Non-Alcoholic Fatty Liver Disease and Extrahepatic Cancers: A Wolf in Sheep’s Clothing?
Abstract
:1. Introduction
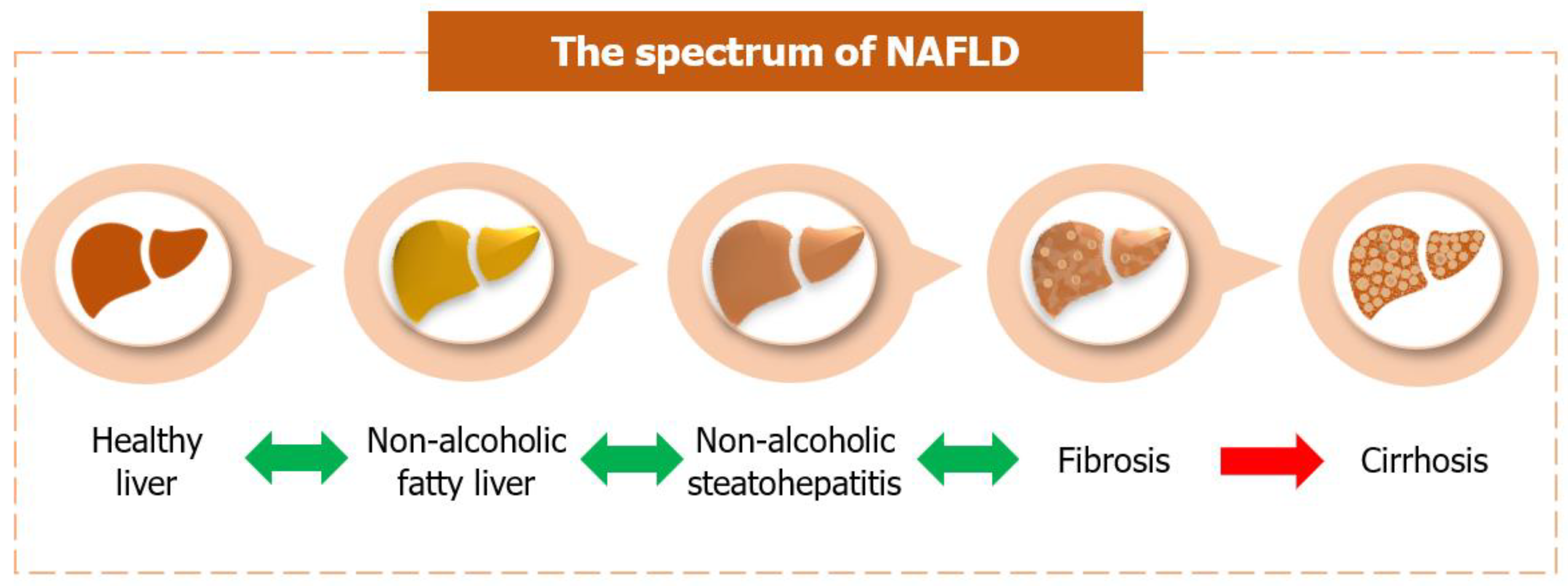
2. Overview of Non-Alcoholic Fatty Liver Disease (NAFLD)
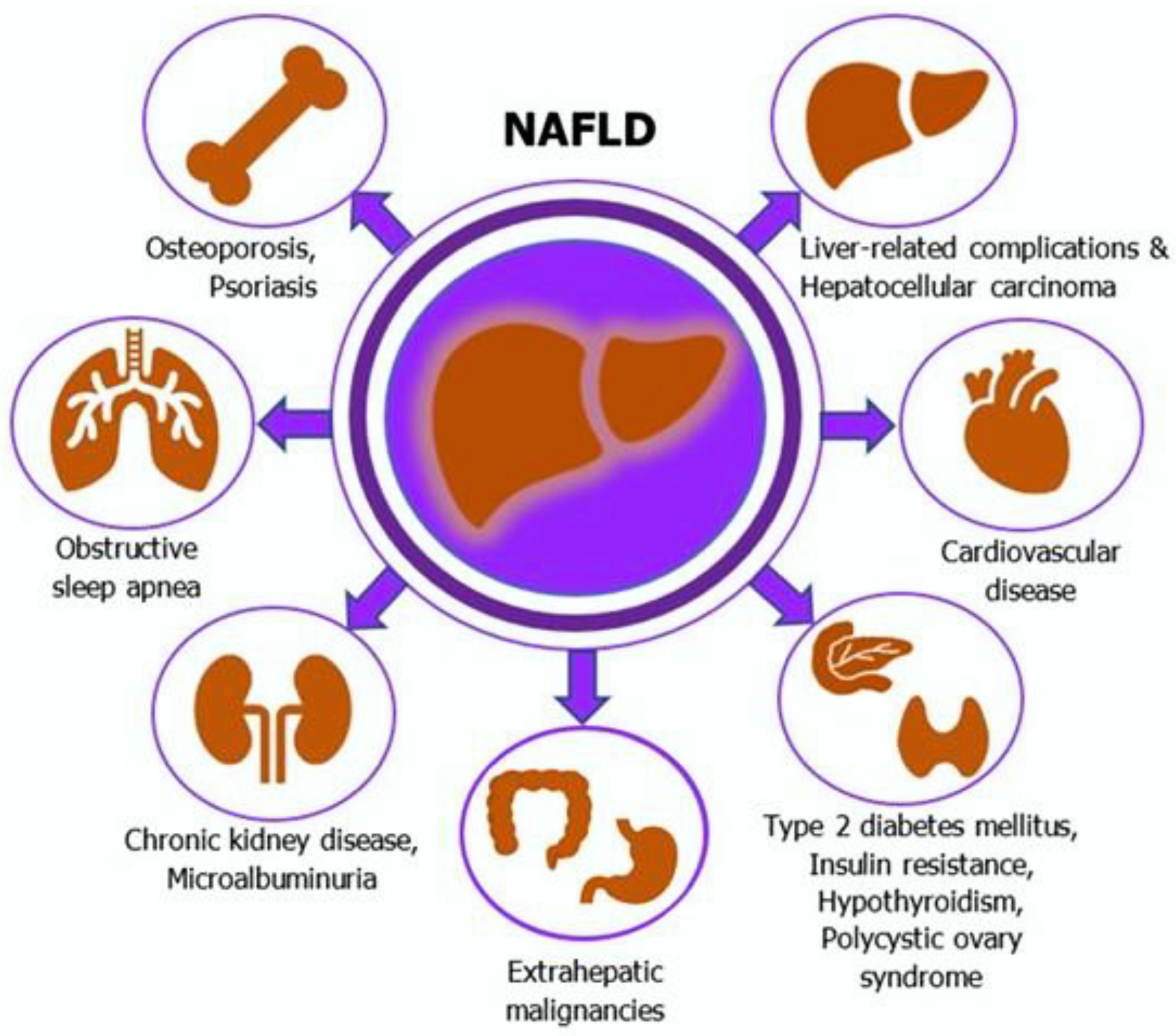
2.1. Epidemiology and Risk Factors
2.2. NAFLD Pathogenesis
2.3. Histological Features
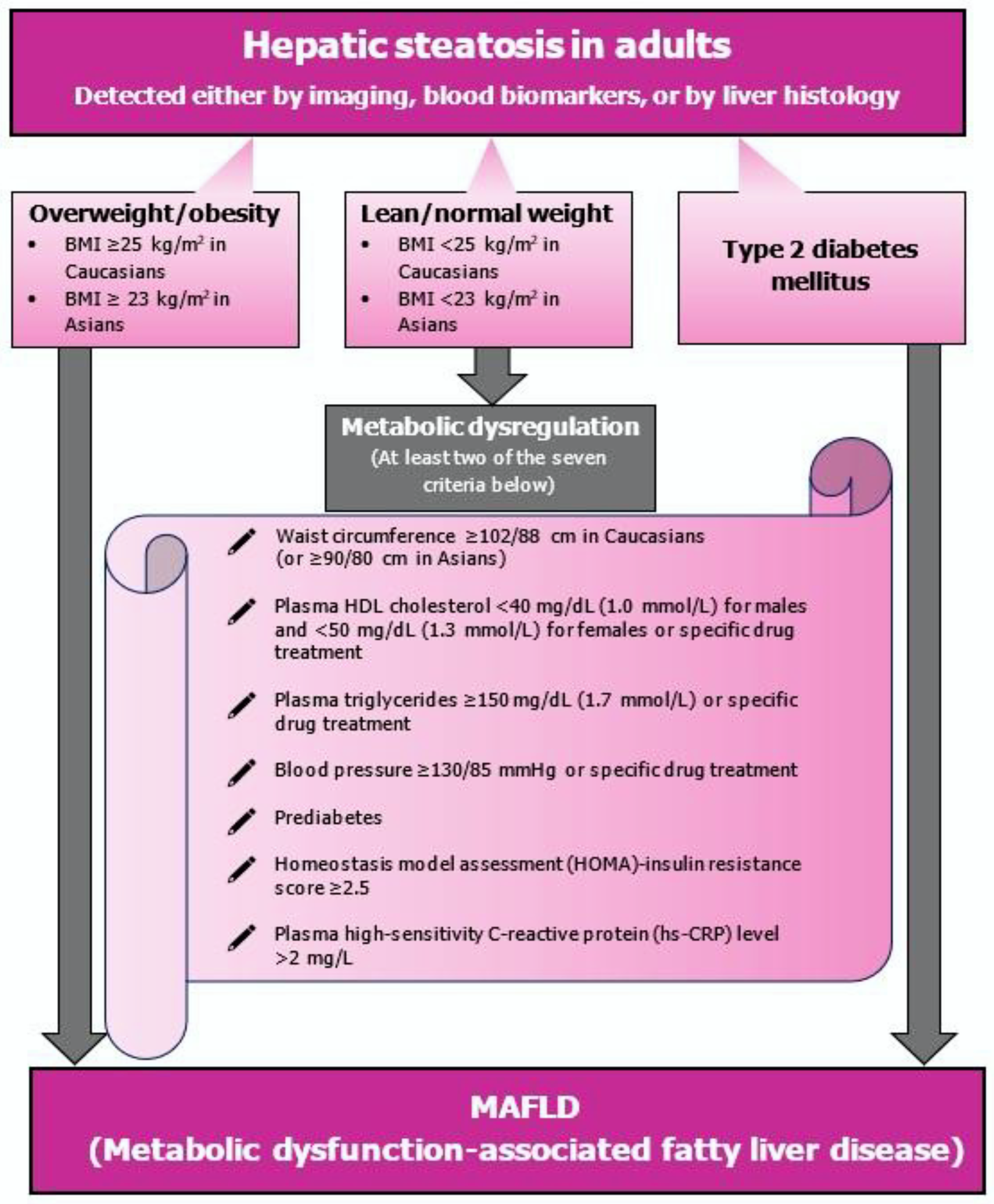
2.4. Clinical Features and Diagnostic Workup
- Hepatic steatosis index (BMI, gender, diabetes and the AST/ALT ratio) [125],
- Lipid accumulation product (waist circumference and triglycerides) [126],
- Triglyceride-glucose (TyG) index (fasting glucose and triglyceride levels) [127],
- Visceral adiposity index (waist circumference, BMI, triglycerides and high-density lipoprotein cholesterol levels) [128].
2.5. Treatment
3. Association between NAFLD and Extrahepatic Cancers
3.1. NAFLD and Colorectal Adenomas
3.2. NAFLD and Colorectal Cancer
3.3. Pathophysiological Links between NAFLD, Colorectal Adenomas and Cancer
3.4. NAFLD and Other Extrahepatic Cancers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lazarus, J.V.; Colombo, M.; Cortez-Pinto, H.; Huang, T.T.-K.; Miller, V.; Ninburg, M.; Schattenberg, J.M.; Seim, L.; Wong, V.W.S.; Zelber-Sagi, S. NAFLD-Sounding the Alarm on a Silent Epidemic. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.J.; Aguilar, M.; Cheung, R.; Perumpail, R.B.; Harrison, S.A.; Younossi, Z.M.; Ahmed, A. Nonalcoholic Steatohepatitis Is the Second Leading Etiology of Liver Disease among Adults Awaiting Liver Transplantation in the United States. Gastroenterology 2015, 148, 547–555. [Google Scholar] [CrossRef]
- McCullough, A.J. Pathophysiology of Nonalcoholic Steatohepatitis. J. Clin. Gastroenterol. 2006, 40, S17–S29. [Google Scholar]
- Serfaty, L.; Lemoine, M. Definition and Natural History of Metabolic Steatosis: Clinical Aspects of NAFLD, NASH and Cirrhosis. Diabetes Metab. 2008, 34, 634–637. [Google Scholar] [CrossRef]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a Continuum: From Obesity to Metabolic Syndrome and Diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef]
- Alam, S.; Mustafa, G.; Alam, M.; Ahmad, N. Insulin Resistance in Development and Progression of Nonalcoholic Fatty Liver Disease. World J. Gastrointest. Pathophysiol. 2016, 7, 211–217. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A Multisystem Disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, M.J.; Adams, L.A.; Canbay, A.; Syn, W.-K. Extrahepatic Complications of Nonalcoholic Fatty Liver Disease: Hepatology. Hepatology 2014, 59, 1174–1197. [Google Scholar] [CrossRef]
- Kim, S.; Keku, T.O.; Martin, C.; Galanko, J.; Woosley, J.T.; Schroeder, J.C.; Satia, J.A.; Halabi, S.; Sandler, R.S. Circulating Levels of Inflammatory Cytokines and Risk of Colorectal Adenomas. Cancer Res. 2008, 68, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Sanna, C.; Rosso, C.; Marietti, M.; Bugianesi, E. Non-Alcoholic Fatty Liver Disease and Extra-Hepatic Cancers. Int. J. Mol. Sci. 2016, 17, 717. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.-S.; Ma, X.-F.; Zhao, J.; Du, S.-X.; Zhang, J.; Dong, M.-Z.; Xin, Y.-N. Association between Nonalcoholic Fatty Liver Disease and Extrahepatic Cancers: A Systematic Review and Meta-Analysis. Lipids Health Dis. 2020, 19, 118. [Google Scholar] [CrossRef] [PubMed]
- Marjot, T.; Moolla, A.; Cobbold, J.F.; Hodson, L.; Tomlinson, J.W. Nonalcoholic Fatty Liver Disease in Adults: Current Concepts in Etiology, Outcomes, and Management. Endocr. Rev. 2020, 41, 66–117. [Google Scholar] [CrossRef] [PubMed]
- Funuyet-Salas, J.; Pérez-San-Gregorio, M.Á.; Martín-Rodríguez, A.; Romero-Gómez, M. Quality of Life and Coping in Nonalcoholic Fatty Liver Disease: Influence of Diabetes and Obesity. Int. J. Environ. Res. Public Health 2021, 18, 3503. [Google Scholar] [CrossRef] [PubMed]
- Tokushige, K.; Ikejima, K.; Ono, M.; Eguchi, Y.; Kamada, Y.; Itoh, Y.; Akuta, N.; Yoneda, M.; Iwasa, M.; Yoneda, M.; et al. Evidence-Based Clinical Practice Guidelines for Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis 2020. Hepatol. Res. 2021, 51, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Hashimoto, E.; Ikejima, K.; Uto, H.; Ono, M.; Sumida, Y.; Seike, M.; Takei, Y.; Takehara, T.; Tokushige, K.; et al. Evidence-Based Clinical Practice Guidelines for Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. J. Gastroenterol. 2015, 50, 364–377. [Google Scholar] [CrossRef]
- Lonardo, A.; Ballestri, S.; Marchesini, G.; Angulo, P.; Loria, P. Nonalcoholic Fatty Liver Disease: A Precursor of the Metabolic Syndrome. Dig. Liver Dis. 2015, 47, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Vanni, E.; Bugianesi, E.; Kotronen, A.; De Minicis, S.; Yki-Järvinen, H.; Svegliati-Baroni, G. From the Metabolic Syndrome to NAFLD or Vice Versa? Dig. Liver Dis. 2010, 42, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.-F.; Schattenberg, J.M.; et al. A New Definition for Metabolic Dysfunction-Associated Fatty Liver Disease: An International Expert Consensus Statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Méndez-Sánchez, N.; Díaz-Orozco, L.; Córdova-Gallardo, J. Redefinition of Fatty Liver Disease from NAFLD to MAFLD Raised Disease Awareness: Mexican Experience. J. Hepatol. 2021, 75, 221–222. [Google Scholar] [CrossRef]
- Fouad, Y.; Waked, I.; Bollipo, S.; Gomaa, A.; Ajlouni, Y.; Attia, D. What’s in a Name? Renaming “NAFLD” to “MAFLD”. Liver Int. 2020, 40, 1254–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eguchi, Y.; Hyogo, H.; Ono, M.; Mizuta, T.; Ono, N.; Fujimoto, K.; Chayama, K.; Saibara, T.; JSG-NAFLD. Prevalence and Associated Metabolic Factors of Nonalcoholic Fatty Liver Disease in the General Population from 2009 to 2010 in Japan: A Multicenter Large Retrospective Study. J. Gastroenterol. 2012, 47, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Summart, U.; Thinkhamrop, B.; Chamadol, N.; Khuntikeo, N.; Songthamwat, M.; Kim, C.S. Gender Differences in the Prevalence of Nonalcoholic Fatty Liver Disease in the Northeast of Thailand: A Population-Based Cross-Sectional Study. F1000Research 2017, 6, 1630. [Google Scholar] [CrossRef] [PubMed]
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Nonalcoholic Steatohepatitis: A Review. JAMA 2020, 323, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Marchesini, G.; Pinto-Cortez, H.; Petta, S. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: Implications for Liver Transplantation: Implications for Liver Transplantation. Transplantation 2019, 103, 22–27. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Mirza, M.S. Obesity, Visceral Fat, and NAFLD: Querying the Role of Adipokines in the Progression of Nonalcoholic Fatty Liver Disease. ISRN Gastroenterol. 2011, 2011, 592404. [Google Scholar] [CrossRef] [Green Version]
- Arner, P. Not All Fat Is Alike. Lancet 1998, 351, 1301–1302. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Pallayova, M.; Taheri, S. Non-Alcoholic Fatty Liver Disease in Obese Adults: Clinical Aspects and Current Management Strategies: Non-Alcoholic Fatty Liver Disease in Obese Adults. Clin. Obes. 2014, 4, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Perumpail, B.J.; Khan, M.A.; Yoo, E.R.; Cholankeril, G.; Kim, D.; Ahmed, A. Clinical Epidemiology and Disease Burden of Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2017, 23, 8263–8276. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.; Fernández-Gil, P.; Hernández-Guerra, M.; Cayón, A.; Mayorga, M.; Domínguez-Diez, A.; Fernández-Escalante, J.C.; Pons-Romero, F. Are There Predictive Factors of Severe Liver Fibrosis in Morbidly Obese Patients with Non-Alcoholic Steatohepatitis? Obes. Surg. 2001, 11, 254–257. [Google Scholar] [CrossRef]
- Dixon, J.B.; Bhathal, P.S.; O’Brien, P.E. Nonalcoholic Fatty Liver Disease: Predictors of Nonalcoholic Steatohepatitis and Liver Fibrosis in the Severely Obese. Gastroenterology 2001, 121, 91–100. [Google Scholar] [CrossRef]
- Beymer, C.; Kowdley, K.V.; Larson, A.; Edmonson, P.; Dellinger, E.P.; Flum, D.R. Prevalence and Predictors of Asymptomatic Liver Disease in Patients Undergoing Gastric Bypass Surgery. Arch. Surg. 2003, 138, 1240–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholam, P.M.; Kotler, D.P.; Flancbaum, L.J. Liver Pathology in Morbidly Obese Patients Undergoing Roux-En-Y Gastric Bypass Surgery. Obes. Surg. 2002, 12, 49–51. [Google Scholar] [CrossRef]
- Peng, L.; Wu, S.; Zhou, N.; Zhu, S.; Liu, Q.; Li, X. Clinical Characteristics and Risk Factors of Nonalcoholic Fatty Liver Disease in Children with Obesity. BMC Pediatr. 2021, 21, 122. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Deutsch, R.; Kahen, T.; Lavine, J.E.; Stanley, C.; Behling, C. Prevalence of Fatty Liver in Children and Adolescents. Pediatrics 2006, 118, 1388–1393. [Google Scholar] [CrossRef]
- Stefan, N.; Häring, H.-U.; Cusi, K. Non-Alcoholic Fatty Liver Disease: Causes, Diagnosis, Cardiometabolic Consequences, and Treatment Strategies. Lancet Diabetes Endocrinol. 2019, 7, 313–324. [Google Scholar] [CrossRef]
- Anderson, E.L.; Howe, L.D.; Jones, H.E.; Higgins, J.P.T.; Lawlor, D.A.; Fraser, A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0140908. [Google Scholar] [CrossRef] [Green Version]
- Dai, W.; Ye, L.; Liu, A.; Wen, S.W.; Deng, J.; Wu, X.; Lai, Z. Prevalence of Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus: A Meta-Analysis: A Meta-Analysis. Medicine 2017, 96, e8179. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The Global Epidemiology of NAFLD and NASH in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Jinjuvadia, R.; Antaki, F.; Lohia, P.; Liangpunsakul, S. The Association between Nonalcoholic Fatty Liver Disease and Metabolic Abnormalities in the United States Population. J. Clin. Gastroenterol. 2017, 51, 160–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.M.; Stepanova, M.; Negro, F.; Hallaji, S.; Younossi, Y.; Lam, B.; Srishord, M. Nonalcoholic Fatty Liver Disease in Lean Individuals in the United States. Medicine 2012, 91, 319–327. [Google Scholar] [CrossRef]
- Fracanzani, A.L.; Petta, S.; Lombardi, R.; Pisano, G.; Russello, M.; Consonni, D.; Di Marco, V.; Cammà, C.; Mensi, L.; Dongiovanni, P.; et al. Liver and Cardiovascular Damage in Patients with Lean Nonalcoholic Fatty Liver Disease, and Association with Visceral Obesity. Clin. Gastroenterol. Hepatol. 2017, 15, 1604–1611.e1. [Google Scholar] [CrossRef]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stål, P.; Hultcrantz, R.; Kechagias, S. Risk for Development of Severe Liver Disease in Lean Patients with Nonalcoholic Fatty Liver Disease: A Long-Term Follow-up Study. Hepatol. Commun. 2018, 2, 48–57. [Google Scholar] [CrossRef]
- Leung, J.C.-F.; Loong, T.C.-W.; Wei, J.L.; Wong, G.L.-H.; Chan, A.W.-H.; Choi, P.C.-L.; Shu, S.S.-T.; Chim, A.M.-L.; Chan, H.L.-Y.; Wong, V.W.-S. Histological Severity and Clinical Outcomes of Nonalcoholic Fatty Liver Disease in Nonobese Patients. Hepatology 2017, 65, 54–64. [Google Scholar] [CrossRef]
- Wei, J.L.; Leung, J.C.-F.; Loong, T.C.-W.; Wong, G.L.-H.; Yeung, D.K.-W.; Chan, R.S.-M.; Chan, H.L.-Y.; Chim, A.M.-L.; Woo, J.; Chu, W.C.-W.; et al. Prevalence and Severity of Nonalcoholic Fatty Liver Disease in Non-Obese Patients: A Population Study Using Proton-Magnetic Resonance Spectroscopy. Am. J. Gastroenterol. 2015, 110, 1306–1314, quiz 1315. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global Burden of NAFLD and NASH: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Non-alcoholic Fatty Liver Disease Study Group; Lonardo, A.; Bellentani, S.; Argo, C.K.; Ballestri, S.; Byrne, C.D.; Caldwell, S.H.; Cortez-Pinto, H.; Grieco, A.; Machado, M.V.; et al. Epidemiological Modifiers of Non-Alcoholic Fatty Liver Disease: Focus on High-Risk Groups. Dig. Liver Dis. 2015, 47, 997–1006. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.M.; Brancati, F.L.; Diehl, A.M. Nonalcoholic Fatty Liver Disease. Gastroenterology 2002, 122, 1649–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, C.P.; James, O.F. Steatohepatitis: A Tale of Two “Hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Fotbolcu, H.; Zorlu, E. Nonalcoholic Fatty Liver Disease as a Multi-Systemic Disease. World J. Gastroenterol. 2016, 22, 4079–4090. [Google Scholar] [CrossRef]
- Peverill, W.; Powell, L.W.; Skoien, R. Evolving Concepts in the Pathogenesis of NASH: Beyond Steatosis and Inflammation. Int. J. Mol. Sci. 2014, 15, 8591–8638. [Google Scholar] [CrossRef] [PubMed]
- Basaranoglu, M.; Neuschwander-Tetri, B.A. Nonalcoholic Fatty Liver Disease: Clinical Features and Pathogenesis. Gastroenterol. Hepatol. 2006, 2, 282–291. [Google Scholar]
- Lewis, G.F.; Carpentier, A.; Adeli, K.; Giacca, A. Disordered Fat Storage and Mobilization in the Pathogenesis of Insulin Resistance and Type 2 Diabetes. Endocr. Rev. 2002, 23, 201–229. [Google Scholar] [CrossRef]
- Haque, M.; Sanyal, A.J. The Metabolic Abnormalities Associated with Non-Alcoholic Fatty Liver Disease. Best Pract. Res. Clin. Gastroenterol. 2002, 16, 709–731. [Google Scholar] [CrossRef]
- Browning, J.D.; Horton, J.D. Molecular Mediators of Hepatic Steatosis and Liver Injury. J. Clin. Investig. 2004, 114, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Papandreou, D.; Rousso, I.; Mavromichalis, I. Update on Non-Alcoholic Fatty Liver Disease in Children. Clin. Nutr. 2007, 26, 409–415. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A.; Caldwell, S.H. Nonalcoholic Steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology 2003, 37, 1202–1219. [Google Scholar] [CrossRef]
- Pessayre, D.; Fromenty, B. NASH: A Mitochondrial Disease. J. Hepatol. 2005, 42, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Gastaldelli, A.; Vanni, E.; Gambino, R.; Cassader, M.; Baldi, S.; Ponti, V.; Pagano, G.; Ferrannini, E.; Rizzetto, M. Insulin Resistance in Non-Diabetic Patients with Non-Alcoholic Fatty Liver Disease: Sites and Mechanisms. Diabetologia 2005, 48, 634–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crespo, J.; Cayón, A.; Fernández-Gil, P.; Hernández-Guerra, M.; Mayorga, M.; Domínguez-Díez, A.; Fernández-Escalante, J.C.; Pons-Romero, F. Gene Expression of Tumor Necrosis Factor Alpha and TNF-Receptors, P55 and P75, in Nonalcoholic Steatohepatitis Patients. Hepatology 2001, 34, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Chitturi, S.; Farrell, G.; Frost, L.; Kriketos, A.; Lin, R.; Fung, C.; Liddle, C.; Samarasinghe, D.; George, J. Serum Leptin in NASH Correlates with Hepatic Steatosis but Not Fibrosis: A Manifestation of Lipotoxicity?: Serum Leptin in NASH Correlates with Hepatic Steatosis but Not Fibrosis: A Manifestation of Lipotoxicity? Hepatology 2002, 36, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Shimomura, I.; Kishida, K.; Nishizawa, H.; Matsuda, M.; Nagaretani, H.; Furuyama, N.; Kondo, H.; Takahashi, M.; Arita, Y.; et al. Diet-Induced Insulin Resistance in Mice Lacking Adiponectin/ACRP30. Nat. Med. 2002, 8, 731–737. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Y.; Keshaw, H.; Xu, L.Y.; Lam, K.S.L.; Cooper, G.J.S. The Fat-Derived Hormone Adiponectin Alleviates Alcoholic and Nonalcoholic Fatty Liver Diseases in Mice. J. Clin. Investig. 2003, 112, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Hui, J.M.; Hodge, A.; Farrell, G.C.; Kench, J.G.; Kriketos, A.; George, J. Beyond Insulin Resistance in NASH: TNF-α or Adiponectin? Hepatology 2004, 40, 46–54. [Google Scholar] [CrossRef]
- Ambroszkiewicz, J.; Chełchowska, M.; Rowicka, G.; Klemarczyk, W.; Strucińska, M.; Gajewska, J. Anti-Inflammatory and pro-Inflammatory Adipokine Profiles in Children on Vegetarian and Omnivorous Diets. Nutrients 2018, 10, 1241. [Google Scholar] [CrossRef] [Green Version]
- Ganz, M.; Szabo, G. Immune and Inflammatory Pathways in NASH. Hepatol. Int. 2013, 7, 771–781. [Google Scholar] [CrossRef] [Green Version]
- Bugianesi, E.; Pagotto, U.; Manini, R.; Vanni, E.; Gastaldelli, A.; de Iasio, R.; Gentilcore, E.; Natale, S.; Cassader, M.; Rizzetto, M.; et al. Plasma Adiponectin in Nonalcoholic Fatty Liver Is Related to Hepatic Insulin Resistance and Hepatic Fat Content, Not to Liver Disease Severity. J. Clin. Endocrinol. Metab. 2005, 90, 3498–3504. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of Inflammation in Nonalcoholic Fatty Liver Disease: The Multiple Parallel Hits Hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Moscatiello, S.; Ciaravella, M.F.; Marchesini, G. Insulin Resistance in Nonalcoholic Fatty Liver Disease. Curr. Pharm. Des. 2010, 16, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.; Virbasius, J.V.; Puri, V. Adipocyte Dysfunctions Linking Obesity to Insulin Resistance and Type 2 Diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cusi, K. Role of Insulin Resistance and Lipotoxicity in Nonalcoholic Steatohepatitis. Clin. Liver Dis. 2009, 13, 545–563. [Google Scholar] [CrossRef]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- Ma, Y.; Lee, G.; Heo, S.-Y.; Roh, Y.-S. Oxidative Stress Is a Key Modulator in the Development of Nonalcoholic Fatty Liver Disease. Antioxidants 2021, 11, 91. [Google Scholar] [CrossRef]
- Prasun, P.; Ginevic, I.; Oishi, K. Mitochondrial Dysfunction in Nonalcoholic Fatty Liver Disease and Alcohol Related Liver Disease. Transl. Gastroenterol. Hepatol. 2021, 6, 4. [Google Scholar] [CrossRef]
- Ucar, F.; Sezer, S.; Erdogan, S.; Akyol, S.; Armutcu, F.; Akyol, O. The Relationship between Oxidative Stress and Nonalcoholic Fatty Liver Disease: Its Effects on the Development of Nonalcoholic Steatohepatitis. Redox Rep. 2013, 18, 127–133. [Google Scholar] [CrossRef]
- Liu, J.; Li, D.; Zhang, T.; Tong, Q.; Ye, R.D.; Lin, L. SIRT3 Protects Hepatocytes from Oxidative Injury by Enhancing ROS Scavenging and Mitochondrial Integrity. Cell Death Dis. 2017, 8, e3158. [Google Scholar] [CrossRef] [Green Version]
- Kirpich, I.A.; Marsano, L.S.; McClain, C.J. Gut-Liver Axis, Nutrition, and Non-Alcoholic Fatty Liver Disease. Clin. Biochem. 2015, 48, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, Y. Review Article: Is Non-Alcoholic Fatty Liver Disease a Spectrum, or Are Steatosis and Non-Alcoholic Steatohepatitis Distinct Conditions? Aliment. Pharmacol. Ther. 2012, 36, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic Variation in PNPLA3 Confers Susceptibility to Nonalcoholic Fatty Liver Disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenti, L.; Alisi, A.; Galmozzi, E.; Bartuli, A.; Del Menico, B.; Alterio, A.; Dongiovanni, P.; Fargion, S.; Nobili, V. I148M Patatin-like Phospholipase Domain-Containing 3 Gene Variant and Severity of Pediatric Nonalcoholic Fatty Liver Disease. Hepatology 2010, 52, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Sumida, Y.; Umemura, A.; Matsuo, K.; Takahashi, M.; Takamura, T.; Yasui, K.; Saibara, T.; Hashimoto, E.; Kawanaka, M.; et al. Genetic Polymorphisms of the Human PNPLA3 Gene Are Strongly Associated with Severity of Non-Alcoholic Fatty Liver Disease in Japanese. PLoS ONE 2012, 7, e38322. [Google Scholar] [CrossRef] [Green Version]
- Longo, M.; Meroni, M.; Paolini, E.; Erconi, V.; Carli, F.; Fortunato, F.; Ronchi, D.; Piciotti, R.; Sabatini, S.; Macchi, C.; et al. TM6SF2/PNPLA3/MBOAT7 Loss-of-Function Genetic Variants Impact on NAFLD Development and Progression Both in Patients and in in Vitro Models. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 759–788. [Google Scholar] [CrossRef]
- He, S.; McPhaul, C.; Li, J.Z.; Garuti, R.; Kinch, L.; Grishin, N.V.; Cohen, J.C.; Hobbs, H.H. A Sequence Variation (I148M) in PNPLA3 Associated with Nonalcoholic Fatty Liver Disease Disrupts Triglyceride Hydrolysis. J. Biol. Chem. 2010, 285, 6706–6715. [Google Scholar] [CrossRef] [Green Version]
- Pingitore, P.; Pirazzi, C.; Mancina, R.M.; Motta, B.M.; Indiveri, C.; Pujia, A.; Montalcini, T.; Hedfalk, K.; Romeo, S. Recombinant PNPLA3 Protein Shows Triglyceride Hydrolase Activity and Its I148M Mutation Results in Loss of Function. Biochim. Biophys. Acta 2014, 1841, 574–580. [Google Scholar] [CrossRef] [Green Version]
- BasuRay, S.; Smagris, E.; Cohen, J.C.; Hobbs, H.H. The PNPLA3 Variant Associated with Fatty Liver Disease (I148M) Accumulates on Lipid Droplets by Evading Ubiquitylation: Basuray et al. Hepatology 2017, 66, 1111–1124. [Google Scholar] [CrossRef] [Green Version]
- Kleiner, D.E.; Makhlouf, H.R. Histology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in Adults and Children. Clin. Liver Dis. 2016, 20, 293–312. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Fukusato, T. Histopathology of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. World J. Gastroenterol. 2014, 20, 15539–15548. [Google Scholar] [CrossRef]
- Ekstedt, M.; Franzén, L.E.; Mathiesen, U.L.; Thorelius, L.; Holmqvist, M.; Bodemar, G.; Kechagias, S. Long-Term Follow-up of Patients with NAFLD and Elevated Liver Enzymes. Hepatology 2006, 44, 865–873. [Google Scholar] [CrossRef]
- Xie, L.; Yui, J.; Hatori, A.; Yamasaki, T.; Kumata, K.; Wakizaka, H.; Yoshida, Y.; Fujinaga, M.; Kawamura, K.; Zhang, M.-R. Translocator Protein (18 KDa), a Potential Molecular Imaging Biomarker for Non-Invasively Distinguishing Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2012, 57, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Sweet, P.H.; Khoo, T.; Nguyen, S. Nonalcoholic Fatty Liver Disease. Prim. Care 2017, 44, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Kudaravalli, P.; John, S. Nonalcoholic Fatty Liver; StatPearls [Internet]: Treasure Island, FL, USA, 2021. [Google Scholar]
- Huang, R.; Zhu, L.; Wang, J.; Xue, L.; Liu, L.; Yan, X.; Huang, S.; Li, Y.; Yan, X.; Zhang, B.; et al. Clinical Features of COVID-19 Patients with Non-Alcoholic Fatty Liver Disease. Hepatol. Commun. 2020, 4, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- Vranić, L.; Radovan, A.; Poropat, G.; Mikolašević, I.; Milić, S. Non-Alcoholic Fatty Liver Disease and COVID-19-Two Pandemics Hitting at the Same Time. Medicina 2021, 57, 1057. [Google Scholar] [CrossRef]
- Mushtaq, K.; Khan, M.U.; Iqbal, F.; Alsoub, D.H.; Chaudhry, H.S.; Ata, F.; Iqbal, P.; Elfert, K.; Balaraju, G.; Almaslamani, M. NAFLD Is a Predictor of Liver Injury in COVID-19 Hospitalized Patients but Not of Mortality, Disease Severity on the Presentation or Progression-The Debate Continues. J. Hepatol. 2021, 74, 482–484. [Google Scholar] [CrossRef]
- Nalbantoglu, I.L.K.; Brunt, E.M. Role of Liver Biopsy in Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2014, 20, 9026–9037. [Google Scholar] [PubMed]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Belt, P.; Neuschwander-Tetri, B.A.; NASH Clinical Research Network (CRN). Nonalcoholic Fatty Liver Disease (NAFLD) Activity Score and the Histopathologic Diagnosis in NAFLD: Distinct Clinicopathologic Meanings. Hepatology 2011, 53, 810–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Bedossa, P.; Poitou, C.; Veyrie, N.; Bouillot, J.-L.; Basdevant, A.; Paradis, V.; Tordjman, J.; Clement, K. Histopathological Algorithm and Scoring System for Evaluation of Liver Lesions in Morbidly Obese Patients. Hepatology 2012, 56, 1751–1759. [Google Scholar] [CrossRef]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Kechagias, S.; Stål, P.; Bedossa, P.; Hultcrantz, R. SAF Score and Mortality in NAFLD after up to 41 Years of Follow-Up. Scand. J. Gastroenterol. 2017, 52, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Matteoni, C.A.; Younossi, Z.M.; Gramlich, T.; Boparai, N.; Liu, Y.C.; McCullough, A.J. Nonalcoholic Fatty Liver Disease: A Spectrum of Clinical and Pathological Severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar] [CrossRef]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic Steatohepatitis: A Proposal for Grading and Staging the Histological Lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Costera, L.; Negre, I.; Samii, K.; Buffet, C. Pain Experienced during Percutaneous Liver Biopsy. Hepatology 1999, 30, 1529–1530. [Google Scholar] [CrossRef]
- Piccinino, F.; Sagnelli, E.; Pasquale, G.; Giusti, G. Complications Following Percutaneous Liver Biopsy. A Multicentre Retrospective Study on 68,276 Biopsies. J. Hepatol. 1986, 2, 165–173. [Google Scholar] [CrossRef]
- Schneier, A.T.; Citti, C.C.; Dieterich, D.T. Management and Diagnosis of Fatty Liver Disease. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 671–683. [Google Scholar] [CrossRef]
- Ratziu, V.; Charlotte, F.; Heurtier, A.; Gombert, S.; Giral, P.; Bruckert, E.; Grimaldi, A.; Capron, F.; Poynard, T.; LIDO Study Group. Sampling Variability of Liver Biopsy in Nonalcoholic Fatty Liver Disease. Gastroenterology 2005, 128, 1898–1906. [Google Scholar] [CrossRef]
- Merriman, R.B.; Ferrell, L.D.; Patti, M.G.; Weston, S.R.; Pabst, M.S.; Aouizerat, B.E.; Bass, N.M. Correlation of Paired Liver Biopsies in Morbidly Obese Patients with Suspected Nonalcoholic Fatty Liver Disease. Hepatology 2006, 44, 874–880. [Google Scholar] [CrossRef]
- Cadranel, J.F.; Rufat, P.; Degos, F. Practices of Liver Biopsy in France: Results of a Prospective Nationwide Survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology 2000, 32, 477–481. [Google Scholar] [CrossRef]
- Bedossa, P.; Dargère, D.; Paradis, V. Sampling Variability of Liver Fibrosis in Chronic Hepatitis C. Hepatology 2003, 38, 1449–1457. [Google Scholar] [CrossRef]
- Demir, M.; Lang, S.; Steffen, H.-M. Nonalcoholic Fatty Liver Disease–Current Status and Future Directions. J. Dig. Dis. 2015, 16, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, R.; Lazo, M.; Bonekamp, S.; Kamel, I.; Brancati, F.L.; Guallar, E.; Clark, J.M. Diagnostic Accuracy and Reliability of Ultrasonography for the Detection of Fatty Liver: A Meta-Analysis. Hepatology 2011, 54, 1082–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, M.S.; Harrison, S.A.; Abdelmalek, M.F.; Anstee, Q.M.; Bedossa, P.; Castera, L.; Dimick-Santos, L.; Friedman, S.L.; Greene, K.; Kleiner, D.E.; et al. Case Definitions for Inclusion and Analysis of Endpoints in Clinical Trials for Nonalcoholic Steatohepatitis through the Lens of Regulatory Science. Hepatology 2018, 67, 2001–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandrin, L.; Fourquet, B.; Hasquenoph, J.-M.; Yon, S.; Fournier, C.; Mal, F.; Christidis, C.; Ziol, M.; Poulet, B.; Kazemi, F.; et al. Transient Elastography: A New Noninvasive Method for Assessment of Hepatic Fibrosis. Ultrasound Med. Biol. 2003, 29, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Imajo, K.; Kessoku, T.; Honda, Y.; Tomeno, W.; Ogawa, Y.; Mawatari, H.; Fujita, K.; Yoneda, M.; Taguri, M.; Hyogo, H.; et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease than Transient Elastography. Gastroenterology 2016, 150, 626–637.e7. [Google Scholar] [CrossRef] [Green Version]
- Loomba, R.; Wolfson, T.; Ang, B.; Hooker, J.; Behling, C.; Peterson, M.; Valasek, M.; Lin, G.; Brenner, D.; Gamst, A.; et al. Magnetic Resonance Elastography Predicts Advanced Fibrosis in Patients with Nonalcoholic Fatty Liver Disease: A Prospective Study. Hepatology 2014, 60, 1920–1928.e7. [Google Scholar] [CrossRef] [Green Version]
- Xiao, G.; Zhu, S.; Xiao, X.; Yan, L.; Yang, J.; Wu, G. Comparison of Laboratory Tests, Ultrasound, or Magnetic Resonance Elastography to Detect Fibrosis in Patients with Nonalcoholic Fatty Liver Disease: A Meta-analysis. Hepatology 2017, 66, 1486–1501. [Google Scholar] [CrossRef] [Green Version]
- Palmeri, M.L.; Wang, M.H.; Rouze, N.C.; Abdelmalek, M.F.; Guy, C.D.; Moser, B.; Diehl, A.M.; Nightingale, K.R. Noninvasive Evaluation of Hepatic Fibrosis Using Acoustic Radiation Force-Based Shear Stiffness in Patients with Nonalcoholic Fatty Liver Disease. J. Hepatol. 2011, 55, 666–672. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Kader, S.M.; El-Den Ashmawy, E.M.S. Non-Alcoholic Fatty Liver Disease: The Diagnosis and Management. World J. Hepatol. 2015, 7, 846–858. [Google Scholar] [CrossRef]
- Torres, D.M.; Williams, C.D.; Harrison, S.A. Features, Diagnosis, and Treatment of Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2012, 10, 837–858. [Google Scholar] [CrossRef]
- Halfon, P.; Munteanu, M.; Poynard, T. FibroTest-ActiTest as a Non-Invasive Marker of Liver Fibrosis. Gastroenterol. Clin. Biol. 2008, 32, 22–39. [Google Scholar] [CrossRef]
- Huang, X.; Xu, M.; Chen, Y.; Peng, K.; Huang, Y.; Wang, P.; Ding, L.; Lin, L.; Xu, Y.; Chen, Y.; et al. Validation of the Fatty Liver Index for Nonalcoholic Fatty Liver Disease in Middle-Aged and Elderly Chinese. Medicine 2015, 94, e1682. [Google Scholar] [CrossRef] [PubMed]
- Koehler, E.M.; Schouten, J.N.L.; Hansen, B.E.; Hofman, A.; Stricker, B.H.; Janssen, H.L.A. External Validation of the Fatty Liver Index for Identifying Nonalcoholic Fatty Liver Disease in a Population-Based Study. Clin. Gastroenterol. Hepatol. 2013, 11, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, D.; Kim, H.J.; Lee, C.-H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.-H.; Cho, S.-H.; Sung, M.-W.; et al. Hepatic Steatosis Index: A Simple Screening Tool Reflecting Nonalcoholic Fatty Liver Disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Bedogni, G.; Kahn, H.S.; Bellentani, S.; Tiribelli, C. A Simple Index of Lipid Overaccumulation Is a Good Marker of Liver Steatosis. BMC Gastroenterol. 2010, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Du, T.; Zhang, J.; Lu, H.; Lin, X.; Xie, J.; Yang, Y.; Yu, X. The Triglyceride and Glucose Index (TyG) Is an Effective Biomarker to Identify Nonalcoholic Fatty Liver Disease. Lipids Health Dis. 2017, 16, 15. [Google Scholar] [CrossRef] [Green Version]
- Fedchuk, L.; Nascimbeni, F.; Pais, R.; Charlotte, F.; Housset, C.; Ratziu, V.; LIDO Study Group. Performance and Limitations of Steatosis Biomarkers in Patients with Nonalcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2014, 40, 1209–1222. [Google Scholar] [CrossRef]
- Joka, D.; Wahl, K.; Moeller, S.; Schlue, J.; Vaske, B.; Bahr, M.J.; Manns, M.P.; Schulze-Osthoff, K.; Bantel, H. Prospective Biopsy-Controlled Evaluation of Cell Death Biomarkers for Prediction of Liver Fibrosis and Nonalcoholic Steatohepatitis. Hepatology 2012, 55, 455–464. [Google Scholar] [CrossRef]
- Tanwar, S.; Trembling, P.M.; Guha, I.N.; Parkes, J.; Kaye, P.; Burt, A.D.; Ryder, S.D.; Aithal, G.P.; Day, C.P.; Rosenberg, W.M. Validation of Terminal Peptide of Procollagen III for the Detection and Assessment of Nonalcoholic Steatohepatitis in Patients with Nonalcoholic Fatty Liver Disease. Hepatology 2013, 57, 103–111. [Google Scholar] [CrossRef]
- Wieckowska, A.; Papouchado, B.G.; Li, Z.; Lopez, R.; Zein, N.N.; Feldstein, A.E. Increased Hepatic and Circulating Interleukin-6 Levels in Human Nonalcoholic Steatohepatitis. Am. J. Gastroenterol. 2008, 103, 1372–1379. [Google Scholar] [CrossRef]
- Wong, V.W.-S.; Hui, A.Y.; Tsang, S.W.-C.; Chan, J.L.-Y.; Tse, A.M.-L.; Chan, K.-F.; So, W.-Y.; Cheng, A.Y.-S.; Ng, W.-F.; Wong, G.L.-H.; et al. Metabolic and Adipokine Profile of Chinese Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2006, 4, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Tacke, F. Roles for Chemokines in Liver Disease. Gastroenterology 2014, 147, 577–594.e1. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Chan, H.L.-Y.; Wong, G.L.-H.; Choi, P.C.-L.; Chan, A.W.-H.; Chan, H.-Y.; Chim, A.M.-L.; Yeung, D.K.-W.; Chan, F.K.-L.; Woo, J.; et al. Non-Invasive Diagnosis of Non-Alcoholic Steatohepatitis by Combined Serum Biomarkers. J. Hepatol. 2012, 56, 1363–1370. [Google Scholar] [CrossRef]
- Zhang, L.; You, W.; Zhang, H.; Peng, R.; Zhu, Q.; Yao, A.; Li, X.; Zhou, Y.; Wang, X.; Pu, L.; et al. PNPLA3 Polymorphisms (Rs738409) and Non-Alcoholic Fatty Liver Disease Risk and Related Phenotypes: A Meta-Analysis: Non-Alcoholic Fatty Liver Disease Risk. J. Gastroenterol. Hepatol. 2015, 30, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Trépo, E.; Nahon, P.; Bontempi, G.; Valenti, L.; Falleti, E.; Nischalke, H.-D.; Hamza, S.; Corradini, S.G.; Burza, M.A.; Guyot, E.; et al. Association between the PNPLA3 (Rs738409 C>G) Variant and Hepatocellular Carcinoma: Evidence from a Meta-Analysis of Individual Participant Data. Hepatology 2014, 59, 2170–2177. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J. Meta-Analysis of the Influence of I148M Variant of Patatin-like Phospholipase Domain Containing 3 Gene (PNPLA3) on the Susceptibility and Histological Severity of Nonalcoholic Fatty Liver Disease. Hepatology 2011, 53, 1883–1894. [Google Scholar] [CrossRef]
- Kozlitina, J.; Smagris, E.; Stender, S.; Nordestgaard, B.G.; Zhou, H.H.; Tybjærg-Hansen, A.; Vogt, T.F.; Hobbs, H.H.; Cohen, J.C. Exome-Wide Association Study Identifies a TM6SF2 Variant That Confers Susceptibility to Nonalcoholic Fatty Liver Disease. Nat. Genet. 2014, 46, 352–356. [Google Scholar] [CrossRef] [Green Version]
- Dogru, T.; Genc, H.; Tapan, S.; Ercin, C.N.; Ors, F.; Aslan, F.; Kara, M.; Sertoglu, E.; Bagci, S.; Kurt, I.; et al. Elevated Asymmetric Dimethylarginine in Plasma: An Early Marker for Endothelial Dysfunction in Non-Alcoholic Fatty Liver Disease? Diabetes Res. Clin. Pract. 2012, 96, 47–52. [Google Scholar] [CrossRef]
- Di Pasqua, L.G.; Berardo, C.; Rizzo, V.; Richelmi, P.; Croce, A.C.; Vairetti, M.; Ferrigno, A. MCD Diet-Induced Steatohepatitis Is Associated with Alterations in Asymmetric Dimethylarginine (ADMA) and Its Transporters. Mol. Cell. Biochem. 2016, 419, 147–155. [Google Scholar] [CrossRef]
- Hernández, A.; Arab, J.P.; Reyes, D.; Lapitz, A.; Moshage, H.; Bañales, J.M.; Arrese, M. Extracellular Vesicles in NAFLD/ALD: From Pathobiology to Therapy. Cells 2020, 9, 817. [Google Scholar] [CrossRef] [Green Version]
- Verdam, F.J.; Dallinga, J.W.; Driessen, A.; de Jonge, C.; Moonen, E.J.C.; van Berkel, J.B.N.; Luijk, J.; Bouvy, N.D.; Buurman, W.A.; Rensen, S.S.; et al. Non-Alcoholic Steatohepatitis: A Non-Invasive Diagnosis by Analysis of Exhaled Breath. J. Hepatol. 2013, 58, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Thoma, C.; Day, C.P.; Trenell, M.I. Lifestyle Interventions for the Treatment of Non-Alcoholic Fatty Liver Disease in Adults: A Systematic Review. J. Hepatol. 2012, 56, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.-S.; Kim, D. Non-Alcoholic Fatty Liver Disease and Lifestyle Modifications, Focusing on Physical Activity. Korean J. Intern. Med. 2018, 33, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Promrat, K.; Kleiner, D.E.; Niemeier, H.M.; Jackvony, E.; Kearns, M.; Wands, J.R.; Fava, J.L.; Wing, R.R. Randomized Controlled Trial Testing the Effects of Weight Loss on Nonalcoholic Steatohepatitis. Hepatology 2010, 51, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Lazo, M.; Solga, S.F.; Horska, A.; Bonekamp, S.; Diehl, A.M.; Brancati, F.L.; Wagenknecht, L.E.; Pi-Sunyer, F.X.; Kahn, S.E.; Clark, J.M.; et al. Effect of a 12-Month Intensive Lifestyle Intervention on Hepatic Steatosis in Adults with Type 2 Diabetes. Diabetes Care 2010, 33, 2156–2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musso, G.; Cassader, M.; Rosina, F.; Gambino, R. Impact of Current Treatments on Liver Disease, Glucose Metabolism and Cardiovascular Risk in Non-Alcoholic Fatty Liver Disease (NAFLD): A Systematic Review and Meta-Analysis of Randomised Trials. Diabetologia 2012, 55, 885–904. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’Dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The Mediterranean Diet Improves Hepatic Steatosis and Insulin Sensitivity in Individuals with Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with Diet, Physical Activity and Exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [Green Version]
- NIH Conference. Gastrointestinal Surgery for Severe Obesity. Consensus Development Conference Panel. Ann. Intern. Med. 1991, 115, 956–961. [Google Scholar] [CrossRef]
- Burguera, B.; Agusti, A.; Arner, P.; Baltasar, A.; Barbe, F.; Barcelo, A.; Breton, I.; Cabanes, T.; Casanueva, F.F.; Couce, M.E.; et al. Critical Assessment of the Current Guidelines for the Management and Treatment of Morbidly Obese Patients. J. Endocrinol. Investig. 2007, 30, 844–852. [Google Scholar] [CrossRef]
- Klein, S.; Mittendorfer, B.; Eagon, J.C.; Patterson, B.; Grant, L.; Feirt, N.; Seki, E.; Brenner, D.; Korenblat, K.; McCrea, J. Gastric Bypass Surgery Improves Metabolic and Hepatic Abnormalities Associated with Nonalcoholic Fatty Liver Disease. Gastroenterology 2006, 130, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Viana, E.C.; Araujo-Dasilio, K.L.; Miguel, G.P.S.; Bressan, J.; Lemos, E.M.; Moyses, M.R.; de Abreu, G.R.; de Azevedo, J.L.M.C.; Carvalho, P.S.; Passos-Bueno, M.R.S.; et al. Gastric Bypass and Sleeve Gastrectomy: The Same Impact on IL-6 and TNF-α. Prospective Clinical Trial. Obes. Surg. 2013, 23, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Mofrad, P.S.; Contos, M.J.; Sargeant, C.; Luketic, V.A.; Sterling, R.K.; Stravitz, R.T.; Shiffman, M.L.; Clore, J.; Mills, A.S. A Pilot Study of Vitamin E versus Vitamin E and Pioglitazone for the Treatment of Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2004, 2, 1107–1115. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef] [Green Version]
- Musso, G.; Cassader, M.; Paschetta, E.; Gambino, R. Thiazolidinediones and Advanced Liver Fibrosis in Nonalcoholic Steatohepatitis: A Meta-Analysis. JAMA Intern. Med. 2017, 177, 633–640. [Google Scholar] [CrossRef]
- Seko, Y.; Sumida, Y.; Sasaki, K.; Itoh, Y.; Iijima, H.; Hashimoto, T.; Ishii, S.; Inagaki, N. Effects of Canagliflozin, an SGLT2 Inhibitor, on Hepatic Function in Japanese Patients with Type 2 Diabetes Mellitus: Pooled and Subgroup Analyses of Clinical Trials. J. Gastroenterol. 2018, 53, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Bajaj, H.S.; Brown, R.E.; Bhullar, L.; Sohi, N.; Kalra, S.; Aronson, R. SGLT2 Inhibitors and Incretin Agents: Associations with Alanine Aminotransferase Activity in Type 2 Diabetes. Diabetes Metab. 2018, 44, 493–499. [Google Scholar] [CrossRef]
- Akuta, N.; Kawamura, Y.; Fujiyama, S.; Sezaki, H.; Hosaka, T.; Kobayashi, M.; Kobayashi, M.; Saitoh, S.; Suzuki, F.; Suzuki, Y.; et al. SGLT2 Inhibitor Treatment Outcome in Nonalcoholic Fatty Liver Disease Complicated with Diabetes Mellitus: The Long-Term Effects on Clinical Features and Liver Histopathology. Intern. Med. 2020, 59, 1931–1937. [Google Scholar] [CrossRef]
- Svegliati-Baroni, G.; Saccomanno, S.; Rychlicki, C.; Agostinelli, L.; De Minicis, S.; Candelaresi, C.; Faraci, G.; Pacetti, D.; Vivarelli, M.; Nicolini, D.; et al. Glucagon-like Peptide-1 Receptor Activation Stimulates Hepatic Lipid Oxidation and Restores Hepatic Signalling Alteration Induced by a High-Fat Diet in Nonalcoholic Steatohepatitis: Effect of Exenatide on the Liver. Liver Int. 2011, 31, 1285–1297. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; LEAN trial team; Abouda, G.; et al. Liraglutide Safety and Efficacy in Patients with Non-Alcoholic Steatohepatitis (LEAN): A Multicentre, Double-Blind, Randomised, Placebo-Controlled Phase 2 Study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, M.J.; Hull, D.; Guo, K.; Barton, D.; Hazlehurst, J.M.; Gathercole, L.L.; Nasiri, M.; Yu, J.; Gough, S.C.; Newsome, P.N.; et al. Glucagon-like Peptide 1 Decreases Lipotoxicity in Non-Alcoholic Steatohepatitis. J. Hepatol. 2016, 64, 399–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eguchi, Y.; Kitajima, Y.; Hyogo, H.; Takahashi, H.; Kojima, M.; Ono, M.; Araki, N.; Tanaka, K.; Yamaguchi, M.; Matsuda, Y.; et al. Pilot Study of Liraglutide Effects in Non-Alcoholic Steatohepatitis and Non-Alcoholic Fatty Liver Disease with Glucose Intolerance in Japanese Patients (LEAN-J): Liraglutide for NASH/NAFLD. Hepatol. Res. 2015, 45, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Philo, L.; Nguyen, P.; Hofflich, H.; Hernandez, C.; Bettencourt, R.; Richards, L.; Salotti, J.; Bhatt, A.; Hooker, J.; et al. Sitagliptin vs. Placebo for Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. J. Hepatol. 2016, 65, 369–376. [Google Scholar] [PubMed] [Green Version]
- Kawakubo, M.; Tanaka, M.; Ochi, K.; Watanabe, A.; Saka-Tanaka, M.; Kanamori, Y.; Yoshioka, N.; Yamashita, S.; Goto, M.; Itoh, M.; et al. Dipeptidyl Peptidase-4 Inhibition Prevents Nonalcoholic Steatohepatitis-Associated Liver Fibrosis and Tumor Development in Mice Independently of Its Anti-Diabetic Effects. Sci. Rep. 2020, 10, 983. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Petta, S.; Mannisto, V.; Mancina, R.M.; Pipitone, R.; Karja, V.; Maggioni, M.; Kakela, P.; Wiklund, O.; Mozzi, E.; et al. Statin Use and Non-Alcoholic Steatohepatitis in at Risk Individuals. J. Hepatol. 2015, 63, 705–712. [Google Scholar] [CrossRef]
- Kargiotis, K.; Athyros, V.G.; Giouleme, O.; Katsiki, N.; Katsiki, E.; Anagnostis, P.; Boutari, C.; Doumas, M.; Karagiannis, A.; Mikhailidis, D.P. Resolution of Non-Alcoholic Steatohepatitis by Rosuvastatin Monotherapy in Patients with Metabolic Syndrome. World J. Gastroenterol. 2015, 21, 7860–7868. [Google Scholar] [CrossRef]
- Torres, D.M.; Jones, F.J.; Shaw, J.C.; Williams, C.D.; Ward, J.A.; Harrison, S.A. Rosiglitazone versus Rosiglitazone and Metformin versus Rosiglitazone and Losartan in the Treatment of Nonalcoholic Steatohepatitis in Humans: A 12-Month Randomized, Prospective, Open-Label Trial. Hepatology 2011, 54, 1631–1639. [Google Scholar] [CrossRef]
- Goh, G.B.; Pagadala, M.R.; Dasarathy, J.; Unalp-Arida, A.; Sargent, R.; Hawkins, C.; Sourianarayanane, A.; Khiyami, A.; Yerian, L.; Pai, R.; et al. Renin-Angiotensin System and Fibrosis in Non-Alcoholic Fatty Liver Disease. Liver Int. 2015, 35, 979–985. [Google Scholar] [CrossRef]
- Hyogo, H.; Ikegami, T.; Tokushige, K.; Hashimoto, E.; Inui, K.; Matsuzaki, Y.; Tokumo, H.; Hino, F.; Tazuma, S. Efficacy of Pitavastatin for the Treatment of Non-Alcoholic Steatohepatitis with Dyslipidemia: An Open-Label, Pilot Study: Pitavastatin and Its Efficacy in NASH. Hepatol. Res. 2011, 41, 1057–1065. [Google Scholar] [CrossRef]
- Lindor, K.D.; Kowdley, K.V.; Heathcote, E.J.; Harrison, M.E.; Jorgensen, R.; Angulo, P.; Lymp, J.F.; Burgart, L.; Colin, P. Ursodeoxycholic Acid for Treatment of Nonalcoholic Steatohepatitis: Results of a Randomized Trial. Hepatology 2004, 39, 770–778. [Google Scholar] [CrossRef]
- Leuschner, U.F.H.; Lindenthal, B.; Herrmann, G.; Arnold, J.C.; Rössle, M.; Cordes, H.-J.; Zeuzem, S.; Hein, J.; Berg, T.; NASH Study Group. High-Dose Ursodeoxycholic Acid Therapy for Nonalcoholic Steatohepatitis: A Double-Blind, Randomized, Placebo-Controlled Trial. Hepatology 2010, 52, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. A Meta-Analysis of Randomized Trials for the Treatment of Nonalcoholic Fatty Liver Disease. Hepatology 2010, 52, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.T.; Cho, Y.K.; Park, J.H.; Kim, H.J.; Park, D.I.; Sohn, C.I.; Jeon, W.K.; Kim, B.I.; Won, K.H.; Jin, W. Relationship of Non-Alcoholic Fatty Liver Disease to Colorectal Adenomatous Polyps: Relationship of NAFLD to Colorectal Cancer. J. Gastroenterol. Hepatol. 2010, 25, 562–567. [Google Scholar] [CrossRef] [Green Version]
- Touzin, N.T.; Bush, K.N.V.; Williams, C.D.; Harrison, S.A. Prevalence of Colonic Adenomas in Patients with Nonalcoholic Fatty Liver Disease. Therap. Adv. Gastroenterol. 2011, 4, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Wong, V.W.-S.; Wong, G.L.-H.; Tsang, S.W.-C.; Fan, T.; Chu, W.C.-W.; Woo, J.; Chan, A.W.-H.; Choi, P.C.-L.; Chim, A.M.-L.; Lau, J.Y.-W.; et al. High Prevalence of Colorectal Neoplasm in Patients with Non-Alcoholic Steatohepatitis. Gut 2011, 60, 829–836. [Google Scholar] [CrossRef]
- Stadlmayr, A.; Aigner, E.; Steger, B.; Scharinger, L.; Lederer, D.; Mayr, A.; Strasser, M.; Brunner, E.; Heuberger, A.; Hohla, F.; et al. Nonalcoholic Fatty Liver Disease: An Independent Risk Factor for Colorectal Neoplasia: Nonalcoholic Fatty Liver Disease. J. Intern. Med. 2011, 270, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.I.; Lim, Y.-S.; Park, H.S. Colorectal Neoplasms in Relation to Non-Alcoholic Fatty Liver Disease in Korean Women: A Retrospective Cohort Study: Colorectal Neoplasms and NAFLD. J. Gastroenterol. Hepatol. 2012, 27, 91–95. [Google Scholar] [CrossRef]
- Min, Y.W.; Yun, H.S.; Chang, W.I.; Kim, J.Y.; Kim, Y.-H.; Son, H.J.; Kim, J.J.; Rhee, J.C.; Chang, D.K. Influence of Non-Alcoholic Fatty Liver Disease on the Prognosis in Patients with Colorectal Cancer. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 78–83. [Google Scholar] [CrossRef]
- Huang, K.-W.; Leu, H.-B.; Wang, Y.-J.; Luo, J.-C.; Lin, H.-C.; Lee, F.-Y.; Chan, W.-L.; Lin, J.-K.; Chang, F.-Y. Patients with Nonalcoholic Fatty Liver Disease Have Higher Risk of Colorectal Adenoma after Negative Baseline Colonoscopy. Colorectal Dis. 2013, 15, 830–835. [Google Scholar] [CrossRef]
- Lin, X.-F.; Shi, K.-Q.; You, J.; Liu, W.-Y.; Luo, Y.-W.; Wu, F.-L.; Chen, Y.-P.; Wong, D.K.-H.; Yuen, M.-F.; Zheng, M.-H. Increased Risk of Colorectal Malignant Neoplasm in Patients with Nonalcoholic Fatty Liver Disease: A Large Study. Mol. Biol. Rep. 2014, 41, 2989–2997. [Google Scholar] [CrossRef]
- You, J.; Huang, S.; Huang, G.-Q.; Zhu, G.-Q.; Ma, R.-M.; Liu, W.-Y.; Shi, K.-Q.; Guo, G.-L.; Chen, Y.-P.; Braddock, M.; et al. Nonalcoholic Fatty Liver Disease: A Negative Risk Factor for Colorectal Cancer Prognosis. Medicine 2015, 94, e479. [Google Scholar] [CrossRef] [PubMed]
- Basyigit, S.; Uzman, M.; Kefeli, A.; Sapmaz, F.P.; Yeniova, A.O.; Nazligul, Y.; Asiltürk, Z. Absence of Non-Alcoholic Fatty Liver Disease in the Presence of Insulin Resistance Is a Strong Predictor for Colorectal Carcinoma. Int. J. Clin. Exp. Med. 2015, 8, 18601–18610. [Google Scholar] [PubMed]
- Bhatt, B.D.; Lukose, T.; Siegel, A.B.; Brown, R.S., Jr.; Verna, E.C. Increased Risk of Colorectal Polyps in Patients with Non-Alcoholic Fatty Liver Disease Undergoing Liver Transplant Evaluation. J. Gastrointest. Oncol. 2015, 6, 459–468. [Google Scholar] [PubMed]
- Lee, T.; Yun, K.E.; Chang, Y.; Ryu, S.; Park, D.I.; Choi, K.; Jung, Y.S. Risk of Colorectal Neoplasia According to Fatty Liver Severity and Presence of Gall Bladder Polyps. Dig. Dis. Sci. 2016, 61, 317–324. [Google Scholar] [CrossRef]
- Pan, S.; Hong, W.; Wu, W.; Chen, Q.; Zhao, Q.; Wu, J.; Jin, Y. The Relationship of Nonalcoholic Fatty Liver Disease and Metabolic Syndrome for Colonoscopy Colorectal Neoplasm. Medicine 2017, 96, e5809. [Google Scholar] [CrossRef]
- Ahn, J.S.; Sinn, D.H.; Min, Y.W.; Hong, S.N.; Kim, H.S.; Jung, S.-H.; Gu, S.; Rhee, P.-L.; Paik, S.W.; Son, H.J.; et al. Non-Alcoholic Fatty Liver Diseases and Risk of Colorectal Neoplasia. Aliment. Pharmacol. Ther. 2017, 45, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.-F.; Zhou, X.-D.; Sun, Y.-J.; Fang, D.-H.; Zhao, Q.; Huang, J.-H.; Jin, Y.; Wu, J.-S. Sex-Influenced Association of Non-Alcoholic Fatty Liver Disease with Colorectal Adenomatous and Hyperplastic Polyps. World J. Gastroenterol. 2017, 23, 5206. [Google Scholar] [CrossRef]
- Yang, Y.J.; Bang, C.S.; Shin, S.P.; Baik, G.H. Clinical Impact of Non-Alcoholic Fatty Liver Disease on the Occurrence of Colorectal Neoplasm: Propensity Score Matching Analysis. PLoS ONE 2017, 12, e0182014. [Google Scholar]
- Kim, G.-A.; Lee, H.C.; Choe, J.; Kim, M.-J.; Lee, M.J.; Chang, H.-S.; Bae, I.Y.; Kim, H.-K.; An, J.; Shim, J.H.; et al. Association between Non-Alcoholic Fatty Liver Disease and Cancer Incidence Rate. J. Hepatol. 2017, 68, 140–146. [Google Scholar] [CrossRef]
- Ze, E.Y.; Kim, B.J.; Jun, D.H.; Kim, J.G.; Kang, H.; Lee, D.Y. The Fatty Liver Index: A Simple and Accurate Predictor of Colorectal Adenoma in an Average-Risk Population: A Simple and Accurate Predictor of Colorectal Adenoma in an Average-Risk Population. Dis. Colon Rectum 2018, 61, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.-F.; Dong, X.-L.; Huang, Q.-K.; Hong, W.-D.; Wu, W.-Z.; Wu, J.-S.; Pan, S. The Combined Effect of Non-Alcoholic Fatty Liver Disease and Metabolic Syndrome on Colorectal Carcinoma Mortality: A Retrospective in Chinese Females. World J. Surg. Oncol. 2018, 16, 163. [Google Scholar] [CrossRef]
- Kim, M.C.; Park, J.G.; Jang, B.I.; Lee, H.J.; Lee, W.K. Liver Fibrosis Is Associated with Risk for Colorectal Adenoma in Patients with Nonalcoholic Fatty Liver Disease. Medicine 2019, 98, e14139. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, M.; Hashimoto, Y.; Obora, A.; Kojima, T.; Fukui, M. Non-Alcoholic Fatty Liver Disease with Obesity as an Independent Predictor for Incident Gastric and Colorectal Cancer: A Population-Based Longitudinal Study. BMJ Open Gastroenterol. 2019, 6, e000295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Liu, S.; Gao, Y.; Ma, H.; Zhan, S.; Yang, Y.; Xin, Y.; Xuan, S. Association between NAFLD and Risk of Colorectal Adenoma in Chinese Han Population. J. Clin. Transl. Hepatol. 2019, 7, 99–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.; Lim, S.-K.; Joo, S.K.; Jeong, D.-H.; Kim, J.H.; Bae, J.M.; Park, J.H.; Chang, M.S.; Lee, D.H.; Jung, Y.J.; et al. Nonalcoholic Steatohepatitis Is Associated with a Higher Risk of Advanced Colorectal Neoplasm. Liver Int. 2019, 39, 1722–1731. [Google Scholar] [CrossRef]
- Allen, A.M.; Hicks, S.B.; Mara, K.C.; Larson, J.J.; Therneau, T.M. The Risk of Incident Extrahepatic Cancers Is Higher in Non-Alcoholic Fatty Liver Disease than Obesity-A Longitudinal Cohort Study. J. Hepatol. 2019, 71, 1229–1236. [Google Scholar] [CrossRef]
- Lee, J.-M.; Park, Y.-M.; Yun, J.-S.; Ahn, Y.-B.; Lee, K.-M.; Kim, D.B.; Lee, J.M.; Han, K.; Ko, S.-H. The Association between Nonalcoholic Fatty Liver Disease and Esophageal, Stomach, or Colorectal Cancer: National Population-Based Cohort Study. PLoS ONE 2020, 15, e0226351. [Google Scholar] [CrossRef]
- Blackett, J.W.; Verna, E.C.; Lebwohl, B. Increased Prevalence of Colorectal Adenomas in Patients with Nonalcoholic Fatty Liver Disease: A Cross-Sectional Study. Dig. Dis. 2020, 38, 222–230. [Google Scholar] [CrossRef]
- Lesmana, C.R.A.; Pakasi, L.S.; Sudoyo, A.W.; Krisnuhoni, E.; Lesmana, L.A. The Clinical Significance of Colon Polyp Pathology in Nonalcoholic Fatty Liver Disease (NAFLD) and Its Impact on Screening Colonoscopy in Daily Practice. Can. J. Gastroenterol. Hepatol. 2020, 2020, 6676294. [Google Scholar] [CrossRef]
- Yu, X.; Xie, L.; Zhou, Y.; Yuan, X.; Wu, Y.; Chen, P. Patients with Non-alcoholic Fatty Liver Disease May Be a High-risk Group for the Development of Colorectal Polyps: A Cross-sectional Study. World Acad. Sci. J. 2020, 2, 1. [Google Scholar] [CrossRef]
- Zhang, X.; Wong, V.W.-S.; Yip, T.C.-F.; Tse, Y.-K.; Liang, L.Y.; Hui, V.W.-K.; Li, G.-L.; Chan, H.L.-Y.; Wong, G.L.-H. Colonoscopy and Risk of Colorectal Cancer in Patients with Nonalcoholic Fatty Liver Disease: A Retrospective Territory-Wide Cohort Study. Hepatol. Commun. 2021, 5, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, S.; Nakano, D.; Kawaguchi, T.; Eslam, M.; Ouchi, A.; Nagata, T.; Kuroki, H.; Kawata, H.; Abe, H.; Nouno, R.; et al. Non-Obese MAFLD Is Associated with Colorectal Adenoma in Health Check Examinees: A Multicenter Retrospective Study. Int. J. Mol. Sci. 2021, 22, 5462. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Jung, Y.S.; Park, J.H.; Park, D.I.; Sohn, C.I. Impact of Nonalcoholic Fatty Liver Disease on the Risk of Metachronous Colorectal Neoplasia after Polypectomy. Korean J. Intern. Med. 2021, 36, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-Y.; Bae, J.-H.; Kwak, M.-S.; Yang, J.-I.; Chung, S.-J.; Yim, J.-Y.; Lim, S.-H.; Chung, G.-E. The Risk of Colorectal Adenoma in Nonalcoholic or Metabolic-Associated Fatty Liver Disease. Biomedicines 2021, 9, 1401. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, H.W.; Kim, S.U.; Chang Kim, H. Metabolic Dysfunction-Associated Fatty Liver Disease Increases Colon Cancer Risk: A Nationwide Cohort Study. Clin. Transl. Gastroenterol. 2022, 13, e00435. [Google Scholar] [CrossRef]
- Mehta, S.J.; Morris, A.M.; Kupfer, S.S. Colorectal Cancer Screening Starting at Age 45 Years-Ensuring Benefits Are Realized by All. JAMA Netw. Open 2021, 4, e2112593. [Google Scholar] [CrossRef]
- Mikolasevic, I.; Orlic, L.; Stimac, D.; Hrstic, I.; Jakopcic, I.; Milic, S. Non-Alcoholic Fatty Liver Disease and Colorectal Cancer. Postgrad. Med. J. 2017, 93, 153–158. [Google Scholar] [CrossRef]
- Muhidin, S.O.; Magan, A.A.; Osman, K.A.; Syed, S.; Ahmed, M.H. The Relationship between Nonalcoholic Fatty Liver Disease and Colorectal Cancer: The Future Challenges and Outcomes of the Metabolic Syndrome. J. Obes. 2012, 2012, 637538. [Google Scholar] [CrossRef]
- Mantovani, A.; Dauriz, M.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Bonora, E.; Targher, G. Association between Nonalcoholic Fatty Liver Disease and Colorectal Tumours in Asymptomatic Adults Undergoing Screening Colonoscopy: A Systematic Review and Meta-Analysis. Metabolism 2018, 87, 1–12. [Google Scholar] [CrossRef]
- Jarrar, M.H.; Baranova, A.; Collantes, R.; Ranard, B.; Stepanova, M.; Bennett, C.; Fang, Y.; Elariny, H.; Goodman, Z.; Chandhoke, V.; et al. Adipokines and Cytokines in Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2008, 27, 412–421. [Google Scholar] [CrossRef]
- Tasci, I.; Dogru, T.; Ercin, C.N.; Erdem, G.; Sonmez, A. Adipokines and Cytokines in Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2008, 28, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, T.; Schwartz, B. Leptin Promotes Motility and Invasiveness in Human Colon Cancer Cells by Activating Multiple Signal-Transduction Pathways. Int. J. Cancer 2008, 123, 2543–2556. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Hosono, K.; Uchiyama, T.; Sakai, E.; Sugiyama, M.; Takahashi, H.; Nakajima, N.; Wada, K.; Takeda, K.; Nakagama, H.; et al. Leptin Acts as a Growth Factor for Colorectal Tumours at Stages Subsequent to Tumour Initiation in Murine Colon Carcinogenesis. Gut 2011, 60, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut Microbiota and Human NAFLD: Disentangling Microbial Signatures from Metabolic Disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Schetter, A.J.; Okayama, H.; Harris, C.C. The Role of MicroRNAs in Colorectal Cancer. Cancer J. 2012, 18, 244–252. [Google Scholar] [CrossRef] [Green Version]
- Baffy, G. MicroRNAs in Nonalcoholic Fatty Liver Disease. J. Clin. Med. 2015, 4, 1977–1988. [Google Scholar] [CrossRef] [Green Version]
- Merry, A.H.H.; Schouten, L.J.; Goldbohm, R.A.; van den Brandt, P.A. Body Mass Index, Height and Risk of Adenocarcinoma of the Oesophagus and Gastric Cardia: A Prospective Cohort Study. Gut 2007, 56, 1503–1511. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Sharma, A.N.; Murad, M.H.; Buttar, N.S.; El-Serag, H.B.; Katzka, D.A.; Iyer, P.G. Central Adiposity Is Associated with Increased Risk of Esophageal Inflammation, Metaplasia, and Adenocarcinoma: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 1399–1412.e7. [Google Scholar] [CrossRef] [Green Version]
- Kubo, A.; Cook, M.B.; Shaheen, N.J.; Vaughan, T.L.; Whiteman, D.C.; Murray, L.; Corley, D.A. Sex-Specific Associations between Body Mass Index, Waist Circumference and the Risk of Barrett’s Oesophagus: A Pooled Analysis from the International BEACON Consortium. Gut 2013, 62, 1684–1691. [Google Scholar] [CrossRef] [Green Version]
- El-Serag, H.B.; Hashmi, A.; Garcia, J.; Richardson, P.; Alsarraj, A.; Fitzgerald, S.; Vela, M.; Shaib, Y.; Abraham, N.S.; Velez, M.; et al. Visceral Abdominal Obesity Measured by CT Scan Is Associated with an Increased Risk of Barrett’s Oesophagus: A Case-Control Study. Gut 2014, 63, 220–229. [Google Scholar] [CrossRef]
- Rothman, K.J. BMI-Related Errors in the Measurement of Obesity. Int. J. Obes. 2008, 32, S56–S59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, J.A.; Reynolds, J.V. Visceral Obesity, Metabolic Syndrome, and Esophageal Adenocarcinoma. Front. Oncol. 2021, 11, 627270. [Google Scholar] [CrossRef] [PubMed]
- Corrao, S.; Natoli, G.; Argano, C. Nonalcoholic Fatty Liver Disease Is Associated with Intrahepatic Cholangiocarcinoma and Not with Extrahepatic Form: Definitive Evidence from Meta-Analysis and Trial Sequential Analysis: Definitive Evidence from Meta-Analysis and Trial Sequential Analysis. Eur. J. Gastroenterol. Hepatol. 2021, 33, 62–68. [Google Scholar] [CrossRef]
- Petrick, J.L.; Yang, B.; Altekruse, S.F.; Van Dyke, A.L.; Koshiol, J.; Graubard, B.I.; McGlynn, K.A. Risk Factors for Intrahepatic and Extrahepatic Cholangiocarcinoma in the United States: A Population-Based Study in SEER-Medicare. PLoS ONE 2017, 12, e0186643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lorenzo, S.; Tovoli, F.; Mazzotta, A.; Vasuri, F.; Edeline, J.; Malvi, D.; Boudjema, K.; Renzulli, M.; Jeddou, H.; D’Errico, A.; et al. Non-Alcoholic Steatohepatitis as a Risk Factor for Intrahepatic Cholangiocarcinoma and Its Prognostic Role. Cancers 2020, 12, 3182. [Google Scholar] [CrossRef]
- Park, J.-H.; Hong, J.Y.; Kwon, M.; Lee, J.; Han, K.; Han, I.W.; Kang, W.; Park, J.K. Association between Non-Alcoholic Fatty Liver Disease and the Risk of Biliary Tract Cancers: A South Korean Nationwide Cohort Study. Eur. J. Cancer 2021, 150, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Greenwood, D.C.; Chan, D.S.M.; Vieira, R.; Vieira, A.R.; Navarro Rosenblatt, D.A.; Cade, J.E.; Burley, V.J.; Norat, T. Body Mass Index, Abdominal Fatness and Pancreatic Cancer Risk: A Systematic Review and Non-Linear Dose-Response Meta-Analysis of Prospective Studies. Ann. Oncol. 2012, 23, 843–852. [Google Scholar] [CrossRef]
- Esposito, K.; Chiodini, P.; Colao, A.; Lenzi, A.; Giugliano, D. Metabolic Syndrome and Risk of Cancer: A Systematic Review and Meta-Analysis. Diabetes Care 2012, 35, 2402–2411. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-F.; Tseng, Y.-C.; Huang, H.-H.; Shih, Y.-L.; Hsieh, T.-Y.; Lin, H.-H. Exploring the Relationship between Nonalcoholic Fatty Liver Disease and Pancreatic Cancer by Computed Tomographic Survey. Intern. Emerg. Med. 2018, 13, 191–197. [Google Scholar] [CrossRef]
- Lee, S.; Jung, Y.; Bae, Y.; Yun, S.P.; Kim, S.; Jo, H.; Seo, H.-I. Prevalence and Risk Factors of Nonalcoholic Fatty Liver Disease in Breast Cancer Patients. Tumori 2017, 103, 187–192. [Google Scholar] [CrossRef]
- Nseir, W.; Abu-Rahmeh, Z.; Tsipis, A.; Mograbi, J.; Mahamid, M. Relationship between Non-Alcoholic Fatty Liver Disease and Breast Cancer. Isr. Med. Assoc. J. 2017, 19, 242–245. [Google Scholar]
- Kwak, M.-S.; Yim, J.Y.; Yi, A.; Chung, G.-E.; Yang, J.I.; Kim, D.; Kim, J.S.; Noh, D.-Y. Nonalcoholic Fatty Liver Disease Is Associated with Breast Cancer in Nonobese Women. Dig. Liver Dis. 2019, 51, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Lee, H.S.; Chang, S.W.; Lee, C.U.; Kim, J.S.; Jung, Y.K.; Kim, J.H.; Seo, Y.S.; Yim, H.J.; Lee, C.H.; et al. Underlying Nonalcoholic Fatty Liver Disease Is a Significant Factor for Breast Cancer Recurrence after Curative Surgery. Medicine 2019, 98, e17277. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, I.S.; Han, K.-D.; Park, H.; Kim, K.H.; Kim, J.-S. Association between Fatty Liver Index and Risk of Breast Cancer: A Nationwide Population-Based Study. Clin. Breast Cancer 2020, 20, e450–e457. [Google Scholar] [CrossRef]
- Huber, Y.; Labenz, C.; Michel, M.; Wörns, M.-A.; Galle, P.R.; Kostev, K.; Schattenberg, J.M. Tumor Incidence in Patients with Non-Alcoholic Fatty Liver Disease. Dtsch. Arztebl. Int. 2020, 117, 719–724. [Google Scholar] [CrossRef]
- MacInnis, R.J.; English, D.R. Body Size and Composition and Prostate Cancer Risk: Systematic Review and Meta-Regression Analysis. Cancer Causes Control 2006, 17, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Arase, Y.; Kobayashi, M.; Suzuki, F.; Suzuki, Y.; Kawamura, Y.; Akuta, N.; Imai, N.; Kobayashi, M.; Sezaki, H.; Matsumoto, N.; et al. Difference in Malignancies of Chronic Liver Disease Due to Non-Alcoholic Fatty Liver Disease or Hepatitis C in Japanese Elderly Patients: Malignancies in Patients with Liver Disease. Hepatol. Res. 2012, 42, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.-M.; Lee, J.-H.; Yoon, J.-H.; Kwak, C.; Lee, Y.J.; Cho, Y.Y.; Lee, Y.B.; Yu, S.J.; Kim, Y.J.; Kim, H.H.; et al. Nonalcoholic Fatty Liver Disease Is a Negative Risk Factor for Prostate Cancer Recurrence. Endocr. Relat. Cancer 2014, 21, 343–353. [Google Scholar] [CrossRef] [Green Version]
- Simon, T.G.; Roelstraete, B.; Sharma, R.; Khalili, H.; Hagström, H.; Ludvigsson, J.F. Cancer Risk in Patients with Biopsy-Confirmed Nonalcoholic Fatty Liver Disease: A Population-Based Cohort Study. Hepatology 2021, 74, 2410–2423. [Google Scholar] [CrossRef]
- Stocks, T.; Bjørge, T.; Ulmer, H.; Manjer, J.; Häggström, C.; Nagel, G.; Engeland, A.; Johansen, D.; Hallmans, G.; Selmer, R.; et al. Metabolic Risk Score and Cancer Risk: Pooled Analysis of Seven Cohorts. Int. J. Epidemiol. 2015, 44, 1353–1363. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.-Y.; Qu, J.-C.; Cao, H.-X.; Chen, G.-Y.; Shi, Y.-H.; Fan, J.-G. Obesity and Nonalcoholic Fatty Liver Disease Associated with Adenocarcinoma in Patients with Lung Cancer. Medicine 2019, 98, e17098. [Google Scholar] [CrossRef] [PubMed]

| Author, Year | Country | Study Design | Study Population | Diagnosis of NAFLD and Colorectal Neoplasms | Main Findings |
|---|---|---|---|---|---|
| Hwang et al., 2010 [174] | South Korea | Cross-sectional study | 2917 participants undergoing routine colonoscopy (556 subjects with adenomatous polyps and 2361 subjects without polyps) | US and colonoscopy | NAFLD prevalence (adenomatous polyp group vs. control group): 41.5% vs. 30.2% (p < 0.001). NAFLD was associated with an increased risk of developing colorectal adenomatous polyps (OR, 1.28; 95% CI, 1.03–1.60; p = 0.029) |
| Touzin et al., 2011 [175] | USA | Retrospective cohort study | 233 patients undergoing screening colonoscopy (94 patients with NAFLD and 139 patients without NAFLD) | Liver biopsy + US, and colonoscopy | Prevalence of colonic adenomas (NAFLD vs. control group): 24.4% vs. 25.1% (p = 1.00). Regarding the prevalence of adenomas, no difference was observed between the two groups |
| Wong et al., 2011 [176] | China | Cross-sectional study | 380 community and consecutive patients undergoing screening colonoscopy (199 patients with NAFLD and 181 patients without NAFLD) | Proton-magnetic resonance spectroscopy/ liver biopsy, and colonoscopy. | Prevalence of colorectal adenomas (NAFLD vs. control group): 34.7% vs. 21.5% (p = 0.043). Prevalence of advanced colorectal neoplasms (NAFLD vs. control group): 18.6% vs. 5.5% (p = 0.002). Among the biopsy-proven NAFLD patients, the prevalence of (a) colorectal adenomas (NASH vs. NAFL group) was 51% vs. 25.6% (p = 0.005), and (b) advanced colorectal neoplasms (NASH vs. NAFL group) was 34.7% vs. 14.0% (p = 0.011). NASH was associated with colorectal adenomas (adjusted OR, 4.89; 95% CI, 2.04–11.70; p < 0.001) and advanced colorectal neoplasms (adjusted OR, 5.34; 95% CI, 1.92–14.84; p = 0.001) |
| Stadlmayr et al., 2011 [177] | Austria | Cross-sectional study | 1211 patients undergoing screening colonoscopy (632 patients with NAFLD and 579 patients without NAFLD) | US and colonoscopy | Prevalence of colorectal lesions (NAFLD vs. control group): 34% vs. 21.7% (p < 0.001). Among men, (a) the prevalence of rectal adenomas (NAFLD vs. control group) was 11% vs. 3.4% (p = 0.004), and (b)CRC prevalence (NAFLD vs. control group) was 1.6% vs. 0.4% (p < 0.001). Hepatic steatosis was independently associated with an increased risk of developing colorectal adenomas (adjusted OR, 1.47; 95% CI, 1.079–2.003; p = 0.015) |
| Lee et al., 2012 [178] | South Korea | Retrospective cohort study | 5517 females undergoing life insurance health examinations (831 participants with NAFLD and 4686 participants without NAFLD) | US and colonoscopy | NAFLD was independently associated with an increased risk of developing colorectal adenomatous polyps (adjusted RR, 1.94; 95% CI, 1.11–3.40) and CRC (adjusted RR, 3.08; 95% CI, 1.02–9.34) |
| Min et al., 2012 [179] | South Korea | Retrospective study | 227 CRC patients (59 patients with NAFLD and 168 patients without NAFLD) | US and colonoscopy | The presence of NAFLD had no influence on the prognosis of CRC patients. There was no significant difference between CRC patients with and without NAFLD regarding the location and differentiation of tumors, CEA, and the total number of synchronous or advanced colorectal adenomas |
| Huang et al., 2013 [180] | Taiwan | Retrospective cohort study | 1522 participants undergoing two consecutive colonoscopies (216 individuals with colorectal adenomas and 1306 individuals without colorectal adenomas after a negative baseline colonoscopy | US and colonoscopy | NAFLD prevalence (adenoma vs. non-adenoma group): 55.6% vs. 38.8% (p < 0.05). NAFLD was an independent risk factor for developing colorectal adenomas after a negative baseline colonoscopy (adjusted OR, 1.45; 95% CI, 1.07–1.98; p = 0.016) |
| Lin et al., 2014 [181] | China | Retrospective and consecutive cohort study | 2315 community subjects undergoing routine colonoscopy (263 patients with NAFLD and 2052 patients without NAFLD) | US and colonoscopy | Prevalence of colorectal lesions (NAFLD vs. control group): 90.9% vs. 93.3%. Prevalence of adenomatous polyps (NAFLD vs. control group): 44.5% vs. 55.7%. Prevalence of colorectal malignant neoplasms (NAFLD vs. control group): 29.3% vs. 18% (p < 0.05). NAFLD was an independent risk factor for developing colorectal malignant neoplasms (adjusted OR, 1.868; 95% CI, 1.360–2.567; p = 0.001) |
| You et al., 2015 [182] | China | Retrospective cohort study | 1314 patients who underwent surgical resection of CRC (127 patients with NAFLD and 1187 patients without NAFLD) | US, and pathological and colonoscopic sample analyses | There was no significant difference in DFS rates between the CRC patient groups with and without NAFLD (p = 0.267). After the adjustment for clinicopathologic covariates, the presence of NAFLD was an independent negative risk factor for OS (HR, 0.593; 95% CI, 0.442–0.921; p = 0.02), but not for DFS (p = 0.270) |
| Basyigit et al., 2015 [183] | Turkey | Cross-sectional study | 127 consecutive patients undergoing colonoscopy (65 patients with NAFLD and 62 patients without NAFLD) | US and colonoscopy | CRC and colorectal adenomas’ prevalence was significantly higher in patients with insulin resistance (p = 0.005 and p = 0.008, respectively). CRC prevalence was significantly lower in NAFLD patients (p = 0.001). The risks of developing colorectal adenomas and cancer were significantly associated with the presence of insulin resistance (OR, 2.338; 95% CI, 1.080–4.993; p = 0.003 and OR, 5.023; 95% CI, 1.789–9.789; p = 0.001, respectively). CRC risk was increased in patients with insulin resistance but without NAFLD (OR, 5.218; 95% CI, 1.538–7.448; p = 0.017) |
| Bhatt et al., 2015 [184] | USA | Retrospective cohort study | 591 patients who completed the liver transplant evaluation process (68 patients with NAFLD and 523 patients without NAFLD) | Liver biopsy/clinical criteria assessment, and colonoscopy | Prevalence of colorectal polyps (NAFLD vs. non-NAFLD group): 59% vs. 40% (p = 0.003). NAFLD was a significant predictor of finding a colorectal polyp (adjusted OR, 2.42; 95% CI, 1.42–4.11; p = 0.001). Prevalence of adenomatous polyps (NAFLD vs. non-NAFLD group): approximately 32% vs. 21% (p = 0.04). NAFLD was a significant predictor of finding colorectal adenomas (adjusted OR, 1.95; 95% CI, 1.09–3.48; p = 0.02) |
| Lee et al., 2016 [185] | South Korea | Cross-sectional study | 44,220 participants undergoing colonoscopy and abdominal US as part of a health screening program (14,655 participants with NAFLD and 29,565 participants without NAFLD) | US and colonoscopy | Adjusted ORs for colorectal neoplasms (patients with NAFLD vs. without NAFLD): 1.13; 95% CI, 1.04–1.24 for mild, 1.12; 95% CI, 0.94–1.33 for moderate, and 1.56; 95% CI, 0.98–2.47 for severe NAFLD (p for trend = 0.007). Adjusted ORs for non-advanced colorectal neoplasms (patients with NAFLD vs. without NAFLD): 1.12; 95% CI, 1.01–1.23 for mild, 1.10; 95% CI, 0.91–1.33 for moderate, and 1.65; 95% CI, 1.02–2.67 for severe NAFLD (p for trend = 0.02). Adjusted ORs for advanced colorectal neoplasms (patients with NAFLD vs. without NAFLD): 1.22; 95% CI, 0.98–1.53 for mild, 1.21; 95% CI, 0.78–1.89 for moderate, and 0.96; 95% CI, 0.23–3.98 for severe NAFLD (p for trend = 0.139). Colorectal neoplasm risk increased with worsening fatty liver severity |
| Pan et al., 2017 [186] | China | Cross-sectional study | 1793 participants undergoing colonoscopy and abdominal US as part of health status check-up (573 participants with NAFLD and 1220 participants without NAFLD) | US and colonoscopy | NAFLD was independently associated with an increased risk of developing colorectal neoplasms (adjusted OR, 2.11; 95% CI, 1.352–2.871; p = 0.001) and CRC (adjusted OR, 2.164; 95% CI, 1.289–3.217; p = 0.005) |
| Ahn et al., 2017 [187] | South Korea | Cross-sectional study | 26,540 participants undergoing colonoscopy and abdominal US as part of a health check-up program (9501 participants with NAFLD and 17,039 participants without NAFLD) | US and colonoscopy | Prevalence of colorectal tumors (NAFLD vs. non-NAFLD group): 38% vs. 28.9% (p < 0.001). Prevalence of advanced colorectal neoplasia (NAFLD vs. non-NAFLD group): 2.8% vs. 1.9% (p < 0.001). NAFLD was independently associated with an increased risk of developing any colorectal neoplasia (adjusted OR, 1.10; 95% CI, 1.03–1.17; p = 0.002), but not advanced colorectal neoplasia (adjusted OR, 1.21; 95% CI, 0.99–1.47; p = 0.053) |
| Chen et al., 2017 [188] | China | Cross-sectional study | 3686 individuals undergoing abdominal US and colonoscopy as part of routine health check-up (779 individuals with NAFLD and 2907 individuals without NAFLD) | US and colonoscopy | NAFLD was independently associated with an increased risk of developing colorectal polyps (adjusted OR, 1.26; 95% CI, 1.05–1.51; p < 0.05) and colorectal adenomas (adjusted OR, 1.28; 95% CI, 1.01–1.64; p < 0.05). Significant association was found between NAFLD and colorectal adenomas in males (adjusted OR, 1.53; 95% CI, 1.18–2.00; p < 0.05), but not in females. NAFLD was also associated with multiple colorectal adenomas (OR, 1.82; 95% CI, 1.29–2.55; p = 0.001), distal adenomas (OR, 1.63; 95% CI, 1.11–2.39; p = 0.013) and bilateral adenomas (OR, 1.89; 95% CI, 1.23–2.91; p = 0.004) |
| Yang et al., 2017 [189] | South Korea | Retrospective cohort study | 1023 patients undergoing surveillance colonoscopy after index colonoscopy (unmatched population: 441 patients with NAFLD and 582 patients without NAFLD; propensity score matched population: 441 patients with NAFLD and 441 patients without NAFLD) | US or CT scan, and colonoscopy | Overall colorectal neoplasm occurrence at 3 years after index colonoscopy (NAFLD vs. non-NAFLD group): 9.1% vs. 5% Overall colorectal neoplasm occurrence at 5 years after index colonoscopy (NAFLD vs. non-NAFLD group): 35.2% vs. 25.3% (p = 0.01). NAFLD was independently associated with an increased risk of developing colorectal neoplasms (adjusted HR, 1.31; 95% CI, 1.01–1.71; p = 0.05) and multiple (≥3) adenomas (adjusted HR, 2.49; 95% CI, 1.20–5.20; p = 0.02), but not advanced colorectal neoplasms (adjusted HR, 1.07; 95% CI, 0.51–2.26; p = 0.85) |
| Kim et al., 2017 [190] | South Korea | Cohort study | 25,947 subjects undergoing screening colonoscopy as part of a health check-up program (8721 subjects with NAFLD and 17,226 subjects without NAFLD) | US and colonoscopy | NAFLD was significantly associated with CRC in males (adjusted HR, 2.01; 95% CI, 1.10–3.68; p = 0.02), but not in females (p = 0.41). The severity of NAFLD was not associated with CRC risk |
| Ze et al., 2018 [191] | South Korea | Retrospective observational study | 2976 consecutive subjects undergoing abdominal US and colonoscopy as part of a health check-up program (1512 subjects with NAFLD and 1464 subjects without NAFLD) | US and colonoscopy | Fatty liver index ≥ 30 was associated with an increased risk of developing colorectal adenomas (OR, 1.269; 95% CI, 1.06–1.49; p = 0.008) |
| Chen et al., 2018 [192] | China | Cross-sectional study | 764 CRC patients who were primarily treated by surgical resection (316 patients with NAFLD and 448 patients without NAFLD) | US and pathological sample analyses | Significant NAFLD was an independent risk factor for CRC-specific mortality in females. Significant NAFLD and metabolic syndrome has a synergistic effect on promoting mortality among CRC patients |
| Kim et al., 2019 [193] | South Korea | Cross-sectional study | 6332 subjects undergoing abdominal US and 1st-time colonoscopy as part of a health screening program (2395 subjects with NAFLD and 3937 subjects without NAFLD) | US and colonoscopy | Prevalence of colorectal adenomas (NAFLD vs. non-NAFLD group): 33.3% vs. 23.8% (p < 0.001). Prevalence of advanced adenomas (NAFLD vs. non-NAFLD group): 5.3% vs. 2.4% (p < 0.001). Prevalence of multiple colorectal adenomas (NAFLD vs. non-NAFLD group): 5.8% vs. 3% (p < 0.001). NAFLD was independently associated with the risk of developing colorectal adenomas (adjusted OR, 1.15; 95% CI, 1.02–1.30; p = 0.027), advanced adenomas (adjusted OR, 1.50; 95% CI, 1.12–2.01; p = 0.006), and multiple adenomas (adjusted OR, 1.32; 95% CI, 1.01–1.73; p = 0.006) |
| Hamaguchi et al., 2019 [194] | Japan | Cohort study | 15,926 individuals participating in a health check-up program (3211 individuals with NAFLD and 12,715 individuals without NAFLD) | US and colonoscopy | CRC incidence rate: 0.37 per 1000 person years in the non-NAFLD group without obesity; 0.72 in the non-NAFLD group with obesity; 0.41 in the NAFLD group without obesity; 1.49 in the NAFLD group with obesity. NAFLD with obesity was independently associated with an increased CRC risk (adjusted HR, 2.96; 95% CI, 1.44–6.09; p = 0.003) |
| Li et al., 2019 [195] | China | Retrospective cohort study | 1089 subjects undergoing colonoscopy (502 subjects with NAFLD and 587 subjects without NAFLD) | US + CAP score using FibroScan probes, and colonoscopy | NAFLD was independently associated with an increased risk of developing colorectal adenomas (OR, 1.425; 95% CI, 1.112–2.042; p = 0.018). NAFLD was associated with an increased adenoma risk in males (OR, 1.473; 95% CI, 1.003–2.162; p = 0.048), but not in females (OR, 1.316; 95% CI, 0.817–2.12; p = 0.259). NAFLD and metabolic syndrome were significantly associated with a high risk of developing adenomas |
| Cho et al., 2019 [196] | South Korea | Prospective cohort study | 476 patients undergoing screening colonoscopy (379 patients with NAFLD and 97 patients without NAFLD) | Liver biopsy and colonoscopy | NAFL was independently associated with an increased risk of developing adenomatous polyps (adjusted OR, 2.76; 95% CI, 1.51–5.06; p = 0.001). NASH was independently associated with an increased risk of developing colorectal adenomatous polyps (adjusted OR, 2.08; 95% CI, 1.12–3.86; p = 0.02) and advanced colorectal neoplasms (adjusted OR, 2.81; 95% CI, 1.01–7.87; p = 0.049) |
| Allen et al., 2019 [197] | USA | Cohort study | 19,163 subjects (4722 subjects with NAFLD and 14,441 age- and sex-matched referent individuals) | NAFLD and cancer was defined utilizing a code-based algorithm (using the NAFLD-specific HICDA, ICD-9-CM and ICD-10-CM codes) | NAFLD was associated with an increased colon cancer risk (IRR, 1.8; 95% CI, 1.1–2.8) |
| Lee et al., 2020 [198] | South Korea | Retrospective cohort study | 8,120,674 subjects who received healthcare checkups (936,159 adults with NAFLD and 7,184,515 adults without NAFLD) | FLI, and endoscopy + ICD-10 codes | NAFLD (FLI ≥ 60) was significantly associated with the risk of developing colon cancer (HR, 1.23; 95% CI, 1.19–1.26) and an increased risk of all-cause mortality in CRC patients (HR, 1.16; 95% CI, 1.10–1.22) |
| Blackett et al., 2020 [199] | USA | Cross-sectional study | 369 patients who underwent liver biopsy and screening or surveillance colonoscopy (123 subjects with NAFLD and 246 matched controls without NAFLD) | Liver biopsy and colonoscopy | Prevalence of colorectal adenomas (NAFLD vs. control group): 40.7% vs. 28.1% (OR, 1.87; 95% CI, 1.15–3.03; p = 0.01). NAFLD was independently associated with an increased risk of detecting colorectal adenomas (adjusted OR, 1.74; 95% CI, 1.05–2.88; p = 0.032), but not advanced neoplastic lesions (adjusted OR, 2.2; 95% CI, 0.93–5.18; p = 0.07). The risk of developing colorectal adenomas was not associated with the severity (steatohepatitis vs. no steatohepatitis) of NAFLD (adjusted OR, 2.47; 95% CI, 0.67–9.1; p = 0.17) |
| Lesmana et al., 2020 [200] | Indonesia | Retrospective database study | 138 subjects undergoing elective colonoscopy (68 subjects with NAFLD and 70 subjects without NAFLD) | US and colonoscopy | Prevalence of colon polyps (NAFLD vs. control group): 44.1% vs. 27.1% (p = 0.037). NAFLD was associated with an increased risk of developing any colon polyp |
| Yu et al., 2020 [201] | China | Cross-sectional study | 1538 patients with colorectal polyps undergoing abdominal US (550 patients with NAFLD and 988 patients without NAFLD) | US and colonoscopy | No significant difference regarding the location and morphology of colorectal polyps between the NAFLD and control groups (p > 0.05). NAFLD was significantly associated with colorectal polyps, especially, in patients with multiple polyps, those with a large size and with villous features (p < 0.05) |
| Zhang et al., 2021 [202] | China | Retrospective cohort study | 8351 NAFLD patients (5308 patients with prior colonoscopy and 3043 patients without prior colonoscopy) | - CRC was identified based on ICD-9-CM diagnosis codes or procedure codes for CRC treatment | Compared to the general population, NAFLD patients who did not undergo colonoscopy had higher incidence rate of CRC (SIR, 2.20; 95% CI, 1.64–2.88; p < 0.001). NAFLD patients who underwent colonoscopy had lower incidence rate of CRC (SIR, 0.54; 95% CI, 0.37–0.75; p < 0.001). After adjustment for demographic and metabolic factors, NAFLD patients with a high fibrosis-4 score (>2.67) had higher risk of developing CRC |
| Fukunaga et al., 2021 [203] | Japan | Cross-sectional study | 124 consecutive health check-up examinees undergoing colonoscopy (58 examinees with NAFLD and 66 examinees without NAFLD; 63 examinees with MAFLD and 61 examinees without MAFLD) | US and colonoscopy | MAFLD was independently associated with colorectal adenomas (OR, 3.191; 95% CI, 1.494–7.070; p = 0.003). Non-obese MAFLD was also associated with colorectal adenomas (OR, 3.351; 95% CI, 1.589–7.262; p ≤ 0.001) |
| Kim et al., 2021 [204] | South Korea | Cohort study | 6182 subjects undergoing abdominal US, endoscopic removal of ≥1 adenomas at the index colonoscopy and a follow-up surveillance colonoscopy (2642 subjects with NAFLD and 3540 subjects without NAFLD) | US and colonoscopy | NAFLD was independently associated with an increased risk of developing metachronous overall colorectal neoplasia in both males (adjusted HR, 1.17; 95% CI, 1.06–1.29) and females (adjusted HR, 1.63; 95% CI, 1.27–2.07). NAFLD was also independently associated with an increased risk of developing metachronous advanced colorectal neoplasia in females (adjusted HR, 2.61; 95% CI, 1.27–5.37) |
| Seo et al., 2021 [205] | South Korea | Retrospective cohort study | A total of 3441 subjects participating in a health check-up program (1127 subjects with MAFLD and 2314 without MAFLD). 3044 subjects were included in the NAFLD analysis (1143 subjects with NAFLD and 1901 subjects without NAFLD) | US and colonoscopy | NAFLD and MAFLD were significantly associated with an increased risk of developing colorectal adenomas in females (adjusted OR, 1.43; 95% CI, 1.01–2.03; p = 0.046 and OR, 1.55; 95% CI, 1.09–2.20; p = 0.015, respectively). NAFLD and MAFLD with an advanced fibrosis index score were also associated with an increased risk of developing adenomas (OR, 1.38; 95% CI, 1.04–1.83; p = 0.027, and OR, 1.45; 95% CI, 1.13–1.96; p = 0.004, respectively) |
| Lee et al., 2022 [206] | South Korea | Cohort study | 8,933,017 participants undergoing routine National Health Insurance Service health examinations (2,517,330 participants with NAFLD and 6,415,687 participants without NAFLD; 3,337,122 participants with MAFLD and 5,595,895 participants without MAFLD) | FLI, and ICD-10 diagnosis codes | The presence of fatty liver disease was significantly associated with an increased CRC risk. The CRC risk was higher in MAFLD patients with liver fibrosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsala, A.; Tsalikidis, C.; Romanidis, K.; Pitiakoudis, M. Non-Alcoholic Fatty Liver Disease and Extrahepatic Cancers: A Wolf in Sheep’s Clothing? Curr. Oncol. 2022, 29, 4478-4510. https://doi.org/10.3390/curroncol29070356
Mitsala A, Tsalikidis C, Romanidis K, Pitiakoudis M. Non-Alcoholic Fatty Liver Disease and Extrahepatic Cancers: A Wolf in Sheep’s Clothing? Current Oncology. 2022; 29(7):4478-4510. https://doi.org/10.3390/curroncol29070356
Chicago/Turabian StyleMitsala, Athanasia, Christos Tsalikidis, Konstantinos Romanidis, and Michail Pitiakoudis. 2022. "Non-Alcoholic Fatty Liver Disease and Extrahepatic Cancers: A Wolf in Sheep’s Clothing?" Current Oncology 29, no. 7: 4478-4510. https://doi.org/10.3390/curroncol29070356
APA StyleMitsala, A., Tsalikidis, C., Romanidis, K., & Pitiakoudis, M. (2022). Non-Alcoholic Fatty Liver Disease and Extrahepatic Cancers: A Wolf in Sheep’s Clothing? Current Oncology, 29(7), 4478-4510. https://doi.org/10.3390/curroncol29070356





