Schorge, J.O.; Del Carmen, M.G.; Keating, N.L. Overall Survival Following Neoadjuvant Chemotherapy vs Primary Cytoreductive Surgery in Women with Epithelial Ovarian Cancer. JAMA Oncol. 2017, 3, 76–82. [Google Scholar] [CrossRef]
- Leiserowitz, G.S.; Lin, J.F.; Tergas, A.I.; Cliby, W.A.; Bristow, R.E. Factors Predicting Use of Neoadjuvant Chemotherapy Compared with Primary Debulking Surgery in Advanced Stage Ovarian Cancer—A National Cancer Database Study. Int. J. Gynecol. Cancer 2017, 27, 675–683. [Google Scholar] [CrossRef]
- Coleridge, S.L.; Bryant, A.; Kehoe, S.; Morrison, J. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst. Rev. 2021, 2, CD005343. [Google Scholar] [CrossRef]
- Melamed, A.; Rauh-Hain, J.; Knisely, A.; Clair, C.S.; Tergas, A.; Collado, F.K.; Hou, J.; Wright, J. The effect of liberal versus restrictive use of neoadjuvant chemotherapy (NACT) for ovarian cancer on postoperative mortality and long-term survival: A quasi-experimental study. Gynecol. Oncol. 2020, 159, 81. [Google Scholar] [CrossRef]
- Mercado, C.; Zingmond, D.; Karlan, B.Y.; Sekaris, E.; Gross, J.; Maggard-Gibbons, M.; Tomlinson, J.S.; Ko, C.Y. Quality of care in advanced ovarian cancer: The importance of provider specialty. Gynecol. Oncol. 2010, 117, 18–22. [Google Scholar] [CrossRef]
- Earle, C.C.; Schrag, D.; Neville, B.A.; Yabroff, K.R.; Topor, M.; Fahey, A.; Trimble, E.L.; Bodurka, D.; Bristow, R.E.; Carney, M.; et al. Effect of Surgeon Specialty on Processes of Care and Outcomes for Ovarian Cancer Patients. JNCI: J. Natl. Cancer Inst. 2006, 98, 172–180. [Google Scholar] [CrossRef]
- Silber, J.H.; Rosenbaum, P.R.; Polsky, D.; Ross, R.N.; Even-Shoshan, O.; Schwartz, J.S.; Armstrong, K.A.; Randall, T.C. Does Ovarian Cancer Treatment and Survival Differ by the Specialty Providing Chemotherapy? J. Clin. Oncol. 2007, 25, 1169–1175. [Google Scholar] [CrossRef]
- Bouchard-Fortier, G.; Gien, L.T.; Sutradhar, R.; Chan, W.C.; Krzyzanowska, M.K.; Liu, S.L.; Ferguson, S.E. Impact of care by gynecologic oncologists on primary ovarian cancer survival: A population-based study. Gynecol. Oncol. 2022, 164, 522–528. [Google Scholar] [CrossRef]
- Falzone, L.; Scandurra, G.; Lombardo, V.; Gattuso, G.; Lavoro, A.; Distefano, A.B.; Scibilia, G.; Scollo, P. A multidisciplinary approach remains the best strategy to improve and strengthen the management of ovarian cancer (Review). Int. J. Oncol. 2021, 59, 1–14. [Google Scholar] [CrossRef]
- Liu, S.L.; Lee, Y.C.; Jivraj, N.; Bowering, V.; Wang, L.; Bhat, G.; Madariaga, A.; Kasherman, L.; Nathwani, K.; Tesfu, A.; et al. Risk stratified multidisciplinary ambulatory management of malignant bowel obstruction (MAMBO) program for women with gynecological cancers: Preliminary results from a prospective single-center study. J. Clin. Oncol. 2020, 38, 6062. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]

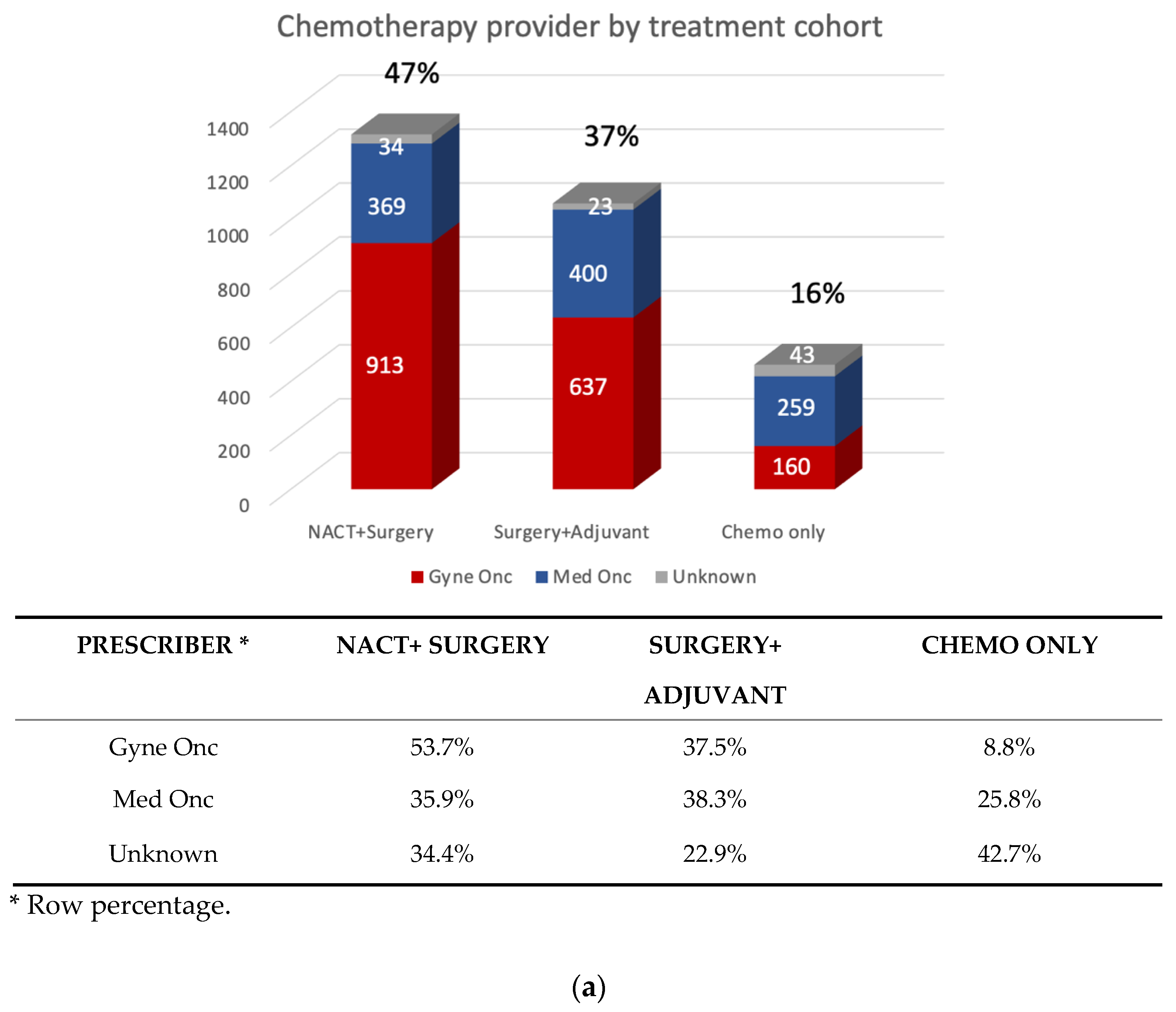
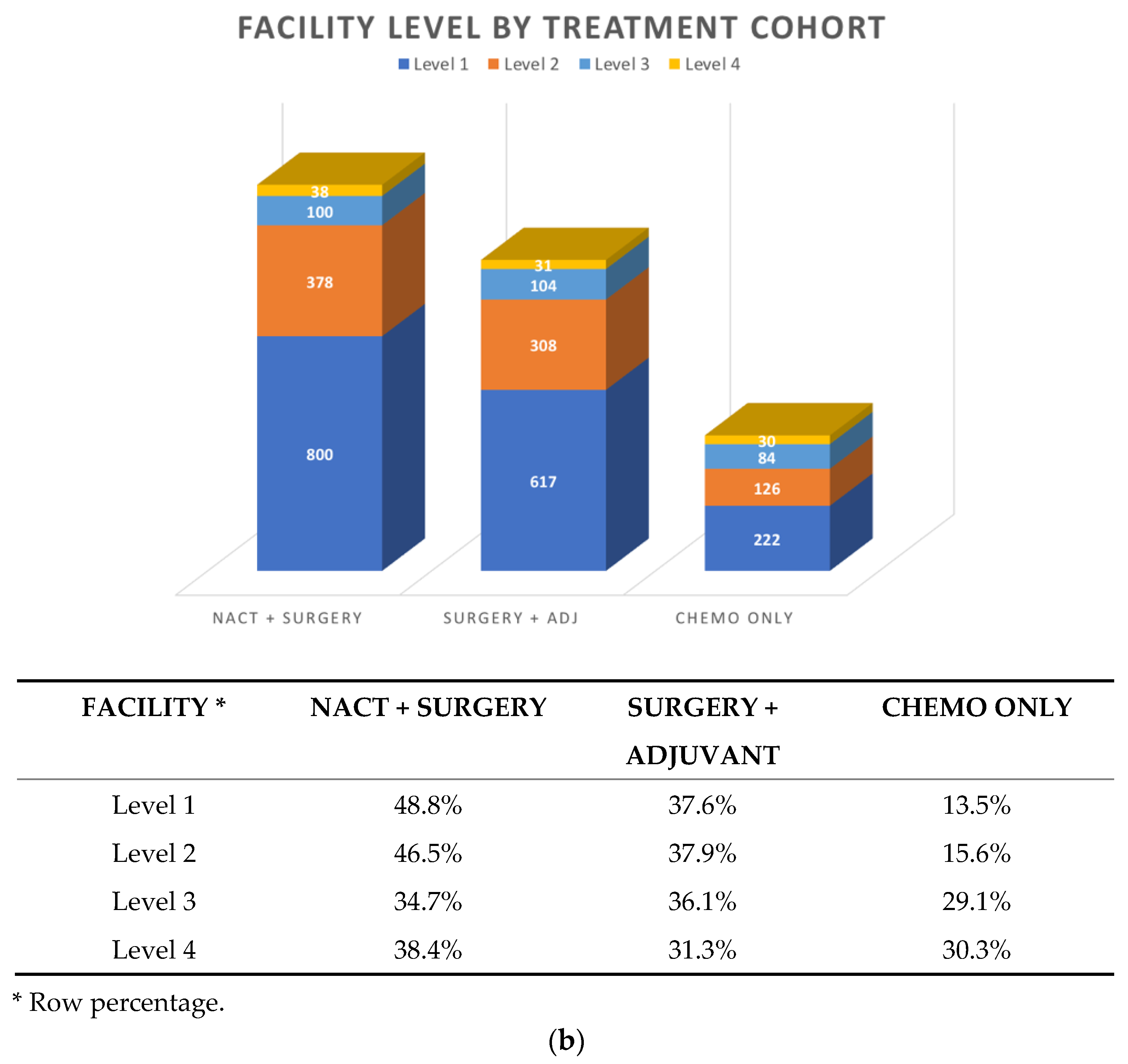
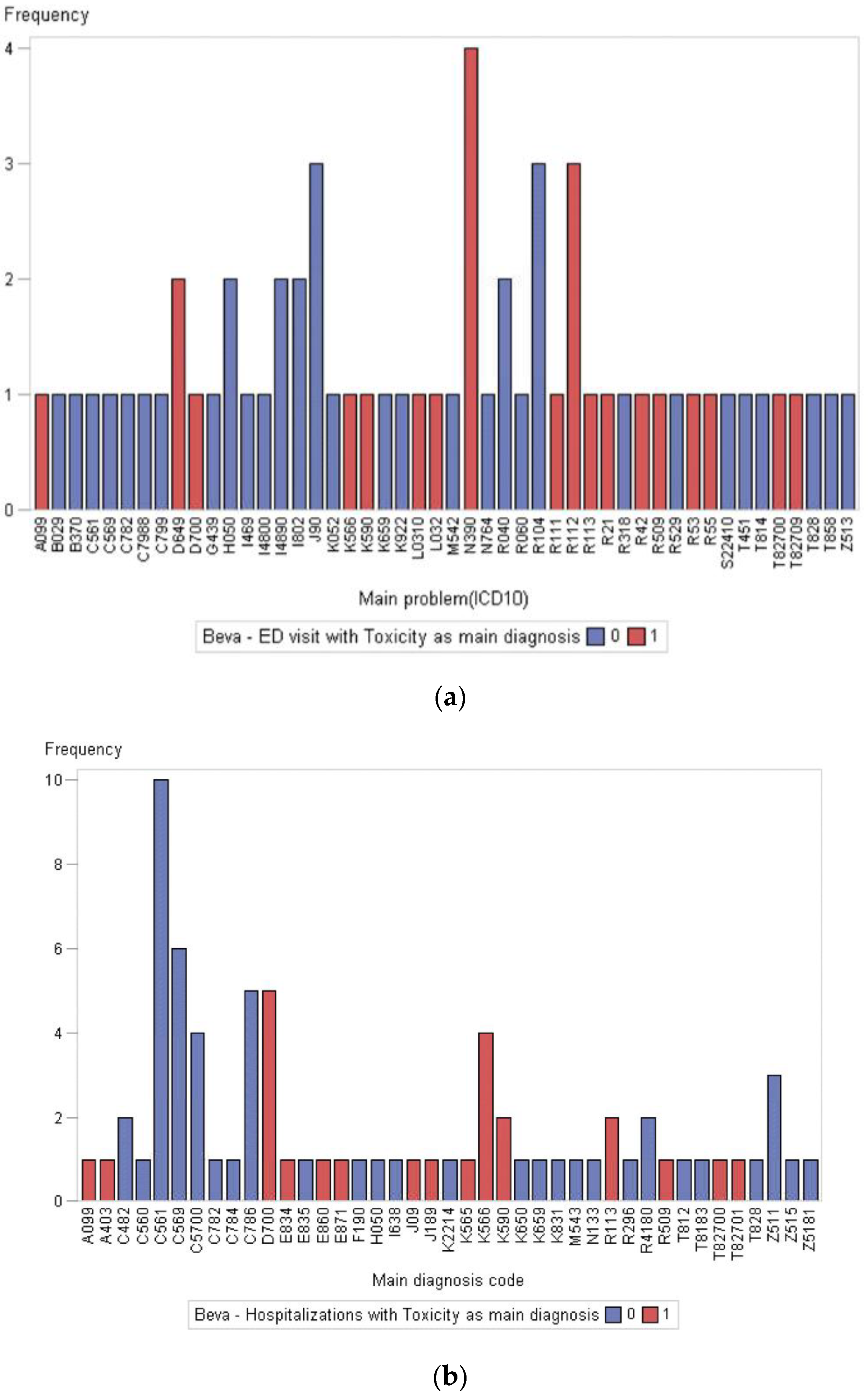
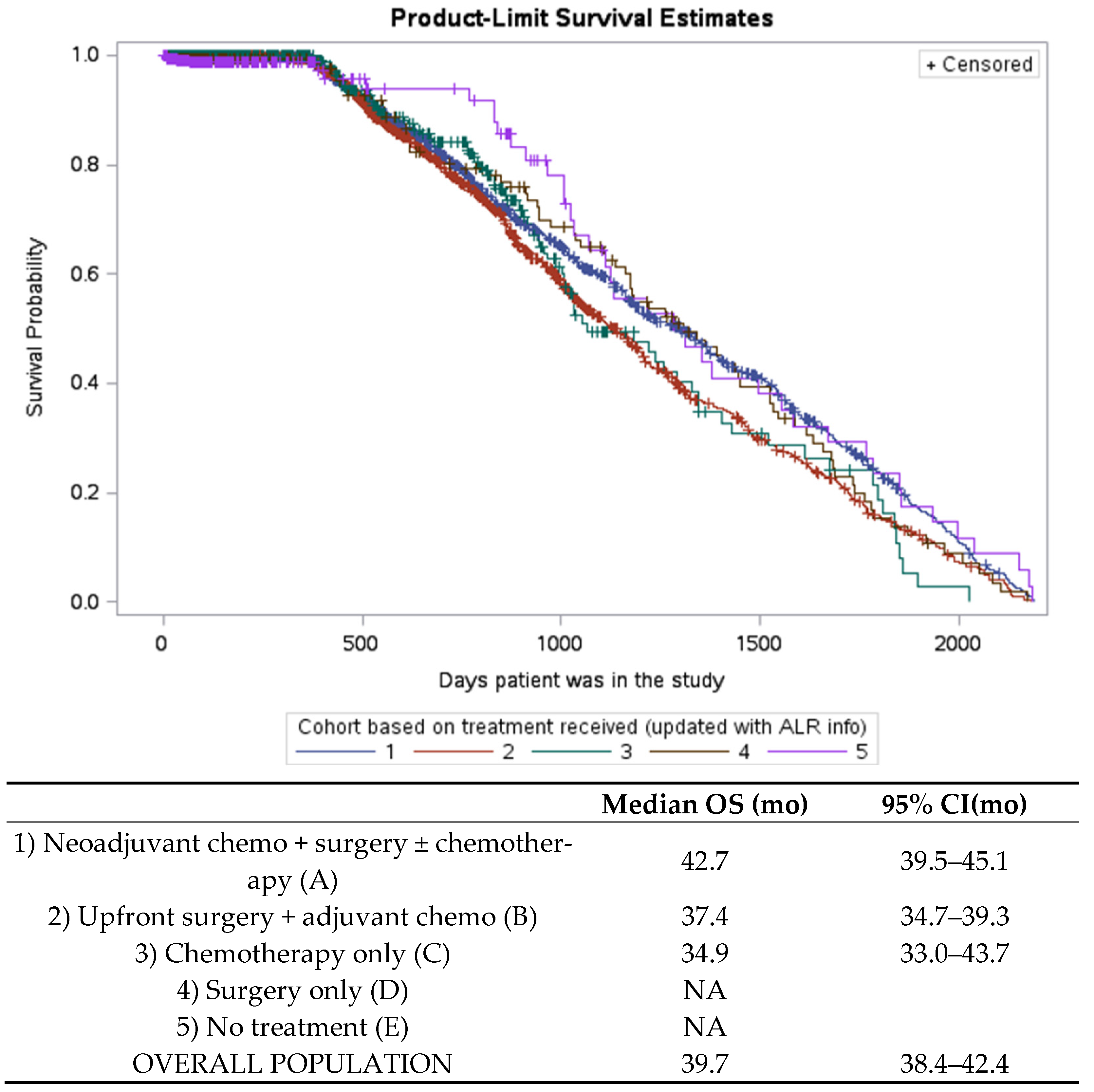
| N (%) | |
|---|---|
| Age | Median: 67 Range: 20–100 |
| Charlson score 0–2 ≥3 | 3634 (97.5%) 92 (2.5%) |
| Rurality score Urban Q1 * Q2 Q3 Q4 Q5 Rural | 3396 (91.0%) 694 (20.4%) 708 (20.8%) 633 (18.6%) 641 (18.8%) 720 (21.2%) 330 (9.0%) |
| Diagnosis year 2014 2015 2016 2017 2018 | 747 (20%) 708 (19%) 754 (20%) 822 (22%) 695 (19%) |
| Histology Serous carcinoma Adenocarcinoma NOS Neoplasm NOS Carcinoma NOS Other | 2395 (64.3%) 418 (11.2%) 314 (8.4%) 164 (4.4%) 435 (11.7%) |
| Stage Advanced (III and IV) Unknown | 3586 (96.2%) 140 (3.8%) |
| Prescriber | Level 1 | Level 2 | Level 3 | Level 4 |
|---|---|---|---|---|
| Gyne Onc | 1384 (84.4%) | 285 (35%) | 20 (7%) | 21 (21%) |
| Med Onc | 187 (11.4%) | 512 (63%) | 255 (88%) | 74 (75%) |
| Unknown/Other | 68 (4.2%) | 15 (2%) | 17 (level 3–4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.L.; Chan, W.C.; Bouchard-Fortier, G.; Lheureux, S.; Ferguson, S.E.; Krzyzanowska, M.K. Patterns of First-Line Systemic Therapy Delivery and Outcomes in Advanced Epithelial Ovarian Cancer in Ontario. Curr. Oncol. 2022, 29, 5988-6009. https://doi.org/10.3390/curroncol29080472
Liu SL, Chan WC, Bouchard-Fortier G, Lheureux S, Ferguson SE, Krzyzanowska MK. Patterns of First-Line Systemic Therapy Delivery and Outcomes in Advanced Epithelial Ovarian Cancer in Ontario. Current Oncology. 2022; 29(8):5988-6009. https://doi.org/10.3390/curroncol29080472
Chicago/Turabian StyleLiu, Shiru L., Wing C. Chan, Geneviève Bouchard-Fortier, Stephanie Lheureux, Sarah E. Ferguson, and Monika K. Krzyzanowska. 2022. "Patterns of First-Line Systemic Therapy Delivery and Outcomes in Advanced Epithelial Ovarian Cancer in Ontario" Current Oncology 29, no. 8: 5988-6009. https://doi.org/10.3390/curroncol29080472
APA StyleLiu, S. L., Chan, W. C., Bouchard-Fortier, G., Lheureux, S., Ferguson, S. E., & Krzyzanowska, M. K. (2022). Patterns of First-Line Systemic Therapy Delivery and Outcomes in Advanced Epithelial Ovarian Cancer in Ontario. Current Oncology, 29(8), 5988-6009. https://doi.org/10.3390/curroncol29080472






