Lung Resections for Elderly Patients with Lung Metastases: A Comparative Study of the Postoperative Complications and Overall Survival
Abstract
:1. Introduction
2. Materials and Methods
2.1. Definition of Complications
2.2. Definition of Comorbidities
2.3. Data Collection and Statistical Analysis
3. Results
3.1. Age Characteristics of the Study Population
3.2. Preoperative Comorbidities in the Study Population
3.3. Primary tumor
3.4. Surgical Characteristics
3.5. Resected Lung Metastases
3.6. Postoperative Morbidity and Hospital Stay
3.7. Postoperative Mortality
3.8. Survival after PM
3.9. Prognostic Factors for Survival and Postoperative Morbidity in the Elderly Population after PM
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pfannschmidt, J.; Egerer, G.; Bischof, M.; Thomas, M.; Dienemann, H. Surgical intervention for pulmonary metastases. Dtsch. Arztebl. Int. 2012, 109, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jemal, A.; Center, M.M.; DeSantis, C.; Ward, E.M. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1893–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yancik, R.; Ries, L.A. Cancer in older persons: An international issue in an aging world. Semin. Oncol. 2004, 31, 128–136. [Google Scholar] [CrossRef]
- Barone, M.; Prioletta, M.; Di Nuzzo, D.; Cipollone, G.; Camplese, P.; Mucilli, F. Pulmonary metastasectomy in elderly colorectal cancer patients: A retrospective single center study. Updates Surg. 2016, 68, 357–367. [Google Scholar] [CrossRef]
- Sponholz, S.; Schirren, M.; Oguzhan, S.; Schirren, J. Morbidity, mortality, and survival in elderly patients undergoing pulmonary metastasectomy for colorectal cancer. Int. J. Colorectal Dis. 2018, 33, 1401–1409. [Google Scholar] [CrossRef]
- Jammer, I.; Wickboldt, N.; Sander, M.; Smith, A.; Schultz, M.J.; Pelosi, P.; Leva, B.; Rhodes, A.; Hoeft, A.; Walder, B.; et al. European Society of Anaesthesiology (ESA) and the European Society of Intensive Care Medicine (ESICM); European Society of Anaesthesiology; European Society of Intensive Care Medicine. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: A statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur. J. Anaesthesiol. 2015, 32, 88–105. [Google Scholar]
- Seely, A.; Ivanovic, J.; Threader, J.; Al-Hussaini Derar Al-Shehab, A.; Ramsay, T.; Gilbert, S.; Maziak, D.E.; Shamji, F.; Sundaresan, R.S. Systematic classification of morbidity and mortality after thoracic surgery. Ann. Thorac. Surg. 2010, 90, 936–942, discussion 942. [Google Scholar] [CrossRef]
- Mirza, S.; Clay, R.D.; Koslow, M.A.; Scanlon, P.D. COPD Guidelines: A Review of the 2018 GOLD Report. Mayo Clin. Proc. 2018, 93, 1488–1502. [Google Scholar] [CrossRef] [Green Version]
- Guerrera, F.; Renaud, S.; Schaeffer, M.; Nigra, V.; Solidoro, P.; Santelmo, N.; Filosso, P.L.; Falcoz, P.E.; Ruffini, E.; Oliaro, A.; et al. Low accuracy of computed tomography and positron emission tomography to detect lung and lymph node metastases of colorectal cancer. Ann. Thorac. Surg. 2017, 104, 1194–1199. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Fuster, A.; Belda-Sanchis, J.; Aguiló, R.; Embun, R.; Mojal, S.; Call, S.; Molins, L.; de Andrés, J.J.R. Morbidity and mortality in a large series of surgical patients with pulmonary metastases of colorectal carcinoma: A prospective multicentre Spanish study (GECMP-CCR-SEPAR). Eur. J. Cardiothorac. Surg. 2014, 45, 671–676. [Google Scholar] [CrossRef] [Green Version]
- Spaggiari, L.; Scanagatta, P. Surgery of non-small cell lung cancer in the elderly. Curr. Opin. Oncol. 2007, 19, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Licker, M.; Schweizer, A.; Ellenberger, C.; Tschopp, J.M.; Diaper, J.; Clergue, F. Perioperative medical management of patients with COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2007, 2, 493–515. [Google Scholar] [PubMed]
- Welter, S.; Jacobs, J.; Krbek, T.; Poettgen, C.; Stamatis, G. Prognostic impact of lymph node involvement in pulmonary metastases from colorectal cancer. Eur. J. Cardiothorac. Surg. 2007, 31, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Welter, S.; Jacobs, J.; Krbek, T.; Krebs, B.; Stamatis, G. Long-term survival after repeated resection of pulmonary metastases from colorectal cancer. Ann. Thorac. Surg. 2007, 84, 203–210. [Google Scholar] [CrossRef]
- Onaitis, M.W.; Petersen, R.P.; Haney, J.C.; Saltz, L.; Park, B.; Flores, R.; Rizk, N.; Bains, M.S.; Dycoco, J.; D’Amico, T.A.; et al. Prognostic factors for recurrence after pulmonary resection of colorectal cancer metastases. Ann. Thorac. Surg. 2009, 87, 1684–1688. [Google Scholar] [CrossRef]
- Poce, R.M.; Navarrete, C.P.; Navarrete, J.A.R.; Fernandez, J.R.; Sanchez, R.A.; Domenech, A.B.; Fernández, A.; de Rota Avecilla Bermúdez, J.L.F. Survival analysis of resection of lung metastases from colorectal cancer. Arch. Bronconeumol. 2009, 45, 235–239. [Google Scholar] [CrossRef]
- Martin, J.; Ginsberg, R.J.; Abolhoda, A.; Bains, M.S.; Downey, R.J.; Korst, R.J.; Weigel, T.L.; Kris, M.G.; Venkatraman, E.S.; Rusch, V.W. Morbidity and mortality after neoadjuvant therapy for lung cancer: Therisks of right pneumonectomy. Ann. Thorac. Surg. 2001, 72, 1149–1154. [Google Scholar] [CrossRef]
- Liu, D.; Labow, D.M.; Dang, N.; Martini, N.; Bains, M.; Burt, M.; Downey RJr Rusch JShah, V.; Ginsberg, R.J. Pulmonary metastasectomy for head and neck cancers. Ann. Surg. Oncol. 1999, 6, 572–578. [Google Scholar] [CrossRef]
- Singer, J.; Brauneck, E.; Zwickl-Traxler, E.; Pecherstorfe, M. Evaluation of personalized cancer therapies based on comprehensive genomic profiling in a middle-sized oncologic center in Austria, the University Clinic Krems. Transl. Oncol. 2021, 14, 101021. [Google Scholar] [CrossRef]
- Renaud, S.; Seitlinger, J.; Lawati, Y.; Guerrera, F.; Falcoz, P.-E.; Massard, G.; Ferri, L.; Spicer, J. Anatomical Resections Improve Survival Following Lung Metastasectomy of Colorectal Cancer Harboring KRAS Mutations. Ann. Surg. 2019, 270, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
| Variable | Elderly (n Patients, %) | Non-Elderly (n Patients, %) | p Value |
|---|---|---|---|
| Cardiac comorbidities | <0.001 | ||
| No | 180 (81.1%) | 504 (93.9%) | |
| yes | 42 (18.9%) | 33 (6.1%) | |
| COPD I–III | 0.2 | ||
| No | 204 (92.3%) | 480 (89.4%) | |
| yes | 17 (7.7%) | 57 (10.6%) | |
| Diabetes mellitus | 0.008 | ||
| No | 198 (89.2%) | 506 (94.6%) | |
| yes | 24 (10.8%) | 29 (5.4%) |
| Histological Type | Elderly (n Patients, %) | Non-Elderly (n Patients, %) |
|---|---|---|
| Melanoma | 16 (7.2%) | 41 (7.6%) |
| Germ cell tumor | 0 | 17 (3.2%) |
| Renal cell carcinoma | 27 (12.2%) | 57 (10.6%) |
| Colorectal cancer | 112 (50.7%) | 244 (45.4%) |
| Thyroid cancer | 6 (2.7%) | 12 (2.2%) |
| Ovarian, cervical, and endometrial cancer | 9 (4.1%) | 9 (1.7%) |
| Breast cancer | 4 (1.8%) | 21 (3.9%) |
| Head and neck cancer | 8 (3.6%) | 23 (4.3%) |
| Osteosarcoma | 2 (0.9%) | 28 (5.2%) |
| Soft-tissue sarcoma | 13 (5.9%) | 48 (8.9%) |
| Other malignancies | 24 (10.9%) | 38 (7.1%) |
| Variable * | Elderly (n Patients, %) | Non-Elderly (n Patients, %) | p Value |
|---|---|---|---|
| Patients | 222 (29.2%) | 538 (70.8%) | - |
| Male patients | 139 (62.6%) | 326 (60.6%) | 0.604 |
| DFI (months) | 45.18 ± 32.70 | 73 ± 88.65 | 0.324 |
| Side of pulmonary metastasectomy | |||
| -unilateral | 160 (75.8%) | 317 (60.7%) | |
| -bilateral | 51 (24.2%) | 205 (39.3%) | <0.001 |
| THT/THT | 41 (18.5%) | 166 (30.9%) | <0.01 |
| VATS | 77 (34.7%) | 115 (21.4%) | <0.001 |
| Open thoracotomy | 145 (65.3%) | 423 (78.6%) | <0.001 |
| Wedge resection | 170 (76.1%) | 412 (76.6%) | 0.8 |
| Segmentectomy | 36 (16.2%) | 97 (18%) | 0.5 |
| Lobectomy | 15 (6.8%) | 25 (4.6%) | 0.23 |
| Pneumonectomy | 1 (0.5%) | 4 (0.7%) | 0.65 |
| Number of resected lung metastases | 2.3 ± 2.6 | 4.1 ± 5.4 | <0.01 |
| Single metastasis | 118 (53.2%) | 218 (40.5%) | 0.001 |
| N1 lymph node metastasis | 6 (2.7%) | 28 (5.2%) | 0.1 |
| N2 lymph node metastasis | 15 (6.8%) | 49 (9.1%) | 0.2 |
| R1 Status | 8 (3.6%) | 39 (7.3%) | 0.04 |
| Repeated pulmonary metastasectomy | 32 (14.4%) | 133 (24.7%) | 0.002 |
| Second Repeated pulmonary metastasectomy | 7 (3.2%) | 50 (9.3%) | 0.003 |
| Variable | Elderly (n Patients, %) | Non-Elderly (n Patients, %) | p Value |
|---|---|---|---|
| Postoperative complication | 44 (19.8%) | 125 (23.2%) | 0.3 |
| Minor complication | 33 (14.4%) | 99 (18.2%) | 0.2 |
| Major complication | 11 (5%) | 26 (4.8%) | 0.2 |
| Pneumonia | 12 (5.4%) | 27 (5%) | 0.8 |
| Atrial fibrillation | 3 (1.4%) | 8 (1.5%) | 0.8 |
| Intestinal obstruction | 0 (0%) | 6 (1.1%) | 0.04 |
| Surgical revision because of complication | 7 (3.2%) | 15 (2.8%) | 0.7 |
| Pulmonary embolism | 1 (0.5%) | 1 (0.2%) | 0.5 |
| Pleural empyema | 1 (0.5%) | 6 (1.1%) | 0.3 |
| Wound-healing disorder | 0 (0%) | 5 (0.9%) | 0.06 |
| Chest tube placement (pneumothorax, pleural effusion) | 4 (1.8%) | 20 (3.7%) | 0.1 |
| Prolonged air leak (>6 days) | 2 (0.9%) | 5 (0.9%) | 0.9 |
| Cardiovascular complication | 6 (2.7%) | 11 (2.0%) | 0.5 |
| Other (e.g., electrolyte disorders) | 6 (2.7%) | 21 (3.7%) | - |
| Length of Hospital stay (days) | 10 ± 5 | 9.6 ± 4.3 | 0.3 |
| In-hospital mortality | 0 | 0 | - |
| 30-day mortality | 2 (1.1%) | 4 (0.8%) | 0.7 |
| 90-day mortality | 8 (4.3%) | 10 (2.1%) | 0.1 |
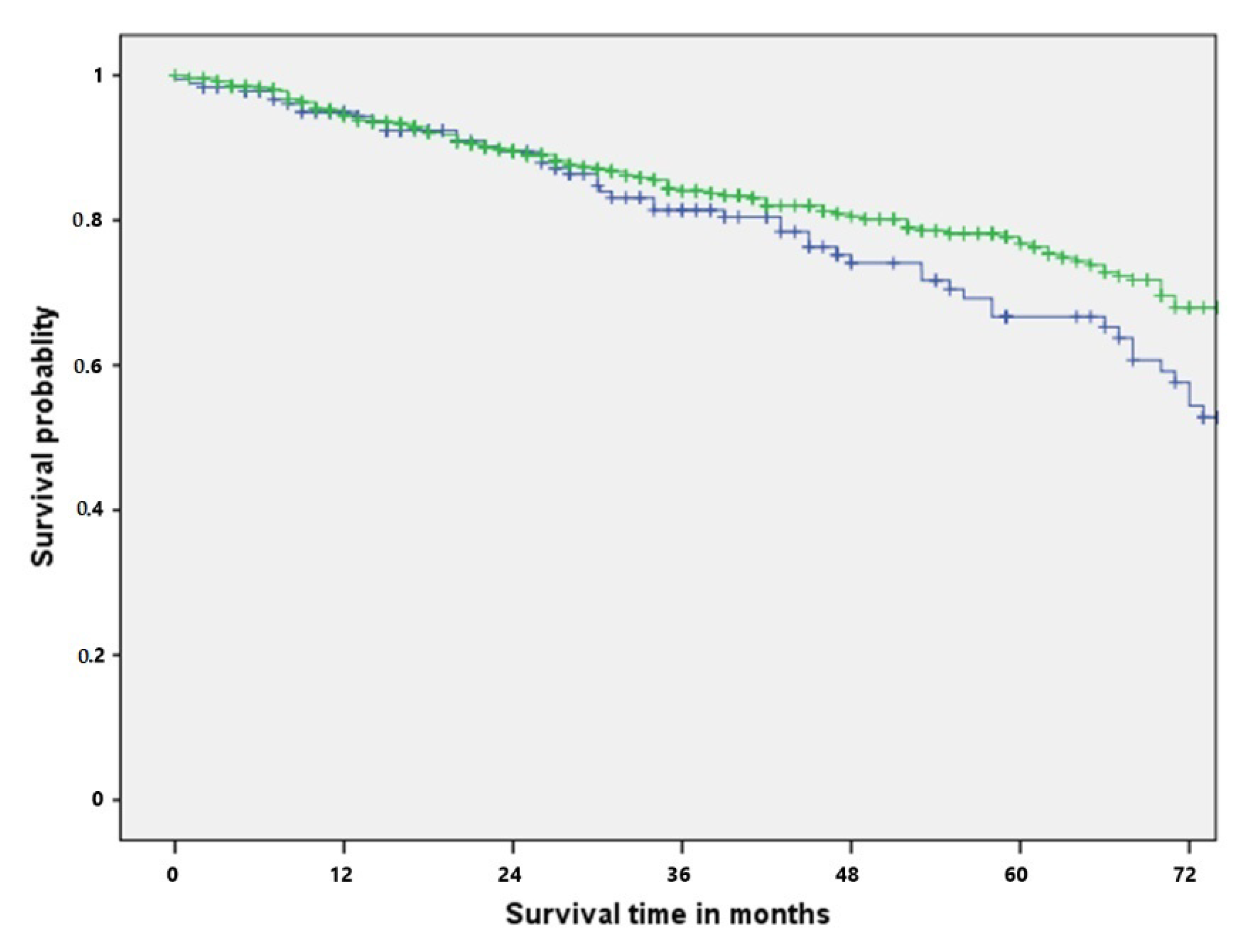
| 3-year survival | 83.1% | 86.2% | 0.117 |
| 5-year survival | 66.7% | 77.7% |
| Variable | Univariate Analysis | ||
|---|---|---|---|
| HR | 95% CI | p Value | |
| Male patients | 1.049 | 0.387–2.845 | 0.925 |
| Cardiac comorbidities | 1.521 | 0.494–4.677 | 0.465 |
| VATS | 1.627 | 0.466–5.678 | 0.445 |
| COPD I–III | 9.201 | 1.890–44.800 | 0.006 |
| Diabetes mellitus | 0.042 | 0.01–53.619 | 0.385 |
| THT/THT | 0.961 | 0.276–3.349 | 0.95 |
| Lobectomy | 0.520 | 0.148–1.831 | 0.309 |
| Variable | Overall Survival | ||
|---|---|---|---|
| Univariate Analysis | |||
| HR | 95% CI | p Value | |
| Male patients | 1.226 | 0.651–2.310 | 0.528 |
| Cardiac comorbidities | 1.105 | 0.489–2.500 | 0.81 |
| COPD I–III | 7.401 | 2.139–25.606 | 0.002 |
| Diabetes mellitus | 0.831 | 0.255–2.704 | 0.759 |
| THT/THT | 1.081 | 0.498–2.346 | 0.844 |
| VATS | 1.650 | 0.729–3.733 | 0.230 |
| Lobectomy | 0.735 | 0.261–2.067 | 0.559 |
| Colorectal cancer | 0.960 | 0.851–1.083 | 0.506 |
| DFI | 1.001 | 0.999–1.002 | 0.309 |
| R Status | 1.281 | 0.926–1.774 | 0.135 |
| N2—lymph node metastases | 1.106 | 0.896–1.365 | 0.347 |
| Postoperative comorbidity | 1.172 | 0.58–2.653 | 0.703 |
| Major postoperative complication | 0.888 | 0.169–4.660 | 0.88 |
| Postoperative cardiovascular complication | 0.6 | 0.09–4.9 | 0.6 |
| >4 resected lung metastases | 0.734 | 0.261–2.064 | 0.558 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.; Ehle, B.; Passlick, B.; Grapatsas, K. Lung Resections for Elderly Patients with Lung Metastases: A Comparative Study of the Postoperative Complications and Overall Survival. Curr. Oncol. 2022, 29, 4511-4521. https://doi.org/10.3390/curroncol29070357
Hassan M, Ehle B, Passlick B, Grapatsas K. Lung Resections for Elderly Patients with Lung Metastases: A Comparative Study of the Postoperative Complications and Overall Survival. Current Oncology. 2022; 29(7):4511-4521. https://doi.org/10.3390/curroncol29070357
Chicago/Turabian StyleHassan, Mohamed, Benjamin Ehle, Bernward Passlick, and Konstantinos Grapatsas. 2022. "Lung Resections for Elderly Patients with Lung Metastases: A Comparative Study of the Postoperative Complications and Overall Survival" Current Oncology 29, no. 7: 4511-4521. https://doi.org/10.3390/curroncol29070357
APA StyleHassan, M., Ehle, B., Passlick, B., & Grapatsas, K. (2022). Lung Resections for Elderly Patients with Lung Metastases: A Comparative Study of the Postoperative Complications and Overall Survival. Current Oncology, 29(7), 4511-4521. https://doi.org/10.3390/curroncol29070357





