High-Volume Hospitals’ Ovarian Cancer Care—Less Individual Approach or Better Treatment Results?
Abstract
:1. Introduction
2. Materials and Methods
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
6.1. Limitations of the Study
6.2. Strengths of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Didkowska, J.; Wojciechowska, U. Zachorowania i Zgony na Nowotwory Złośliwe w Polsce. Krajowy Rejestr Nowotworów, Centrum Onkologii—Instytut im. Marii Skłodowskiej—Curie. Available online: http://onkologia.org.pl/raporty/ (accessed on 9 May 2022).
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Hillner, B.E.; Smith, T.J.; Desch, C.E. Hospital and Physician Volume or Specialization and Outcomes in Cancer Treatment: Importance in Quality of Cancer Care. J. Clin. Oncol. 2000, 18, 2327–2340. [Google Scholar] [CrossRef]
- Liu, J.H.; Zingmond, D.S.; McGory, M.L.; SooHoo, N.F.; Ettner, S.L.; Brook, R.H.; Ko, C.Y. Disparities in the Utilization of High-Volume Hospitals for Complex Surgery. J. Am. Med. Assoc. 2006, 296, 1973–1980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, A.J.; Gray, B.H.; Schlesinger, M. Racial and Ethnic Differences in the Use of High-Volume Hospitals and Surgeons. Arch. Surg. 2010, 145, 179–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goff, B.A.; Matthews, B.J.; Wynn, M.; Muntz, H.G.; Lishner, D.M.; Baldwin, L.M. Ovarian Cancer: Patterns of Surgical Care across the United States. Gynecol. Oncol. 2006, 103, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Goff, B.A.; Matthews, B.J.; Larson, E.H.; Andrilla, C.H.A.; Wynn, M.; Lishner, D.M.; Baldwin, L.M. Predictors of Comprehensive Surgical Treatment in Patients with Ovarian Cancer. Cancer 2007, 109, 2031–2042. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.E.; Zahurak, M.L.; Diaz-Montes, T.P.; Giuntoli, R.L.; Armstrong, D.K. Impact of Surgeon and Hospital Ovarian Cancer Surgical Case Volume on In-Hospital Mortality and Related Short-Term Outcomes. Gynecol. Oncol. 2009, 115, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.E.; Palis, B.E.; Chi, D.S.; Cliby, W.A. The National Cancer Database Report on Advanced-Stage Epithelial Ovarian Cancer: Impact of Hospital Surgical Case Volume on Overall Survival and Surgical Treatment Paradigm. Gynecol. Oncol. 2010, 118, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Kumpulainen, S.; Grénman, S.; Kyyrönen, P.; Pukkala, E.; Sankila, R. Evidence of Benefit from Centralized Treatment of Ovarian Cancer: A Nationwide Population-Based Survival Analysis in Finland. Int. J. Cancer 2002, 102, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Tingulstad, S.; Skjeldestad, F.E.; Hagen, B. The Effect of Centralization of Primary Surgery on Survival in Ovarian Cancer Patients. Obstet. Gynecol. 2003, 102, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Marth, C.; Hiebl, S.; Oberaigner, W.; Winter, R.; Leodolter, S.; Sevelda, P. Influence of Department Volume on Survival for Ovarian Cancer: Results from a Prospective Quality Assurance Program of the Austrian Association for Gynecologic Oncology. Int. J. Gynecol. Cancer 2009, 19, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Wimberger, P.; Lehmann, N.; Kimmig, R.; Burges, A.; Meier, W.; du Bois, A. Prognostic Factors for Complete Debulking in Advanced Ovarian Cancer and Its Impact on Survival. An Exploratory Analysis of a Prospectively Randomized Phase III Study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVAR). Gynecol. Oncol. 2007, 106, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.E.; Chang, J.; Ziogas, A.; Anton-Culver, H. Adherence to Treatment Guidelines for Ovarian Cancer as a Measure of Quality Care. Obstet. Gynecol. 2013, 121, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.E.; Ueda, S.; Gerardi, M.A.; Ajiboye, O.B.; Ibeanu, O.A. Analysis of Racial Disparities in Stage IIIC Epithelial Ovarian Cancer Care and Outcomes in a Tertiary Gynecologic Oncology Referral Center. Gynecol. Oncol. 2011, 122, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Chornokur, G.; Amankwah, E.K.; Schildkraut, J.M.; Phelan, C.M. Global Ovarian Cancer Health Disparities. Gynecol. Oncol. 2013, 129, 258–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goff, B. Symptoms Associated with Ovarian Cancer. Clin. Obstet. Gynecol. 2012, 55, 36–42. [Google Scholar] [CrossRef] [PubMed]
- The Role of the Obstetrician-Gynecologist in the Early Detection of Epithelial Ovarian Cancer in Women at Average Risk. Obstet. Gynecol. 2017, 130, e146–e149. [CrossRef] [PubMed] [Green Version]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.M.; van der Burg, M.E.L.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant Chemotherapy or Primary Surgery in Stage IIIC or IV Ovarian Cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of Surgical Outcome as Prognostic Factor in Advanced Epithelial Ovarian Cancer: A Combined Exploratory Analysis of 3 Prospectively Randomized Phase 3 Multicenter Trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour Les Etudes Des Cancers de l’Ovaire (GINECO). Cancer 2009, 115, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Basta, A.; Bidziński, M.; Bieńkiewicz, A.; Blecharz, P.; Bodnar, L.; Jach, R.; Knapp, P.; Kojs, Z.; Kotarski, J.; Markowska, J.; et al. Recommendations of the Polish Gynecological Oncology Society for the Diagnosis and Treatment of Ovarian Cancer. Curr. Gynecol. Oncol. 2017, 15, 5–23. [Google Scholar] [CrossRef]
- Bristow, R.E.; Chang, J.; Ziogas, A.; Randall, L.M.; Anton-Culver, H. High-Volume Ovarian Cancer Care: Survival Impact and Disparities in Access for Advanced-Stage Disease. Gynecol. Oncol. 2014, 132, 403–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
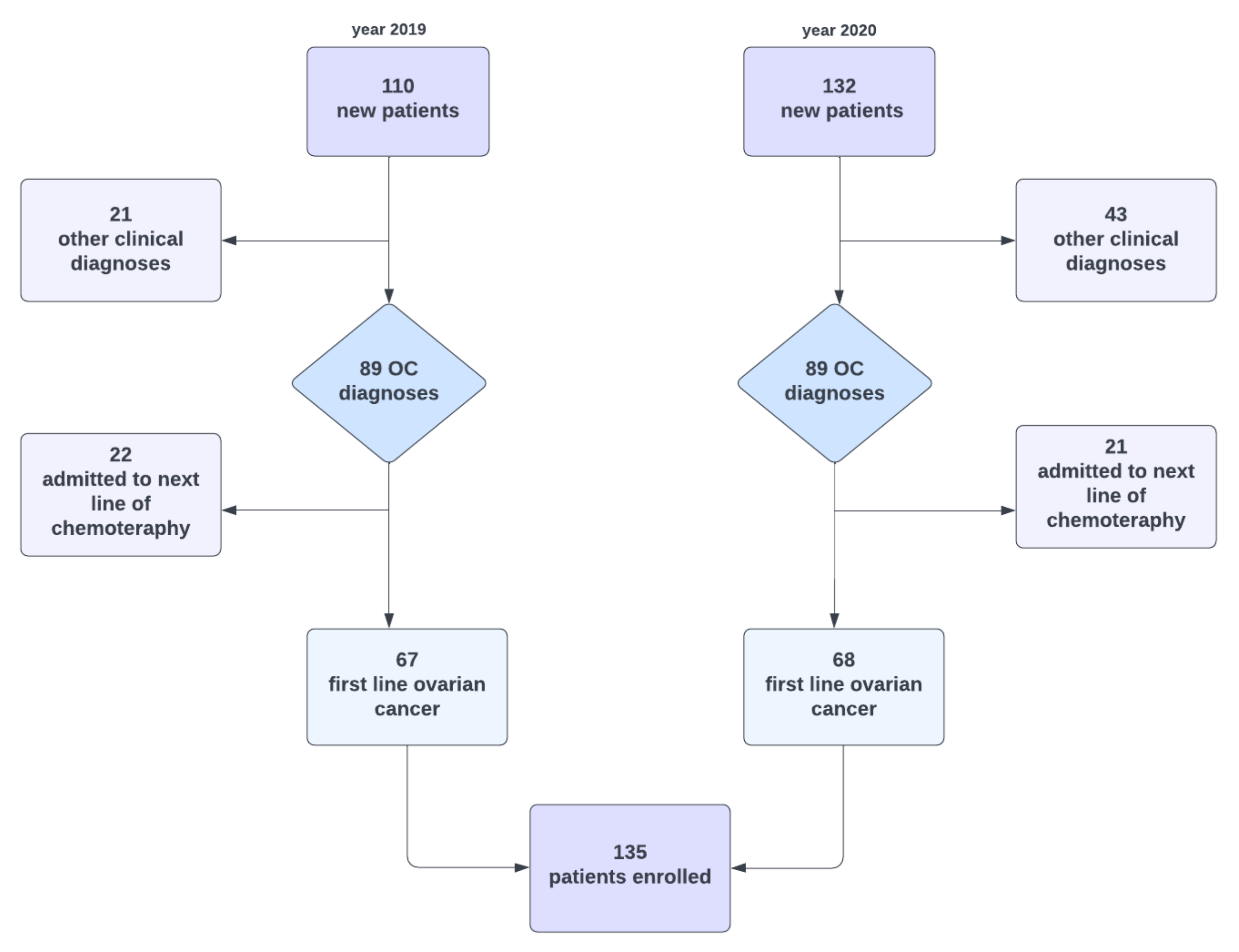

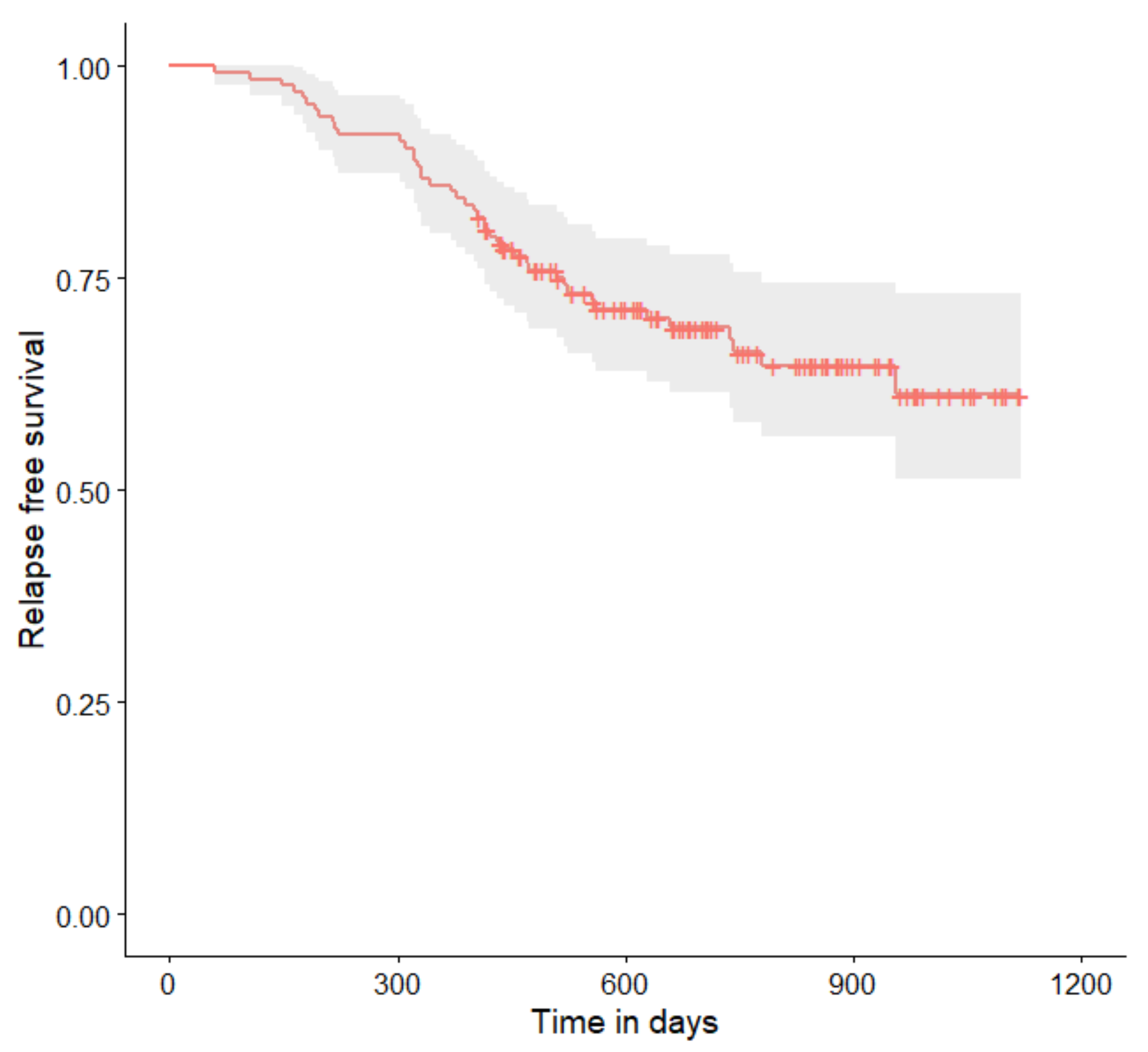

| Characteristic | Value, n (%) |
|---|---|
| Year of measurement | |
| 2019 | 67 (49.6) |
| 2020 | 68 (50.4) |
| Place of the first intervention | |
| SKPP | 95 (70.4) |
| other hospitals | 40 (29.6) |
| Type of the first intervention | |
| SKPP | |
| PDS–laparotomy | 48 (50.5) |
| laparoscopy | 24 (25.3) |
| Biopsy–laparotomy | 18 (18.9) |
| no operation | 5 (5.3) |
| Other hospitals | |
| PDS–laparotomy | 20 (50.0) |
| laparoscopy | 2 (5.0) |
| Biopsy–laparotomy | 17 (42.5) |
| no operation | 1 (2.5) |
| Final decision | |
| PDS after laparotomy or laparoscopy | 69 (51.0) |
| NACT after laparotomy | 31 (23.0) |
| NACT after laparoscopy | 26 (19.3) |
| chemotherapy | 5 (3.7) |
| NACT without primary surgery | 4 (3.0) |
| FIGO staging system | |
| I | 17 (12.6) |
| II | 6 (4.5) |
| III | 69 (51.1) |
| IV | 35 (25.9) |
| non-staging | 8 (5.9) |
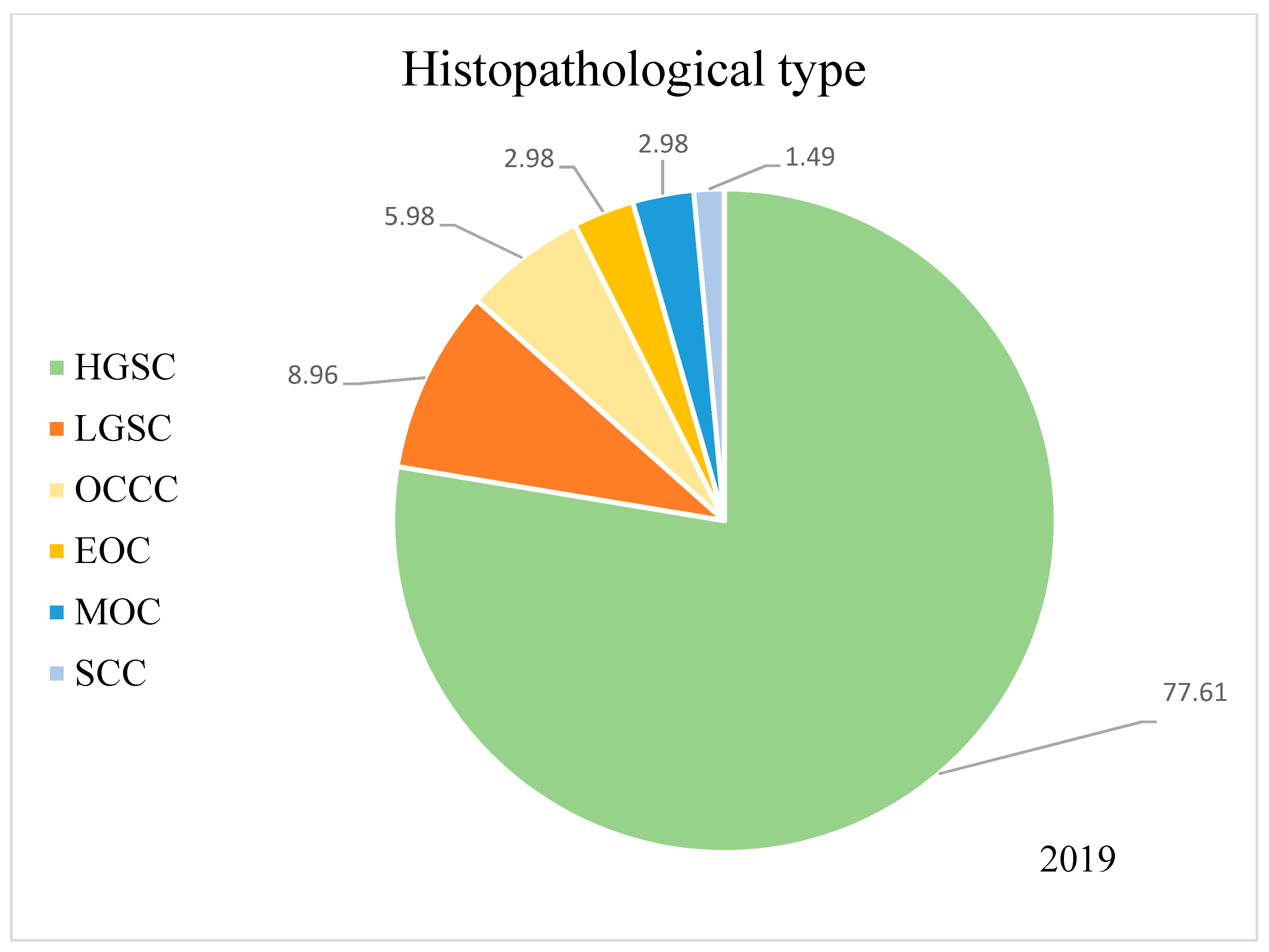
| Histopathology | |
| HGSC | 105 (77.8) |
| other types | 30 (22.2) |
| Stage of the cytoreduction (n = 111) | |
| total (R0) | 52 (46.8) |
| optimal (R1) | 26 (23.5) |
| suboptimal (R2) | 33 (29.7) |
| Characteristic, n (%) | SKPP Patients | Transferred Patients | p |
|---|---|---|---|
| FIGO | |||
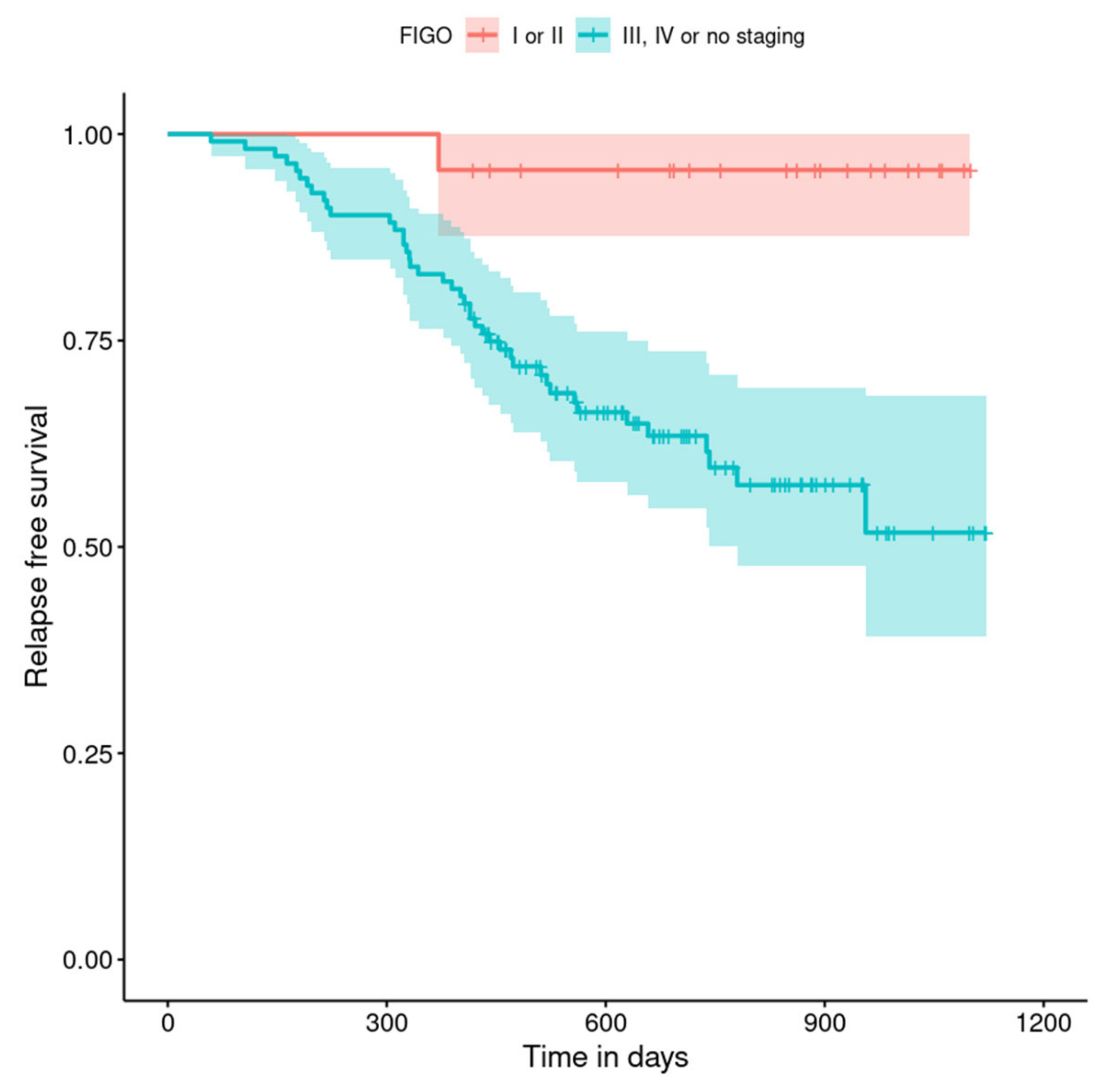
| I or II | 17 (17.9) | 6 (15.0) | 0.828 |
| III or IV | 73 (76.8) | 31 (77.5) | |
| non-staging | 5 (5.3) | 3 (7.5) | |
| Type of the first intervention | |||
| PDS–laparotomy | 48 (50.5) | 20 (50.0) | 0.006 |
| laparoscopy | 24 (25.3) | 2 (5.0) | |
| Biopsy–laparotomy | 18 (18.9) | 17 (42.5) | |
| no operation | 5 (5.3) | 1 (2.5) | |
| Final decision | |||
| PDS after laparoscopy and laparotomy | 48 (50.5) | 21 (52.5) | |
| NACT after laparotomy | 18 (18.9) | 13 (32.5) | |
| NACT after laparoscopy | 24 (25.3) | 2 (5.0) | 0.032 |
| chemotherapy | 2 (2.1) | 3 (7.5) | |
| NACT without primary surgery | 3 (3.2) | 1 (2.5) | |
| Stage of the cytoreduction | |||
| total (R0) | 41 (46.6) | 11 (47.8) | 0.539 |
| optimal (R1) | 19 (21.6) | 7 (30.4) | |
| suboptimal (R2) | 28 (31.8) | 5 (21.7) |
| Characteristic, n (%) | 2019 Group | 2020 Group | p |
|---|---|---|---|
| FIGO staging system | |||
| I or II | 14 (20.9) | 9 (13.2) | 0.420 |
| III or IV | 50 (74.6) | 54 (79.4) | |
| Non-staging | 3 (4.5) | 5 (7.4) | |
| Type of the first intervention | |||
| PDS–laparotomy | 31 (46.3) | 37 (54.4) | 0.060 |
| laparoscopy | 10 (14.9) | 16 (23.5) | |
| Biopsy–laparotomy | 24 (35.8) | 11 (16.2) | |
| no operation | 2 (3.0) | 4 (5.9) | |
| Final decision | |||
| PDS after laparoscopy and laparotomy | 30 (44.8) | 39 (57.4) | |
| NACT after laparotomy | 22 (32.8) | 9 (13.2) | |
| NACT after laparoscopy | 10 (14.9) | 16 (23.5) | 0.002 |
| chemotherapy | 5 (7.5) | 0 (0.0) | |
| NACT without primary surgery | 0 (0.0) | 4 (5.9) | |
| Place of the first intervention | |||
| SKPP | 43 (64.2) | 52 (76.5) | 0.169 |
| Other hospitals | 24 (35.8) | 16 (23.5) | |
| Stage of the cytoreduction | |||
| total (R0) | 27 (54.0) | 25 (41.0) | 0.208 |
| optimal (R1) | 8 (16.0) | 18 (29.5) | |
| suboptimal (R2) | 15 (30.0) | 18 (29.5) |
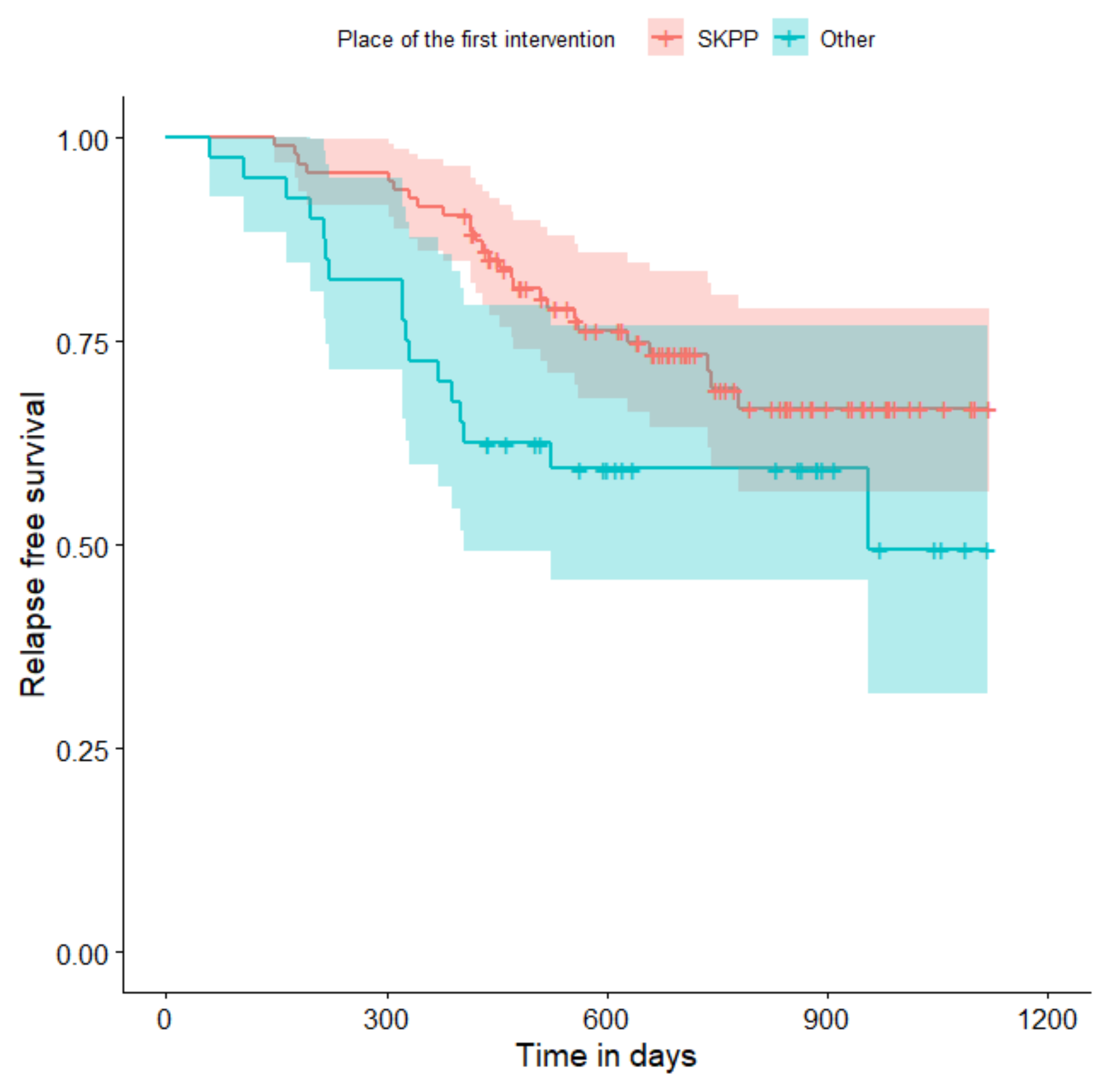
| Variable | HR | 95% CI | p |
|---|---|---|---|
| Place of the first intervention (SKPP vs. other hospitals) | 1.89 | 1.02; 3.48 | 0.043 |
| FIGO (I or II vs. III, IV or non-staging) | 11.84 | 1.62; 86.32 | 0.015 |
| Histopathology (HGSC vs. other) | 0.50 | 0.21; 1.18 | 0.111 |
| Type of the first intervention (PDS vs. …) | |||
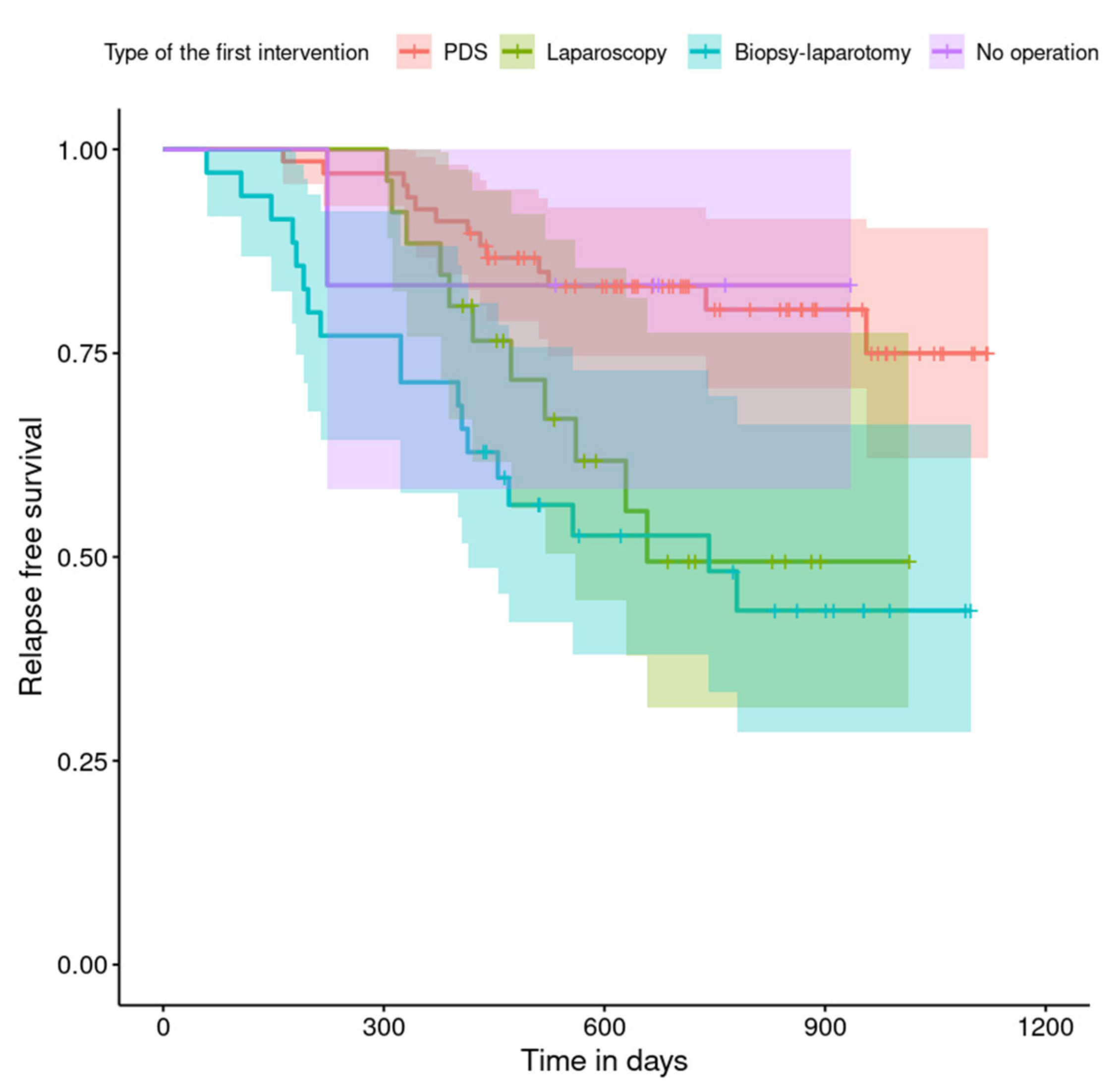
| laparoscopy | 2.67 | 1.19; 5.99 | 0.017 |
| Biopsy–laparotomy | 3.65 | 1.79; 7.47 | <0.001 |
| no operation | 0.95 | 0.12; 7.26 | 0.959 |
| Type of the first intervention (laparoscopy vs. …) | |||
| Biopsy–laparotomy | 1.37 | 0.64; 2.90 | 0.415 |
| no operation | 0.35 | 0.05; 2.75 | 0.321 |
| Final decision (PDS vs. …) | |||
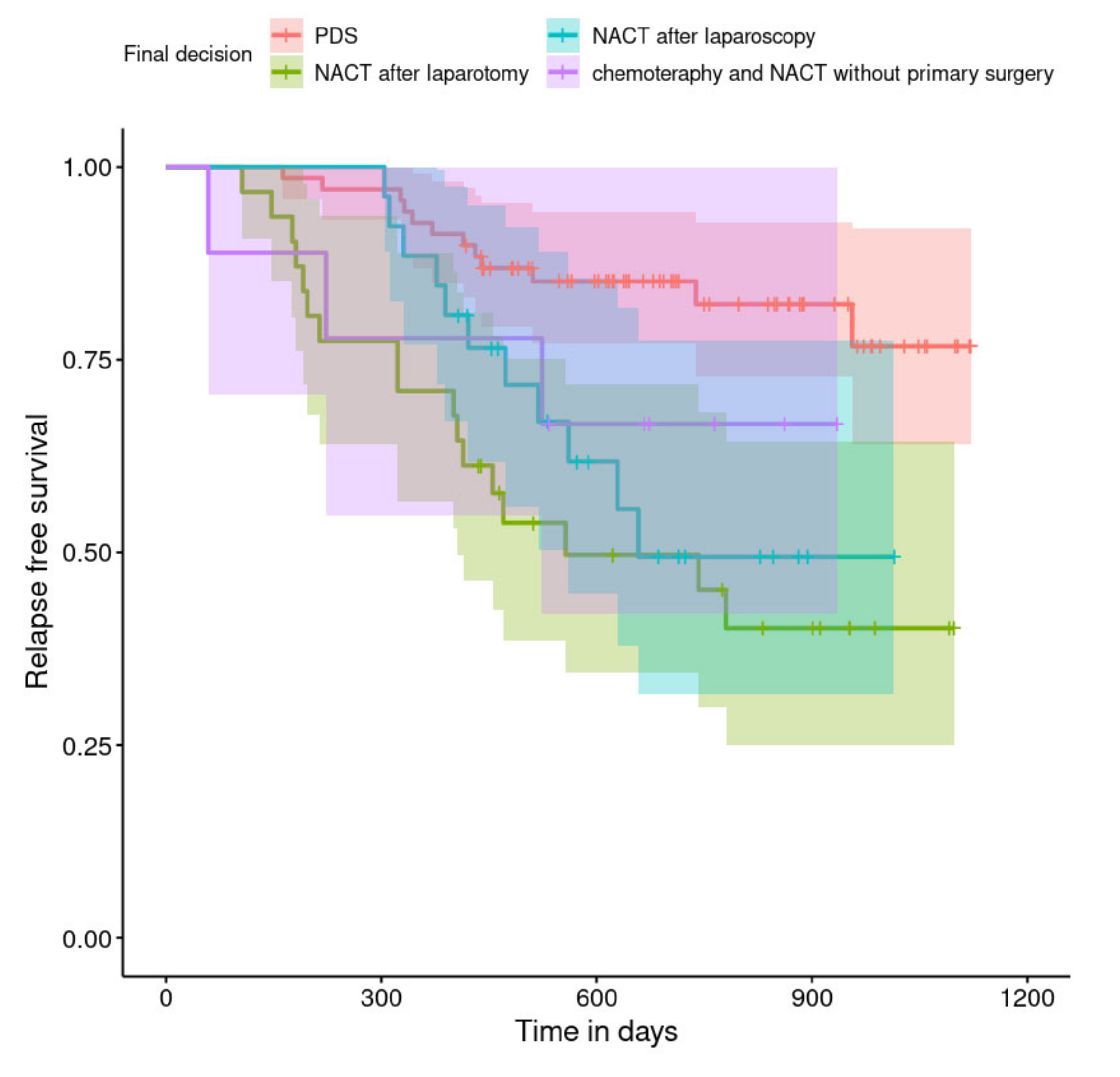
| NACT after laparotomy | 4.27 | 2.04; 8.97 | <0.001 |
| NACT after laparoscopy | 2.93 | 1.29; 6.67 | 0.010 |
| chemotherapy or NACT without primary surgery | 2.26 | 0.64; 8.04 | 0.207 |
| Stage of the cytoreduction (total—R0 vs. …) | |||
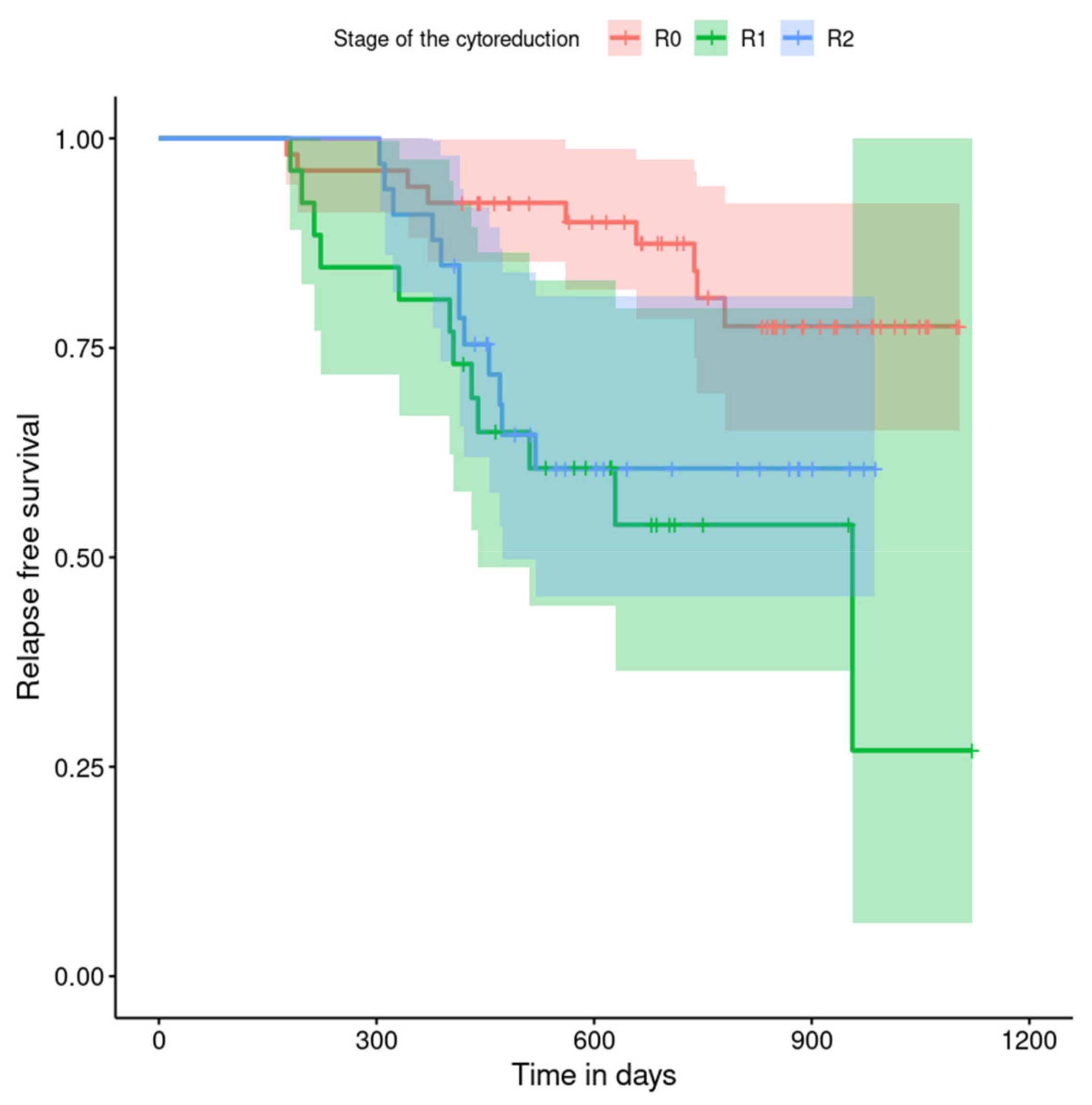
| optimal (R1) | 3.81 | 1.59; 9.12 | 0.003 |
| suboptimal (R2) | 2.75 | 1.15; 6.57 | 0.023 |
| Variable | HR | 95% CI | p |
|---|---|---|---|
| Final decision (PDS vs. …) | |||
| NACT after laparotomy | 7.10 | 2.82; 17.92 | <0.001 |
| NACT after laparoscopy | 4.27 | 1.68; 10.85 | 0.002 |
| Chemotherapy or NACT without primary surgery | 1.91 | 0.24; 15.41 | 0.546 |
| Stage of the cytoreduction (R0 vs. …) | |||
| R1 | 3.62 | 1.48; 8.84 | 0.004 |
| R2 | 1.70 | 0.69; 4.17 | 0.248 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millert-Kalińska, S.; Pruski, D.; Przybylski, M.; Stawicka-Niełacna, M.; Mądry, E.; Mądry, R. High-Volume Hospitals’ Ovarian Cancer Care—Less Individual Approach or Better Treatment Results? Curr. Oncol. 2022, 29, 5278-5294. https://doi.org/10.3390/curroncol29080419
Millert-Kalińska S, Pruski D, Przybylski M, Stawicka-Niełacna M, Mądry E, Mądry R. High-Volume Hospitals’ Ovarian Cancer Care—Less Individual Approach or Better Treatment Results? Current Oncology. 2022; 29(8):5278-5294. https://doi.org/10.3390/curroncol29080419
Chicago/Turabian StyleMillert-Kalińska, Sonja, Dominik Pruski, Marcin Przybylski, Małgorzata Stawicka-Niełacna, Edyta Mądry, and Radosław Mądry. 2022. "High-Volume Hospitals’ Ovarian Cancer Care—Less Individual Approach or Better Treatment Results?" Current Oncology 29, no. 8: 5278-5294. https://doi.org/10.3390/curroncol29080419
APA StyleMillert-Kalińska, S., Pruski, D., Przybylski, M., Stawicka-Niełacna, M., Mądry, E., & Mądry, R. (2022). High-Volume Hospitals’ Ovarian Cancer Care—Less Individual Approach or Better Treatment Results? Current Oncology, 29(8), 5278-5294. https://doi.org/10.3390/curroncol29080419







