Do Sustainable Palliative Single Fraction Radiotherapy Practices Proliferate or Perish 2 Years after a Knowledge Translation Campaign?
Abstract
:1. Background
2. Methods
2.1. Data Sources and Data Extraction
2.2. Statistics
3. Result
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rich, S.E.; Chow, R.; Raman, S.; Liang Zeng, K.; Lutz, S.; Lam, H.; Silva, M.F.; Chow, E. Update of the systematic review of palliative radiation therapy fractionation for bone metastases. Radiother. Oncol. 2018, 126, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Bedard, G.; Hoskin, P.; Chow, E. Overall response rates to radiation therapy for patients with painful uncomplicated bone metastases undergoing initial treatment and retreatment. Radiother. Oncol. 2014, 112, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.; van der Linden, Y.M.; Roos, D.; Hartsell, W.F.; Hoskin, P.; Wu, J.S.Y.; Brundage, M.D.; Nabid, A.; Tissing-Tan, C.J.A.; Oei, B.; et al. Single versus multiple fractions of repeat radiation for painful bone metastases: A randomised, controlled, non-inferiority trial. Lancet Oncol. 2014, 15, 164–171. [Google Scholar] [CrossRef]
- Pin, Y.; Paix, A.; Le Fèvre, C.; Antoni, D.; Blondet, C.; Noël, G. A systematic review of palliative bone radiotherapy based on pain relief and retreatment rates. Crit. Rev. Oncol. Hematol. 2018, 123, 132–137. [Google Scholar] [CrossRef]
- Howell, D.D.; James, J.L.; Hartsell, W.F.; Suntharalingam, M.; MacHtay, M.; Suh, J.H.; Demas, W.F.; Sandler, H.M.; Kachnic, L.A.; Berk, L.B. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of painful vertebral bone metastases—Equivalent efficacy, less toxicity, more convenient: A subset analysis of Radiation Therapy Oncology Group trial 97-14. Cancer 2013, 119, 888–896. [Google Scholar] [CrossRef]
- Van den Hout, W.B.; van der Linden, Y.M.; Steenland, E.; Wiggenraad, R.G.J.; Kievit, J.; de Haes, H.; Leer, J.W.H. Single- versus multiple-fraction radiotherapy in patients with painful bone metastases: Cost-utility analysis based on a randomized trial. J. Natl. Cancer Inst. 2003, 95, 222–229. [Google Scholar] [CrossRef]
- Konski, A.; James, J.; Hartsell, W.; Leibenhaut, M.H.; Janjan, N.; Curran, W.; Roach, M.; Watkins-Bruner, D. Economic analysis of Radiation Therapy Oncology Group 97-14: Multiple versus single fraction radiation treatment of patients with bone metastases. Am. J. Clin. Oncol. Cancer Clin. Trials 2009, 32, 423–428. [Google Scholar] [CrossRef]
- Steenland, E.; Leer, J.; Van Houwelingen, H.; Post, W.J.; Van den Hout, W.B.; Kievit, J.; De Haes, H.; Martijn, H.; Oei, B.; Vonk, E.; et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: A global analysis of the Dutch Bone Metastasis Study. Radiother. Oncol. 1999, 52, 101–109. [Google Scholar] [CrossRef]
- Pollicino, C.A.; Turner, S.L.; Roos, D.E.; O’Brien, P.C. Costing the components of pain management. Analysis of Trans-Tasman Radiation Oncology Group trial (TROG 96.05): One versus five fractions for neuropathic bone pain. Radiother. Oncol. 2005, 76, 264–269. [Google Scholar] [CrossRef]
- Lutz, S.; Berk, L.; Chang, E.; Chow, E.; Hahn, C.; Hoskin, P.; Howell, D.; Konski, A.; Kachnic, L.; Lo, S.; et al. Palliative radiotherapy for bone metastases: An ASTRO evidence-based guideline. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 965–976. [Google Scholar] [CrossRef]
- Wu, J.S.Y.; Wong, R.K.S.; Lloyd, N.S.; Johnston, M.; Bezjak, A.; Whelan, T. Radiotherapy fractionation for the palliation of uncomplicated painful bone metastases—An evidence-based practice guideline. BMC Cancer 2004, 4, 71. [Google Scholar] [CrossRef] [Green Version]
- Janjan, N.; Lutz, S.T.; Bedwinek, J.M.; Hartsell, W.F.; Ng, A.; Pieters, R.S.; Ratanatharathorn, V.; Silberstein, E.B.; Taub, R.J.; Yasko, A.W.; et al. Therapeutic guidelines for the treatment of bone metastasis: A report from the American college of radiology appropriateness criteria expert panel on radiation oncology. J. Palliat. Med. 2009, 12, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Fairchild, A.; Barnes, E.; Ghosh, S.; Ben-Josef, E.; Roos, D.; Hartsell, W.; Holt, T.; Wu, J.; Janjan, N.; Chow, E. International Patterns of Practice in Palliative Radiotherapy for Painful Bone Metastases: Evidence-Based Practice? Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Hartsell, W.F.; Konski, A.A.; Lo, S.S.; Hayman, J.A. Single Fraction Radiotherapy for Bone Metastases: Clinically Effective, Time Efficient, Cost Conscious and Still Underutilized in the United States? Clin. Oncol. 2009, 21, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.; Rahal, R.; Brundage, M.; Fung, S.; Louzado, C.; Milosevic, M.; Xu, J.; Bryant, H. Use of low-value radiotherapy practices in Canada: An analysis of provincial cancer registry data. Curr. Oncol. 2016, 23, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Curran, J.A.; Grimshaw, J.M.; Hayden, J.A.; Campbell, B. Knowledge translation research: The science of moving research into policy and practice. J. Contin. Educ. Health Prof. 2011, 31, 174–180. [Google Scholar] [CrossRef]
- Levinson, W.; Huynh, T. Engaging physicians and patients in conversations about unnecessary tests and procedures: Choosing Wisely Canada. Cmaj 2014, 186, 325–326. [Google Scholar] [CrossRef] [Green Version]
- Mitera, G.; Earle, C.; Latosinsky, S.; Booth, C.; Bezjak, A.; Desbiens, C.; Delouya, G.; Laing, K.; Camuso, N.; Porter, G. Choosing Wisely Canada cancer list: Ten low-value or harmful practices that should be avoided in cancer care. J. Oncol. Pract. 2015, 11, e296–e303. [Google Scholar] [CrossRef]
- Shahhat, S.; Hanumanthappa, N.; Chung, Y.T.; Beck, J.; Koul, R.; Bashir, B.; Cooke, A.; Dubey, A.; Butler, J.; Nashed, M.; et al. Do Coordinated Knowledge Translation Campaigns Persuade Radiation Oncologists to Use Single-Fraction Radiation Therapy Compared With Multiple-Fraction Radiation Therapy for Bone Metastases? Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 365–373. [Google Scholar] [CrossRef]
- Cheon, P.M.; Wong, E.; Thavarajah, N.; Dennis, K.; Lutz, S.; Zeng, L.; Chow, E. A definition of “uncomplicated bone metastases” based on previous bone metastases radiation trials comparing single-fraction and multi-fraction radiation therapy. J. Bone Oncol. 2015, 4, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Ashworth, A.; Kong, W.; Chow, E.; MacKillop, W.J. Fractionation of palliative radiation therapy for bone metastases in Ontario: Do practice guidelines guide practice? Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Ashoor, H.M.; Cardoso, R.; MacDonald, H.; Cogo, E.; Kastner, M.; Perrier, L.; McKibbon, A.; Grimshaw, J.M.; Straus, S.E. Sustainability of knowledge translation interventions in healthcare decision-making: A scoping review. Implement. Sci. 2015, 11, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tricco, A.C.; Antony, J.; Ivers, N.M.; Ashoor, H.M.; Khan, P.A.; Blondal, E.; Ghassemi, M.; MacDonald, H.; Chen, M.H.; Ezer, L.K.; et al. Effectiveness of quality improvement strategies for coordination of care to reduce use of health care services: A systematic review and meta-analysis. Cmaj 2014, 186, E568–E578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, R.; Chan, M.; Minhas, N.; Kandola, G.; Tiwana, M.; Lefresne, S.; Halperin, R.; Schellenberg, D.; Wai, E.; Ahmed, N.; et al. Programmatic Comparison and Dissemination of an Audit of Single-fraction Radiation Therapy Prescribing Practices for Bone Metastases is Associated with a Meaningful and Lasting Change in Practice on a Population Level. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 325–329. [Google Scholar] [CrossRef]
- Biswal, M.; Rajpoot, S.; Dhaliwal, N.; Appananavar, S.B.; Taneja, N.; Gupta, A.K. Evaluation of the short-term and long-term effect of a short series of hand hygiene campaigns on improving adherence in a tertiary care hospital in India. Am. J. Infect. Control 2014, 42, 1009–1010. [Google Scholar] [CrossRef]
- Seto, W.H.; Yuen, S.W.S.; Cheung, C.W.Y.; Ching, P.T.Y.; Cowling, B.J.; Pittet, D. Hand hygiene promotion and the participation of infection control link nurses: An effective innovation to overcome campaign fatigue. Am. J. Infect. Control 2013, 41, 1281–1283. [Google Scholar] [CrossRef]
- Beasley, J.W.; Wetterneck, T.B.; Temte, J.; Lapin, J.A.; Smith, P.; Rivera-Rodriguez, A.J.; Karsh, B.T. Information chaos in primary care: Implications for physician performance and patient safety. J. Am. Board Fam. Med. 2011, 24, 745–751. [Google Scholar] [CrossRef] [Green Version]
- Glassman, P.A.; Simon, B.; Belperio, P.; Lanto, A. Improving recognition of drug interactions benefits and barriers to using automated drug alerts. Med. Care 2002, 40, 1161–1171. [Google Scholar] [CrossRef]
- Van Der Sijs, H.; Aarts, J.; Vulto, A.; Berg, M. Overriding of drug safety alerts in computerized physician order entry. J. Am. Med. Inform. Assoc. 2006, 13, 138–147. [Google Scholar] [CrossRef]
- Baseman, J.G.; Revere, D.; Painter, I.; Toyoji, M.; Thiede, H.; Duchin, J. Public health communications and alert fatigue. BMC Health Serv. Res. 2013, 13, 295. [Google Scholar] [CrossRef] [Green Version]
- Guan, M.; Li, Y.; Scoles, J.D.; Zhu, Y. COVID-19 Message Fatigue: How Does It Predict Preventive Behavioral Intentions and What Types of Information are People Tired of Hearing About? Health Commun. 2022. [Google Scholar] [CrossRef] [PubMed]
- White, M.; Kwasnicka, D.; Dombrowski, S.U.; White, M. Theoretical explanations for maintenance of behaviour change: A systematic review of behaviour theories. Health Psychol. Rev. 2016, 10, 277–296. [Google Scholar]
- Lennox, L.; Maher, L.; Reed, J. Navigating the sustainability landscape: A systematic review of sustainability approaches in healthcare. Implement. Sci. 2018, 13, 27. [Google Scholar] [CrossRef]
- Tiwana, M.S.; Barnes, M.; Yurkowski, E.; Roden, K.; Olson, R.A. Incidence and treatment patterns of complicated bone metastases in a population-based radiotherapy program. Radiother. Oncol. 2016, 118, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, P.J.; Hopkins, K.; Misra, V.; Holt, T.; Mcmenemin, R.; Dubois, D.; Mckinna, F.; Foran, B.; Madhavan, K.; Macgregor, C.; et al. Effect of Single-Fraction vs Multifraction Radiotherapy on Ambulatory Status among Patients with Spinal Canal Compression from Metastatic Cancer: The SCORAD Randomized Clinical Trial. JAMA 2019, 322, 2084–2094. [Google Scholar] [CrossRef] [PubMed]
| Variable | Whole Cohort (n = 1008) | SFRT (n = 638) | MFRT (n = 370) | p-Value | |
|---|---|---|---|---|---|
| Patient Characteristics | |||||
| Age (Median, Range) | 67 (5–96) | 69 (5–96) | 65 (5–93) | 0.0008 | |
| Charlson Comorbidity Index | 0 | 540 (53.6) | 325 (50.9) | 215 (58.1) | 0.034 |
| 1 | 215 (21.3) | 139 (21.8) | 76 (20.5) | ||
| 2 | 139 (13.8) | 89 (14.0) | 50 (13.5) | ||
| ≥3 | 114 (11.3) | 85 (13.3) | 29 (7.8) | ||
| Gender | Female | 423 (42.0) | 257 (40.3) | 166 (44.9) | 0.155 |
| Male | 585 (58.0) | 381 (59.7) | 204 (55.1) | ||
| ECOG Performance Status | 0–1 | 475 (47.1) | 294 (46.1) | 181 (48.9) | 0.193 |
| 2 | 253 (25.1) | 172 (27.0) | 81 (21.9) | ||
| 3–4 | 235 (23.3) | 148 (23.2) | 87 (23.5) | ||
| Unknown | 45 (4.5) | 24 (3.8) | 21 (5.7) | ||
| Disease Characteristics | |||||
| Tumour Type | Prostate | 263 (26.1) | 205 (32.1) | 58 (15.7) | <0.0001 |
| Breast | 174 (17.3) | 107 (16.8) | 67 (18.1) | ||
| Lung | 238 (23.6) | 150 (23.5) | 88 (23.8) | ||
| Hematological | 82 (8.1) | 40 (6.3) | 42 (11.4) | ||
| Non-prostate GU | 88 (8.7) | 39 (6.1) | 49 (13.2) | ||
| Gastrointestinal | 75 (7.4) | 43 (6.7) | 32 (8.7) | ||
| Other | 88 (8.7) | 54 (8.5) | 34 (9.2) | ||
| Site of Radiotherapy | Skull and spine | 450 (44.6) | 234 (36.7) | 216 (58.4) | <0.0001 |
| Upper Extremity | 93 (9.2) | 77 (12.1) | 16 (4.3) | ||
| Chest (including ribs) | 68 (6.8) | 48 (7.5) | 20 (5.4) | ||
| Pelvis and proximal femur | 326 (32.3) | 229 (35.9) | 97 (26.2) | ||
| Lower extremity | 71 (7.0) | 50 (7.8) | 21 (5.7) | ||
| Complicated Bone Metastasis | No | 689 (68.4) | 496 (77.7) | 193 (52.2) | <0.0001 |
| Yes | 319 (31.7) | 142 (22.3) | 177 (47.8) | ||
| Fracture | No | 746 (74.0) | 509 (79.8) | 237 (64.1) | <0.0001 |
| Yes | 262 (26.0) | 129 (20.2) | 133 (36.0) | ||
| Soft Tissue Component | No | 671 (66.6) | 501 (78.5) | 170 (46.0) | <0.0001 |
| Yes | 337 (33.4) | 137 (21.5) | 200 (54.1) | ||
| Cord Compression | No | 923 (91.6) | 616 (96.6) | 307 (83.0) | <0.0001 |
| Yes | 85 (8.4) | 22 (3.5) | 63 (17.0) | ||
| Cauda Equina Compression | No | 978 (97.0) | 633 (99.2) | 345 (93.2) | <0.0001 |
| Yes | 30 (3.0) | 5 (0.8) | 25 (6.8) | ||
| Treatment Characteristics | |||||
| Retreatment | No | 882 (87.5 | 551 (86.4) | 331 (89.5) | 0.152 |
| Yes | 126 (12.5) | 87 (13.6) | 39 (10.5) | ||
| Post-Operative Radiotherapy | No | 958 (95.0) | 618 (96.9) | 340 (91.9) | <0.0001 |
| Yes | 50 (5.0) | 20 (3.1) | 30 (8.1) | ||
| Treatment Location | Winnipeg | 865 (85.8) | 561 (87.9) | 304 (82.2) | 0.011 |
| Brandon | 143 (14.2) | 77 (12.1) | 66 (17.8) | ||
| RO Years in Practice (yrs) | ≤6 | 260 (25.8) | 142 (22.3) | 118 (31.9) | <0.0001 |
| 7–16 | 367 (36.4) | 207 (32.5) | 160 (43.2) | ||
| ≥17 | 381 (37.8) | 289 (45.3) | 92 (24.9) | ||
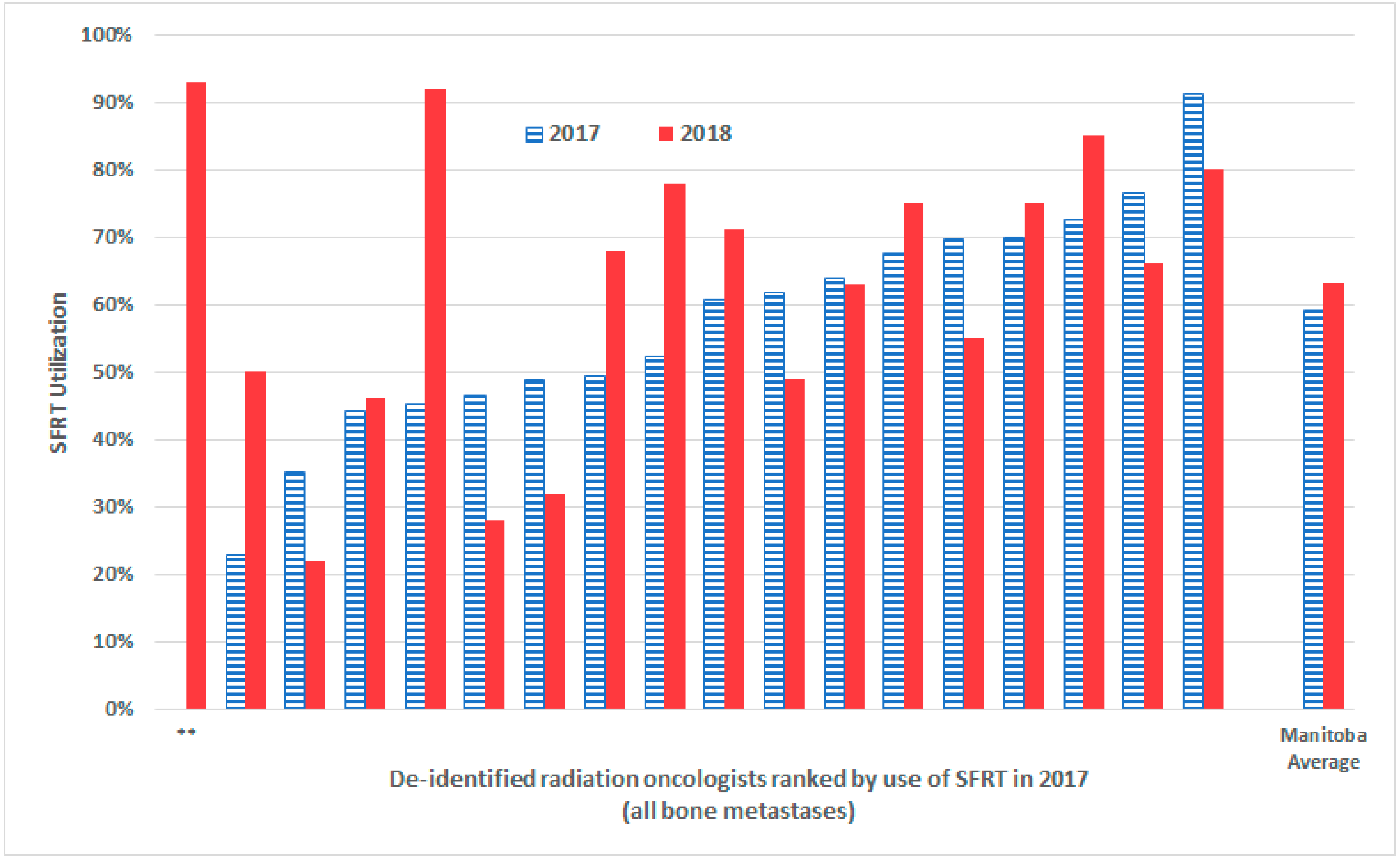
| De-Identified Radiation Oncologist | 2016 %SFRT Utilization (Pre-Campaign) | 2017 %SFRT Utilization (Absolute % Change from Previous Year) | 2018 %SFRT Utilization (Absolute % Change from Previous Year) |
|---|---|---|---|
| A | 21% | 46% (+25%) | 28% (−18%) |
| B | 25% | 49% (+24%) | 32% (−17%) |
| C | 42% | 70% (+28%) | 55% (−15%) |
| D | 23% | 35% (+12%) | 22% (−13%) |
| E | 44% | 61% (+17%) | 49% (−12%) |
| F | 77% | 91% (+14%) | 80% (−11%) |
| G | 32% | 76% (+44%) | 66% (−10%) |
| H | 22% | 64% (+44%) | 63% (−1%) |
| I | 24% | 44% (+20%) | 46% (+2%) |
| J | 34% | 70% (+36%) | 75% (+5%) |
| K | 53% | 68% (+15%) | 75% (+7%) |
| L | 50% | 61% (+11%) | 71% (+10%) |
| M | 55% | 73% (+18%) | 85% (+12%) |
| N | 34% | 52% (+18%) | 78% (+16%) |
| O | 16% | 49% (+33%) | 68% (+19%) |
| P | 0% | 23% (+23%) | 50% (+27%) |
| Q | 23% | 45% (+22%) | 92% (+47%) |
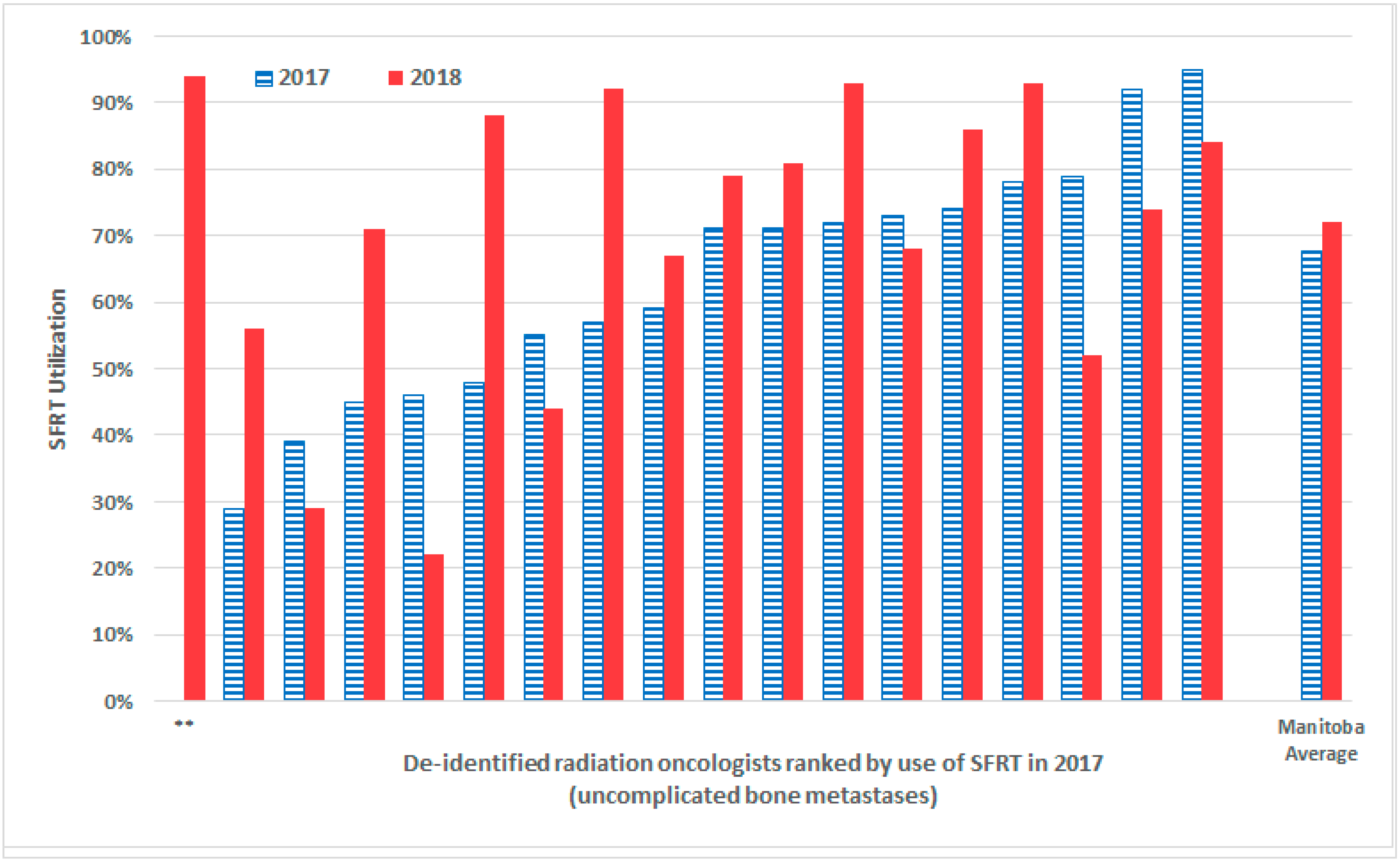
| De-Identified Radiation Oncologist | 2016 %SFRT Utilization (Pre-Campaign) | 2017 %SFRT Utilization (Absolute % Change from Previous Year) | 2018 %SFRT Utilization (Absolute % Change from Previous Year) |
|---|---|---|---|
| A | 67% | 79% (+12%) | 52% (−27%) |
| B | 14% | 46% (+34%) | 22% (−24%) |
| C | 38% | 92% (+54%) | 74% (−18%) |
| D | 36% | 55% (+19%) | 44% (−11%) |
| E | 80% | 95% (+15%) | 84% (−11%) |
| F | 26% | 39% (+13%) | 29% (−10%) |
| G | 26% | 73% (+47%) | 68% (−5%) |
| H | 10% | 59% (+49%) | 67% (+8%) |
| I | 77% | 71%% (−6%) | 79% (+8%) |
| J | 46% | 71% (+25%) | 81% (+10%) |
| K | 64% | 74% (+10%) | 86% (+12%) |
| L | 67% | 78% (+11%) | 93% (+15%) |
| M | 48% | 72% (+24%) | 93% (+21%) |
| N | 24% | 45% (+21%) | 71% (+26%) |
| O | 0% | 29% (+29%) | 56% (+27%) |
| P | 29% | 57% (+28%) | 92% (+35%) |
| Q | 43% | 48% (+5%) | 88% (+40%) |
| Variable | Multivariable Odds Ratio (95%CI) | p-Value | |
|---|---|---|---|
| Age (years) | 5 to ≤57 | Ref | Ref |
| 58 to ≤66 | 0.96 (0.61 to 1.50) | 0.608 | |
| 67 to ≤75 | 0.84 (0.52 to 1.35) | 0.519 | |
| ≥76 | 0.78 (0.48 to 1.27) | 0.484 | |
| Sex | Female | Ref | Ref |
| Male | 1.13 (0.75 to 1.69) | 0.558 | |
| ECOG Performance Status | 0–1 | Ref | Ref |
| 2 | 0.59 (0.40 to 0.87) | 0.007 | |
| 3–4 | 0.57 (0.38 to 0.86) | 0.007 | |
| Charlson Score | 0 | Ref | Ref |
| 1 | 0.73 (0.48 to 1.10) | 0.129 | |
| 2 | 0.77 (0.47 to 1.24) | 0.28 | |
| ≥3 | 0.49 (0.27 to 0.86) | 0.014 | |
| Tumour Type | Prostate | Ref | Ref |
| Breast | 1.71 (0.86 to 3.40) | 0.127 | |
| Lung | 1.63 (0.98 to 2.72) | 0.06 | |
| Hematological | 3.66 (1.90 to 7.05) | <0.0001 | |
| Non-Prostate GU | 2.92 (1.54 to 5.52) | <0.0001 | |
| Gastrointestinal | 1.73 (0.88 to 3.42) | 0.115 | |
| Other | 2.00 (1.05 to 3.78) | 0.034 | |
| Treatment Site | Skull/Spine | Ref | Ref |
| Upper Extremity | 0.30 (0.16 to 0.58) | <0.0001 | |
| Thorax | 0.40 (0.20 to 0.78) | 0.007 | |
| Pelvis | 0.61 (0.43 to 0.89) | 0.01 | |
| Lower Extremity | 0.54 (0.27 to 1.08) | 0.08 | |
| Complicated Bone Metastasis | Uncomplicated | Ref | Ref |
| Complicated | 1.69 (1.18 to 2.41) | 0.004 | |
| Soft Tissue Extension | No | Ref | Ref |
| Yes | 3.80 (2.68 to 5.40) | <0.0001 | |
| Retreatment | No | Ref | Ref |
| Yes | 0.67 (0.41 to 1.09) | 0.105 | |
| Post-Operative Radiotherapy | No | Ref | Ref |
| Yes | 2.77 (1.27 to 6.01) | 0.01 | |
| Treatment Location | Winnipeg | Ref | Ref |
| Brandon | 1.30 (0.78 to 2.15) | 0.308 | |
| Radiation Oncologist Years in Practice | ≤6 | Ref | Ref |
| 7 to 16 | 0.80 (0.51 to 1.24) | 0.312 | |
| ≥17 | 0.30 (0.19 to 0.48) | <0.0001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahhat, S.; Hanumanthappa, N.; Chung, Y.T.; Beck, J.; Koul, R.; Bashir, B.; Cooke, A.; Dubey, A.; Butler, J.; Nashed, M.; et al. Do Sustainable Palliative Single Fraction Radiotherapy Practices Proliferate or Perish 2 Years after a Knowledge Translation Campaign? Curr. Oncol. 2022, 29, 5097-5109. https://doi.org/10.3390/curroncol29070404
Shahhat S, Hanumanthappa N, Chung YT, Beck J, Koul R, Bashir B, Cooke A, Dubey A, Butler J, Nashed M, et al. Do Sustainable Palliative Single Fraction Radiotherapy Practices Proliferate or Perish 2 Years after a Knowledge Translation Campaign? Current Oncology. 2022; 29(7):5097-5109. https://doi.org/10.3390/curroncol29070404
Chicago/Turabian StyleShahhat, Shaheer, Nikesh Hanumanthappa, Youn Tae Chung, James Beck, Rashmi Koul, Bashir Bashir, Andrew Cooke, Arbind Dubey, Jim Butler, Maged Nashed, and et al. 2022. "Do Sustainable Palliative Single Fraction Radiotherapy Practices Proliferate or Perish 2 Years after a Knowledge Translation Campaign?" Current Oncology 29, no. 7: 5097-5109. https://doi.org/10.3390/curroncol29070404
APA StyleShahhat, S., Hanumanthappa, N., Chung, Y. T., Beck, J., Koul, R., Bashir, B., Cooke, A., Dubey, A., Butler, J., Nashed, M., Hunter, W., Ong, A. D., Rathod, S., Tran, K., & Kim, J. O. (2022). Do Sustainable Palliative Single Fraction Radiotherapy Practices Proliferate or Perish 2 Years after a Knowledge Translation Campaign? Current Oncology, 29(7), 5097-5109. https://doi.org/10.3390/curroncol29070404





