The Screening and COnsensus Based on Practices and Evidence (SCOPE) Program Results of a Survey on Daily Practice Patterns for Patients with Metastatic Colorectal Cancer—A Swiss Perspective in the Context of an International Viewpoint
Abstract
:1. Introduction
2. Materials and Methods
- Case 1: A fit and active 54-year-old male with a left-sided, KRAS-wildtype colon adenocarcinoma
- Case 2: A 68-year-old female with a KRAS-mutated left-sided colon adenocarcinoma, comorbidities, and previous tolerability issues
- Case 3: An 82-year-old male with a KRAS-wildtype right-sided colon adenocarcinoma had comorbidities, limited support, and limited hospital accessibility
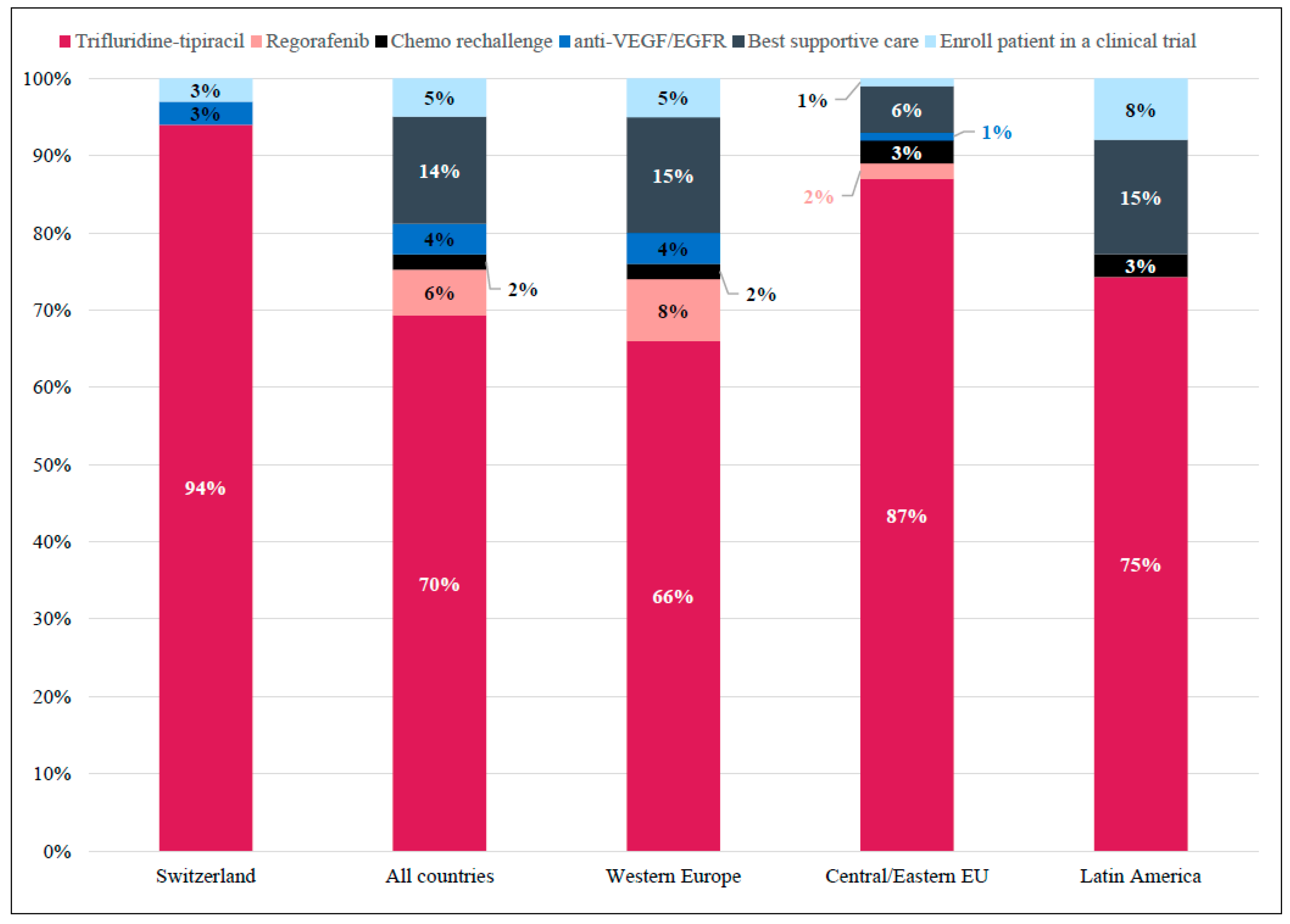
3. Results
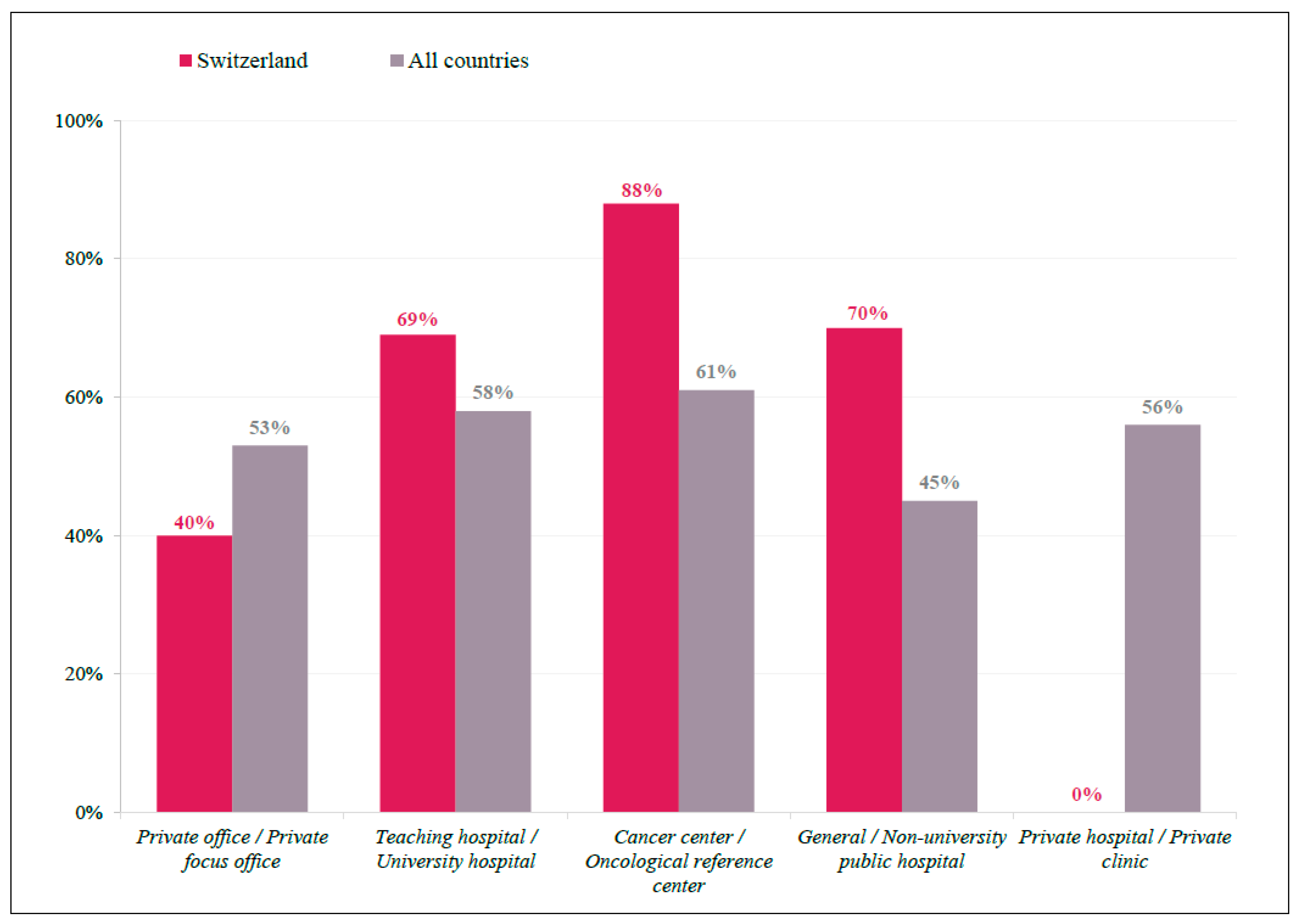
3.1. Participant Demographics
3.2. Practice Patterns
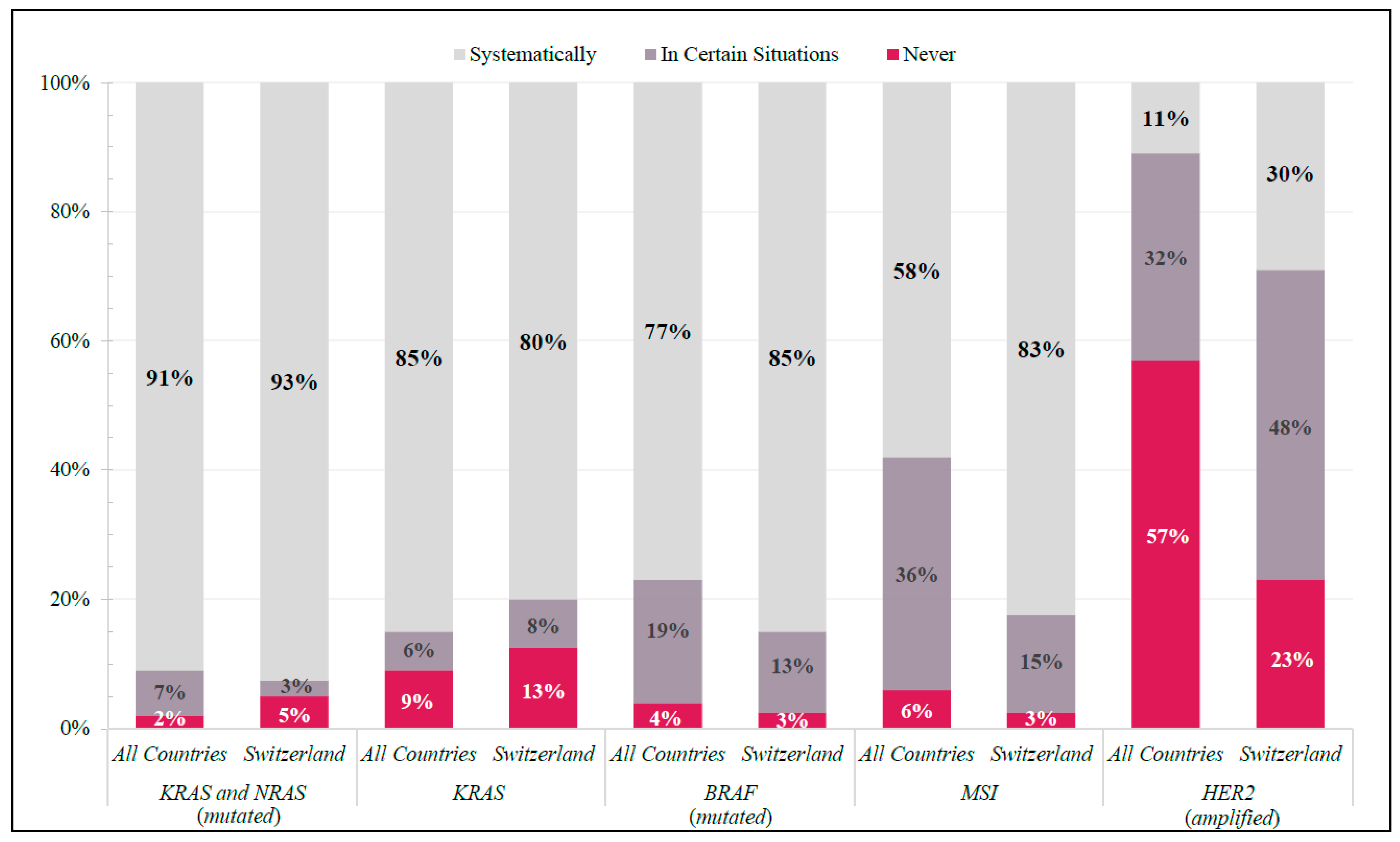
3.2.1. Molecular Diagnostics
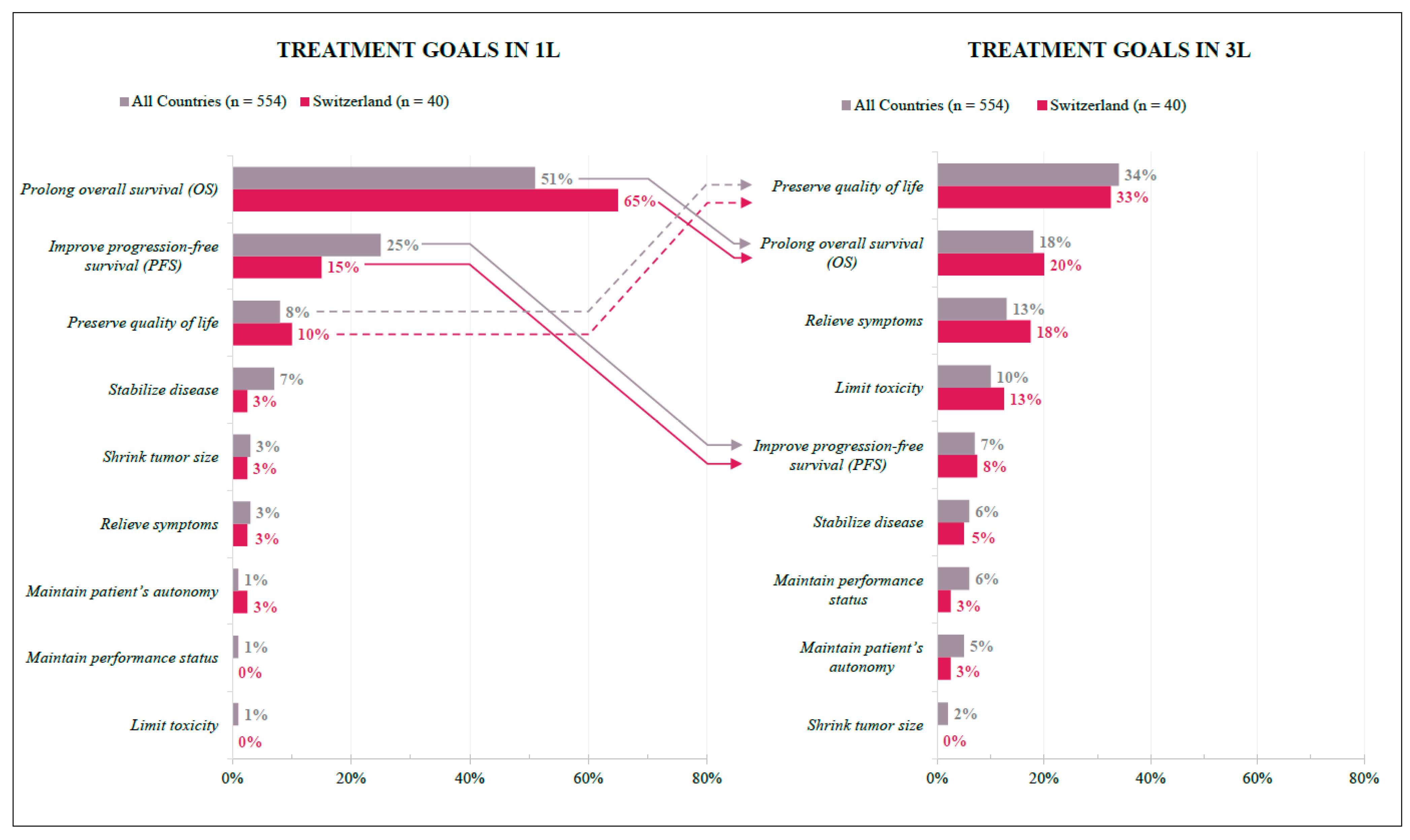
3.2.2. Treatment Goals
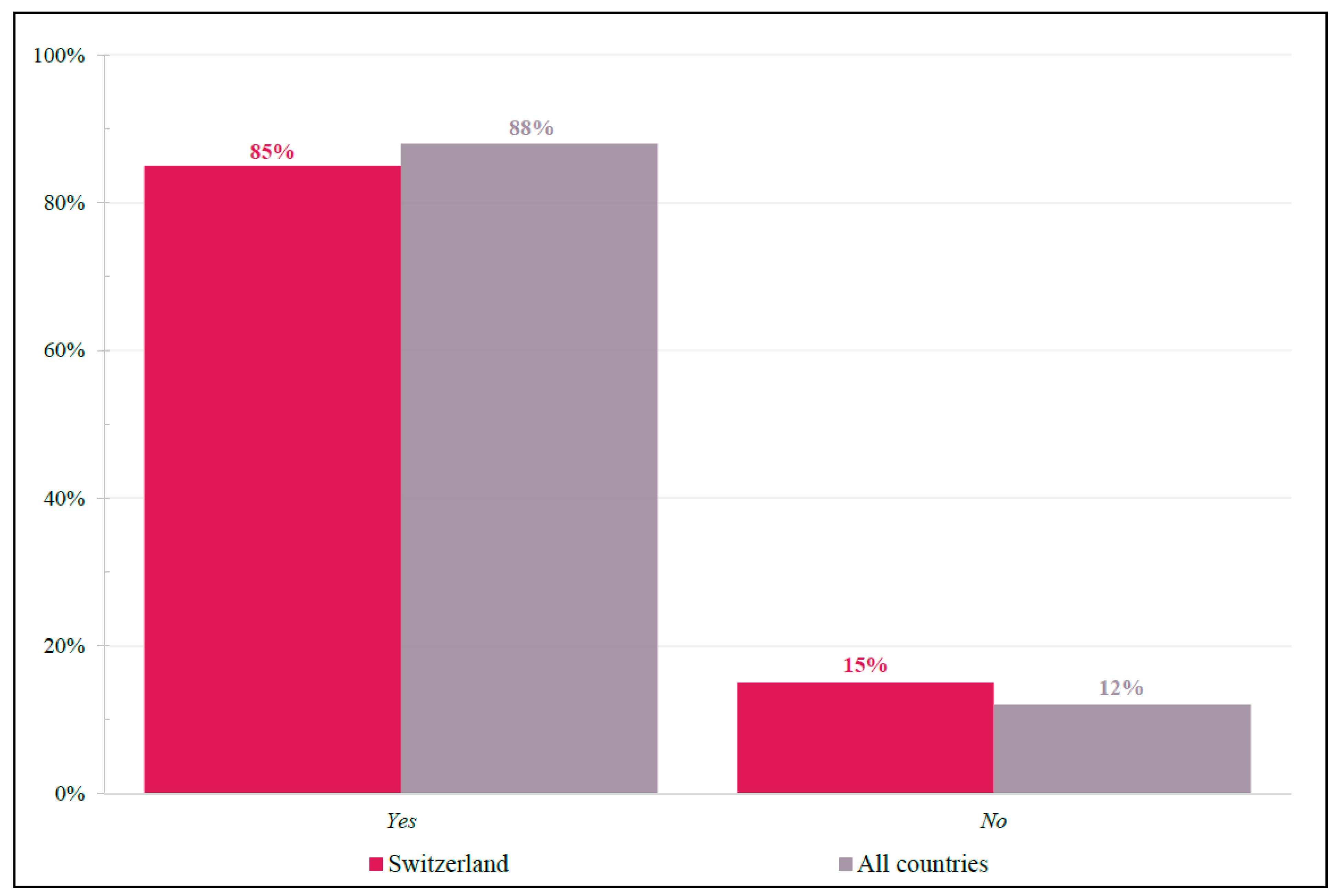
3.2.3. Patient-Centric Factors Influencing Treatment Choice in 3L and Beyond
3.3. Patient Cases
- Case 1: A fit and active 54-year-old male with a left-sided, KRAS-wildtype colon adenocarcinoma
- Case 2: A 68-year-old female with a KRAS-mutant left-sided colon adenocarcinoma, comorbidities, and previous chemotherapy tolerability issues; never exposed to FTD/TPI
- Case 3: An 82-year-old male with a KRAS-wildtype right-sided colon adenocarcinoma who has comorbidities, limited support, and difficult hospital accessibility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Falco, V.; Napolitano, S.; Roselló, S.; Huerta, M.; Cervantes, A.; Ciardiello, F.; Troiani, T. How we treat metastatic colorectal cancer. ESMO Open 2020, 4, e000813. [Google Scholar] [CrossRef] [PubMed]
- Siebenhüner, A.; De Dosso, S.; Meisel, A.; Wagner, A.D.; Borner, M. Metastatic Colorectal Carcinoma after Second Progression and the Role of Trifluridine-Tipiracil (TAS-102) in Switzerland. Oncol. Res. Treat. 2020, 43, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.C.; Huang, K.C.; Chen, W.S.; Jiang, J.K.; Yang, S.H.; Wang, H.S.; Chang, S.C.; Lan, Y.T.; Lin, C.C.; Lin, H.H.; et al. Preference criteria for regorafenib in treating refractory metastatic colorectal cancer are the small tumor burden, slow growth and poor/scanty spread. Sci. Rep. 2021, 11, 15370. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, H.; Mader, R.M.; Müllauer, L.; Erhart, F.; Kautzky-Willer, A.; Prager, G.W. Precision Medicine for the Management of Therapy Refractory Colorectal Cancer. J. Pers. Med. 2020, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Spitale, A.; Ortelli, L.; Mazzucchelli, L.; Bordoni, A. Quality indicators of colorectal cancer care in southern Switzerland: Results from a population-based study. Swiss Med. Wkly. 2017, 147, w14530. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Messersmith, W.A. NCCN Guidelines Updates: Management of Metastatic Colorectal Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 599–601. [Google Scholar] [CrossRef]
- Pox, C.P.; Schmiegel, W. German S3-guideline colorectal carcinoma. Dtsch. Med. Wochenschr. 2013, 138, 2545. [Google Scholar] [CrossRef] [PubMed]
- Prager, G.; Köhne, C.H.; O’Connor, J.M.; Rivera, F.; Santini, D.; Wasan, H.; Phelip, J.M. The Screening and COnsensus Based on Practices and Evidence (SCOPE) Program-Results of a Survey on Daily Practice Patterns for Patients with mCRC. Curr. Oncol. 2021, 28, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.; Prager, G.W.; Quintela, A.; Stein, A.; Moreno Vera, S.; Mounedji, N.; Taieb, J. Beyond second-line therapy in patients with metastatic colorectal cancer: A systematic review. Ann. Oncol. 2018, 29, 835–856. [Google Scholar] [CrossRef] [PubMed]
- Bekaii-Saab, T.; Kim, R.; Kim, T.W.; O’Connor, J.M.; Strickler, J.H.; Malka, D.; Sartore-Bianchi, A.; Bi, F.; Yamaguchi, K.; Yoshino, T.; et al. Third- or Later-line Therapy for Metastatic Colorectal Cancer: Reviewing Best Practice. Clin. Colorectal Cancer 2019, 18, e117–e129. [Google Scholar] [CrossRef] [PubMed]
- Modest, D.P.; Pant, S.; Sartore-Bianchi, A. Treatment sequencing in metastatic colorectal cancer. Eur. J. Cancer 2019, 109, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Montes, A.; Grávalos, C.; Pericay, C.; Safont, M.J.; Benavides, M.; Élez, E.; García-Alfonso, P.; García-Paredes, B.; Carrato, A.; Aranda, E. Current Options for Third-line and Beyond Treatment of Metastatic Colorectal Cancer. Spanish TTD Group Expert Opinion. Clin. Colorectal Cancer 2020, 19, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Kafatos, G.; Banks, V.; Burdon, P.; Neasham, D.; Lowe, K.A.; Anger, C.; Manuguid, F.; Trojan, J. Impact of biomarkers and primary tumor location on the metastatic colorectal cancer first-line treatment landscape in five European countries. Future Oncol. 2021, 17, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Fernau, S.; Mehlis, K.; Schildmann, J.; Krause, S.; Winkler, E.C. The Role of Physicians in Rationing Cancer Care. Attitudes of German Oncologists. Oncol. Res. Treat. 2017, 40, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Feller, A.; Schmidlin, K.; Bordoni, A.; Bouchardy, C.; Bulliard, J.L.; Camey, B.; Konzelmann, I.; Maspoli, M.; Wanner, M.; Zwahlen, M.; et al. Socioeconomic and demographic inequalities in stage at diagnosis and survival among colorectal cancer patients: Evidence from a Swiss population-based study. Cancer Med. 2018, 7, 1498–1510. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Mayer, R.J.; Laurent, S.; Winkler, R.; Grávalos, C.; Benavides, M.; Longo-Munoz, F.; Portales, F.; Ciardiello, F.; Siena, S.; et al. The subgroups of the phase III RECOURSE trial of trifluridine/tipiracil (TAS-102) versus placebo with best supportive care in patients with metastatic colorectal cancer. Eur. J. Cancer 2018, 90, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Hofheinz, R.D.; Kubicka, S.; Arnold, D. Treatment decisions in metastatic colorectal cancer—Beyond first and second line combination therapies. Cancer Treat. Rev. 2017, 59, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Nast, A.; Dreno, B.; Bettoli, V.; Degitz, K.; Erdmann, R.; Finlay, A.Y.; Ganceviciene, R.; Haedersdal, M.; Layton, A.; Lopez-Estebaranz, J.L.; et al. European evidence-based (S3) guidelines for the treatment of acne. J. Eur. Acad. Dermatol. Venereol. 2012, 26 (Suppl. 1), 1–29. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Amonkar, M.; Al-Jassar, G.; Lambert, J.; Malmenäs, M.; Chase, M.; Sun, L.; Kollmar, L.; Vichnin, M. Mismatch Repair/Microsatellite Instability Testing Practices among US Physicians Treating Patients with Advanced/Metastatic Colorectal Cancer. J. Clin. Med. 2019, 8, 558. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siebenhüner, A.R.; Lo Presti, G.; Helbling, D.; Szturz, P.; Astaras, C.; Buccella, Y.; De Dosso, S. The Screening and COnsensus Based on Practices and Evidence (SCOPE) Program Results of a Survey on Daily Practice Patterns for Patients with Metastatic Colorectal Cancer—A Swiss Perspective in the Context of an International Viewpoint. Curr. Oncol. 2022, 29, 5604-5615. https://doi.org/10.3390/curroncol29080442
Siebenhüner AR, Lo Presti G, Helbling D, Szturz P, Astaras C, Buccella Y, De Dosso S. The Screening and COnsensus Based on Practices and Evidence (SCOPE) Program Results of a Survey on Daily Practice Patterns for Patients with Metastatic Colorectal Cancer—A Swiss Perspective in the Context of an International Viewpoint. Current Oncology. 2022; 29(8):5604-5615. https://doi.org/10.3390/curroncol29080442
Chicago/Turabian StyleSiebenhüner, Alexander R., Giorgia Lo Presti, Daniel Helbling, Petr Szturz, Christoforos Astaras, Yannick Buccella, and Sara De Dosso. 2022. "The Screening and COnsensus Based on Practices and Evidence (SCOPE) Program Results of a Survey on Daily Practice Patterns for Patients with Metastatic Colorectal Cancer—A Swiss Perspective in the Context of an International Viewpoint" Current Oncology 29, no. 8: 5604-5615. https://doi.org/10.3390/curroncol29080442
APA StyleSiebenhüner, A. R., Lo Presti, G., Helbling, D., Szturz, P., Astaras, C., Buccella, Y., & De Dosso, S. (2022). The Screening and COnsensus Based on Practices and Evidence (SCOPE) Program Results of a Survey on Daily Practice Patterns for Patients with Metastatic Colorectal Cancer—A Swiss Perspective in the Context of an International Viewpoint. Current Oncology, 29(8), 5604-5615. https://doi.org/10.3390/curroncol29080442





