Biopsychosocial Markers of Body Image Concerns in Patients with Head and Neck Cancer: A Prospective Longitudinal Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
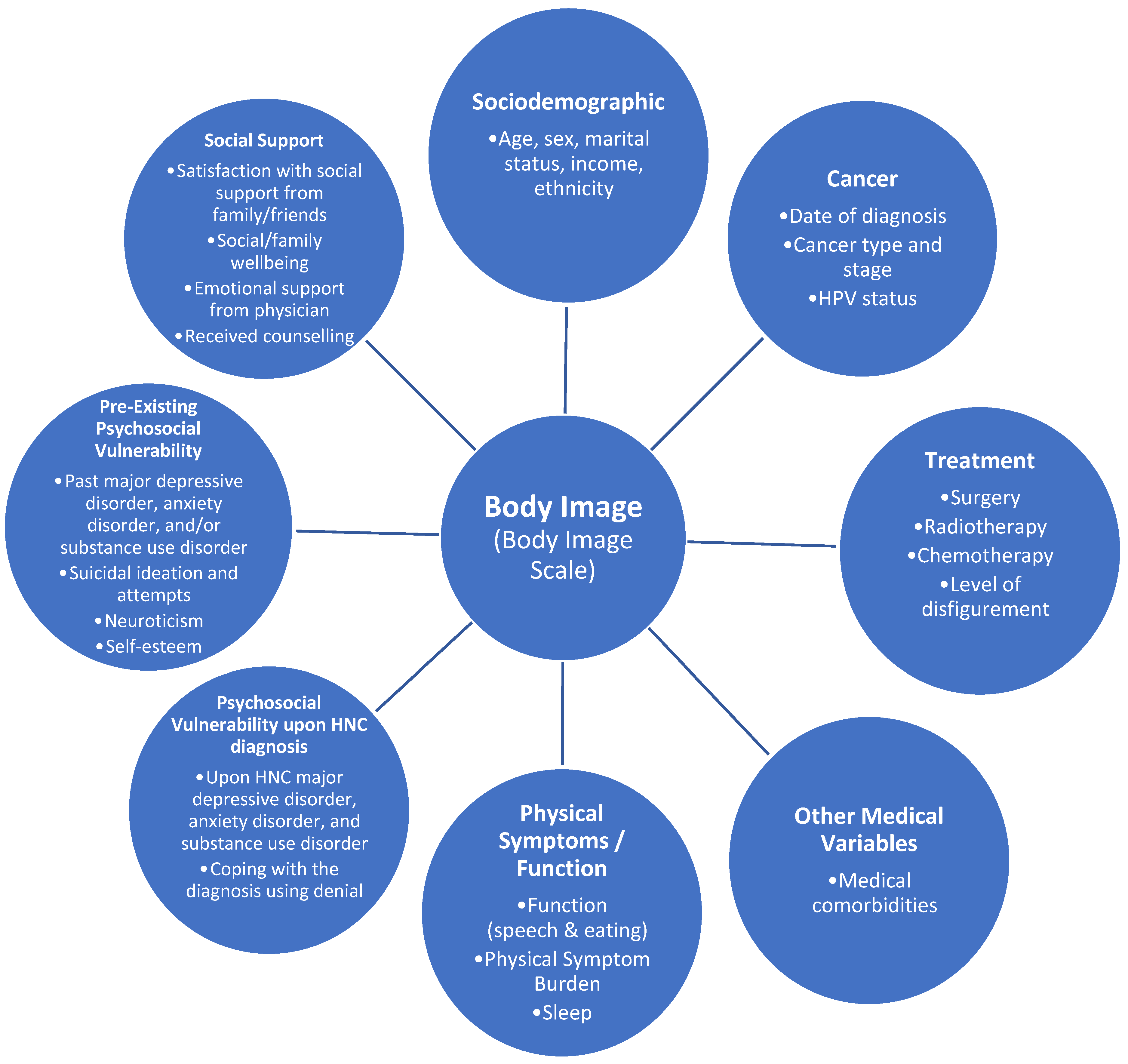
2.3. Measures
2.4. Statistical Analyses
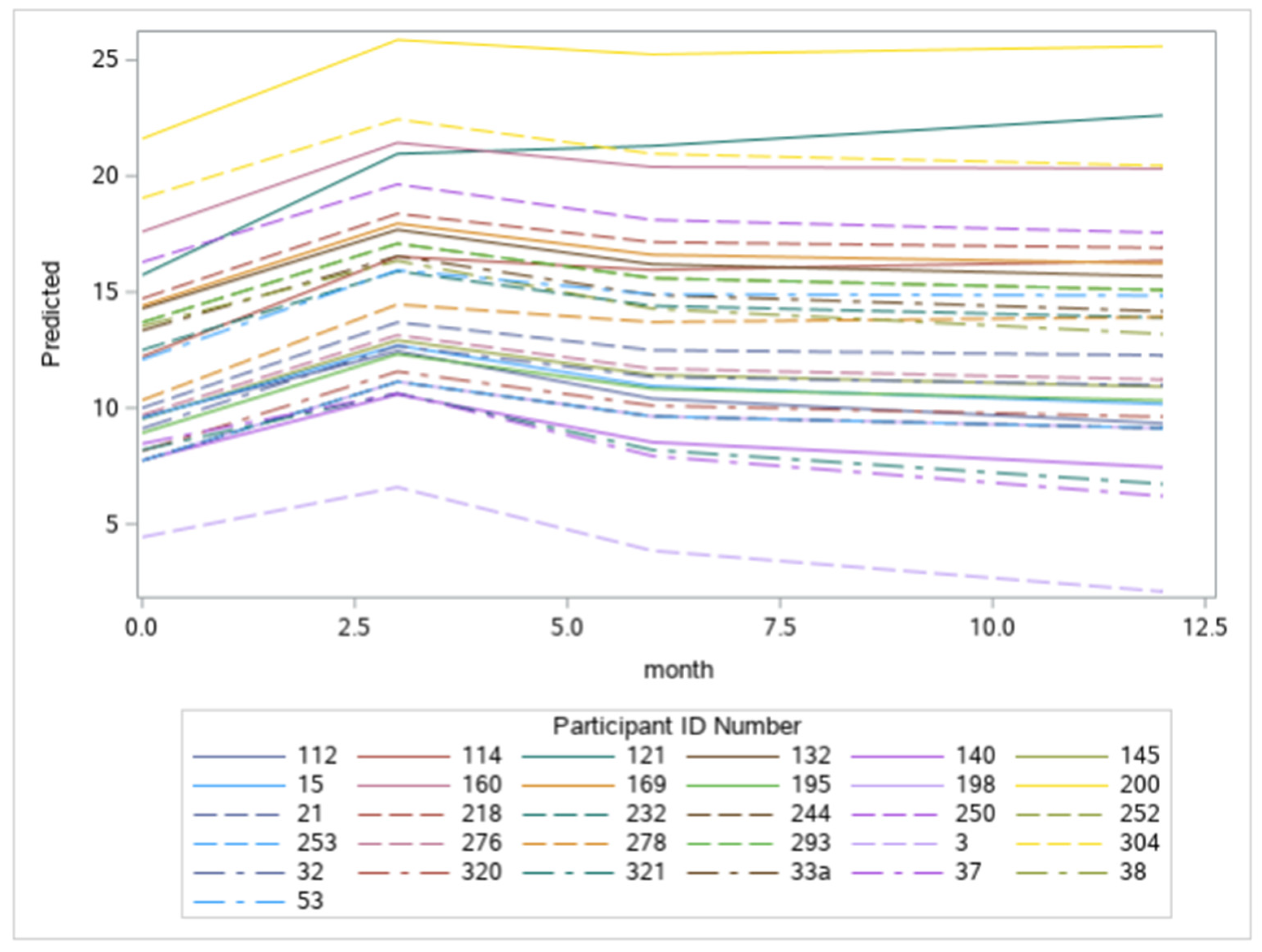
3. Results
4. Discussion
4.1. Limitations
4.2. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alias, A.; Henry, M. Psychosocial Effects of Head and Neck Cancer. Oral Maxillofac. Surg. Clin. N. Am. 2018, 30, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Alias, A. Body image and functional loss. In Principles and Practices of Body Image Care for Cancer Patients; Fingeret, M.C., Teo, I., Eds.; Oxford University Press: New York, NY, USA, 2018; pp. 162–180. [Google Scholar]
- Sundaram, C.S.; Dhillon, H.M.; Butow, P.N.; Sundaresan, P.; Rutherford, C. A systematic review of body image measures for people diagnosed with head and neck cancer (HNC). Support. Care Cancer 2019, 27, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Fingeret, M.C.; Teo, I. Body Image Care for Cancer Patients (Principles and Practices); Oxford University Press: New York, NY, USA, 2018. [Google Scholar]
- Paterson, C.L.; Lengacher, C.A.; Donovan, K.A.; Kip, K.E.; Tofthagen, C.S. Body Image in Younger Breast Cancer Survivors—A Systematic Review. Cancer Nurs. 2016, 39, E39–E58. [Google Scholar] [CrossRef] [Green Version]
- Henry, M.; Albert, J.G.; Frenkiel, S.; Hier, M.; Zeitouni, A.; Kost, K.; Mlynarek, A.; Black, M.; MacDonald, C.; Richardson, K.; et al. Body Image Concerns in Patients with Head and Neck Cancer: A Longitudinal Study. Front. Psychol. 2022, 13, 816587. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Tami, P.; Ramsay, D.; Melanson, L.; MacLean, L.; Nersesian, S.; Ramjeesingh, R. Body image in older breast cancer survivors: A systematic review. Psycho-Oncology 2020, 29, 823–832. [Google Scholar] [CrossRef]
- Neilson, K.; Pollard, A.; Boonzaier, A.; Corry, J.; Castle, D.; Smith, D.; Trauer, T.; Couper, J. A longitudinal study of distress (depression and anxiety) up to 18 months after radiotherapy for head and neck cancer. Psycho-Oncology 2012, 22, 1843–1848. [Google Scholar] [CrossRef] [PubMed]
- Wilson, I.B. Linking Clinical Variables with Health-Related Quality of Life. JAMA 1995, 273, 59–65. [Google Scholar] [CrossRef]
- Ingram, R.E.; Miranda, J.; Segal, Z.V. Cognitive Vulnerability to Depression; Guilford Press: New York, NY, USA, 1998. [Google Scholar]
- Ingram, R.E.; Atchley, R.A.; Segal, Z.V. Vulnerability to Depression: From Cognitive Neuroscience to Prevention and Treatment; Guilford Press: New York, NY, USA, 2011. [Google Scholar]
- First, M.B.; Spitzer, R.L.; Gibbon, M.; Williams, J.B.W. User’s Guide for the Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Research Version (SCID-I for DSM-IV-TR); New York State Psychiatric Institute: New York, NY, USA, 2002; 136p. [Google Scholar]
- Graboyes, E.M.; Hill, E.G.; Marsh, C.H.; Maurer, S.; Day, T.A.; Hornig, J.D.; Lentsch, E.J.; Neskey, D.M.; Skoner, J.; Sterba, K.R. Temporal Trajectory of Body Image Disturbance in Patients with Surgically Treated Head and Neck Cancer. Otolaryngol. Head Neck Surg. 2020, 162, 304–312. [Google Scholar] [CrossRef]
- Hopwood, P.; Fletcher, I.; Lee, A.; Al Ghazal, S. A body image scale for use with cancer patients. Eur. J. Cancer 2001, 37, 189–197. [Google Scholar] [CrossRef]
- Chopra, D.; De La Garza, R.; Lacourt, T.E. Clinical relevance of a Body Image Scale cut point of 10 as an indicator of psychological distress in cancer patients: Results from a psychiatric oncology clinic. Support. Care Cancer 2020, 29, 231–237. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.R.; Irish, J.C.; Devins, G.M.; Rodin, G.M.; Gullane, P.J. Reliability and validity of an observer-rated disfigurement scale for head and neck cancer patients. Head Neck 2000, 22, 132–141. [Google Scholar] [CrossRef]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, L.L.; Zimmerman, G.J.; Cella, D.F.; Long, S.A. Quality of Life and Functional Status Measures in Patients with Head and Neck Cancer. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 482–487. [Google Scholar] [CrossRef]
- Markey, C.H.; Dunaev, J.L.; August, K.J. Body image experiences in the context of chronic pain: An examination of associations among perceptions of pain, body dissatisfaction, and positive body image. Body Image 2020, 32, 103–110. [Google Scholar] [CrossRef]
- Rosenberg, S.M.; Tamimi, R.M.; Gelber, S.; Ruddy, K.J.; Kereakoglow, S.; Borges, V.F.; Come, S.E.; Schapira, L.; Winer, E.P.; Partridge, A.H. Body image in recently diagnosed young women with early breast cancer. Psycho-Oncology 2012, 22, 1849–1855. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. Hospital Anxiety and Depression Scale; PsycTESTS Dataset; American Psychological Association: Washington, DC, USA, 1983. [Google Scholar] [CrossRef] [Green Version]
- Beck, A.T.; Steer, R.A. Manual for the Beck Scale for Suicide Ideation; Psychological Corporation: San Antonio, TX, USA, 1991. [Google Scholar]
- Zimmermann, T.; Scott, J.L.; Heinrichs, N. Individual and dyadic predictors of body image in women with breast cancer. Psycho-Oncology 2009, 19, 1061–1068. [Google Scholar] [CrossRef]
- Cherpitel, C.J. The Rapid Alcohol Problems Screen (RAPS): Methods and Application. In Comprehensive Handbook of Alcohol Related Pathology: Selective Methods Used in Alcohol Research; Preedy, V.R., Watson, R.R., Eds.; Academic Press: London, UK; Elsevier Science: London, UK, 2005; Chapter 105; Volume 3, pp. 1415–1427. [Google Scholar]
- Barrett, P.; Petrides, K.; Eysenck, S.; Eysenck, H. The Eysenck Personality Questionnaire: An examination of the factorial similarity of P, E, N, and L across 34 countries. Pers. Individ. Differ. 1998, 25, 805–819. [Google Scholar] [CrossRef]
- Carver, C.S. You want to measure coping but your protocol’s too long: Consider the Brief COPE. Int. J. Behav. Med. 1997, 4, 92–100. [Google Scholar] [CrossRef]
- Sarason, I.G.; Sarason, B.R.; Shearin, E.N.; Pierce, G.R. A Brief Measure of Social Support: Practical and Theoretical Implications. J. Soc. Pers. Relatsh. 1987, 4, 497–510. [Google Scholar] [CrossRef]
- Canadian Incidence Study of Reported Child Abuse and Neglect-2008; PsycEXTRA Dataset; American Psychological Association: Washington, DC, USA, 2010. [CrossRef]
- Boyes, A.; Girgis, A.; Lecathelinais, C. Brief assessment of adult cancer patients’ perceived needs: Development and validation of the 34-item Supportive Care Needs Survey (SCNS-SF34). J. Eval. Clin. Pract. 2009, 15, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.A.; Sterba, K.R.; Brennan, E.; Maurer, S.; Hill, E.G.; Day, T.A.; Graboyes, E.M. A Systematic Review of Patient-Reported Outcome Measures Assessing Body Image Disturbance in Patients with Head and Neck Cancer. Otolaryngol. Head Neck Surg. 2019, 160, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-C.; Huang, B.-S.; Lin, C.-Y.; Fan, K.-H.; Chang, J.T.-C.; Wu, S.-C.; Lai, Y.-H. Psychosocial effects of a skin camouflage program in female survivors with head and neck cancer: A randomized controlled trial. Psycho-Oncology 2016, 26, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Alleva, J.M.; Diedrichs, P.C.; Halliwell, E.; Peters, M.L.; Dures, E.; Stuijfzand, B.G.; Rumsey, N. More than my RA: A randomized trial investigating body image improvement among women with rheumatoid arthritis using a functionality-focused intervention program. J. Consult. Clin. Psychol. 2018, 86, 666–676. [Google Scholar] [CrossRef]
- Song, L.; Han, X.; Zhang, J.; Tang, L. Body image mediates the effect of stoma status on psychological distress and quality of life in patients with colorectal cancer. Psycho-Oncology 2020, 29, 796–802. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Classification of Functioning, Disability and Health (ICF). Available online: https://www.who.int/standards/classifications/international-classification-of-functioning-disability-and-health (accessed on 28 March 2022).
- Rubenstein, L.M.; Freed, R.D.; Shapero, B.G.; Fauber, R.L.; Alloy, L.B. Cognitive attributions in depression: Bridging the gap between research and clinical practice. J. Psychother. Integr. 2016, 26, 103–115. [Google Scholar] [CrossRef]
- Vos, M.S.; De Haes, J.C.J.M. Denial in cancer patients, an explorative review. Psycho-Oncology 2006, 16, 12–25. [Google Scholar] [CrossRef]
- Rhoten, B.A.; Murphy, B.; Ridner, S.H. Body image in patients with head and neck cancer: A review of the literature. Oral Oncol. 2013, 49, 753–760. [Google Scholar] [CrossRef]
- Rodriguez, A.M.; Frenkiel, S.; Desroches, J.; De Simone, A.; Chiocchio, F.; Macdonald, C.; Black, M.; Zeitouni, A.; Hier, M.; Kost, K.; et al. Development and validation of the McGill body image concerns scale for use in head and neck oncology (MBIS-HNC): A mixed-methods approach. Psycho-Oncology 2019, 28, 116–121. [Google Scholar] [CrossRef] [Green Version]
- Melissant, H.C.; Jansen, F.; Eerenstein, S.E.; Cuijpers, P.; Laan, E.; Lissenberg-Witte, B.I.; Schuit, A.S.; Sherman, K.A.; Leemans, C.R.; Leeuw, I.M.V.-D. Body image distress in head and neck cancer patients: What are we looking at? Support. Care Cancer 2020, 29, 2161–2169. [Google Scholar] [CrossRef]
- Henry, M.; Ho, A.; Lambert, S.; Carnevale, F.A.; Greenfield, B.; Macdonald, C.; Mlynarek, A.; Zeitouni, A.; Rosberger, Z.; Hier, M.; et al. Looking beyond Disfigurement: The experience of Patients with Head and Neck Cancer. J. Palliat. Care 2018, 30, 5–15. [Google Scholar] [CrossRef]
- Screening for Distress. Canadian Partnership Against Cancer. Available online: https://www.partnershipagainstcancer.ca/topics/screening-distress/ (accessed on 28 March 2022).
- Shunmugasundaram, C.; Rutherford, C.; Butow, P.N.; Sundaresan, P.; Dhillon, H.M. What are the optimal measures to identify anxiety and depression in people diagnosed with head and neck cancer (HNC): A systematic review. J. Patient Rep. Outcomes 2020, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Skoogh, J.; Ylitalo, N.; Omerov, P.; Hauksdóttir, A.; Nyberg, U.; Wilderäng, U.; Johansson, B.; Gatz, M.; Steineck, G. ‘A no means no’—Measuring depression using a single-item question versus Hospital Anxiety and Depression Scale (HADS-D). Ann. Oncol. 2010, 21, 1905–1909. [Google Scholar] [CrossRef] [PubMed]
- Turon, H.; Carey, M.; Boyes, A.; Hobden, B.; Dilworth, S.; Sanson-Fisher, R. Agreement between a single-item measure of anxiety and depression and the Hospital Anxiety and Depression Scale: A cross-sectional study. PLoS ONE 2019, 14, e0210111. [Google Scholar] [CrossRef] [Green Version]
- Mina, D.S.; Van Rooijen, S.J.; Minnella, E.M.; Alibhai, S.M.H.; Brahmbhatt, P.; Dalton, S.O.; Gillis, C.; Grocott, M.P.W.; Howell, D.; Randall, I.M.; et al. Multiphasic Prehabilitation Across the Cancer Continuum: A Narrative Review and Conceptual Framework. Front. Oncol. 2021, 10, 598425. [Google Scholar] [CrossRef]
- Crevenna, R.; Palma, S.; Licht, T. Cancer prehabilitation—A short review. Memo-Mag. Eur. Med. Oncol. 2021, 14, 39–43. [Google Scholar] [CrossRef]
- Guidelines for Recovery-Oriented Practice. Mental Health Commission of Canada. Available online: https://mentalhealthcommission.ca/resource/guidelines-for-recovery-oriented-practice/ (accessed on 28 March 2022).
- Recovery Practices for Mental Health and Well-Being. WHO Quality Rights Specialized Training. 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/329602/9789241516747-eng.pdf (accessed on 28 March 2022).
- Caroline, G.-L.; Kennedy, E.B.; Byrne, N.; Gérin-Lajoie, C.; Katz, M.R.; Keshavarz, H.; Sellick, S.; Green, E. Systematic review and meta-analysis of collaborative care interventions for depression in patients with cancer. Psycho-Oncology 2016, 26, 573–587. [Google Scholar] [CrossRef]
- Clarke, A.; Thompson, A.R.; Jenkinson, E.; Rumsey, N.; Newell, R. CBT for Appearance Anxiety: Psychosocial Interventions for Anxiety Due to Visible Difference; Wiley Blackwell: New York, NY, USA, 2014. [Google Scholar]
- Choi, S.; Choi, J. Effects of the teach-back method among cancer patients: A systematic review of the literature. Support. Care Cancer 2021, 29, 7259–7268. [Google Scholar] [CrossRef]
- Talevski, J.; Shee, A.W.; Rasmussen, B.; Kemp, G.; Beauchamp, A. Teach-back: A systematic review of implementation and impacts. PLoS ONE 2020, 15, e0231350. [Google Scholar] [CrossRef]
| Time-Dependent Variables | N Total Sample | Mean | SD |
|---|---|---|---|
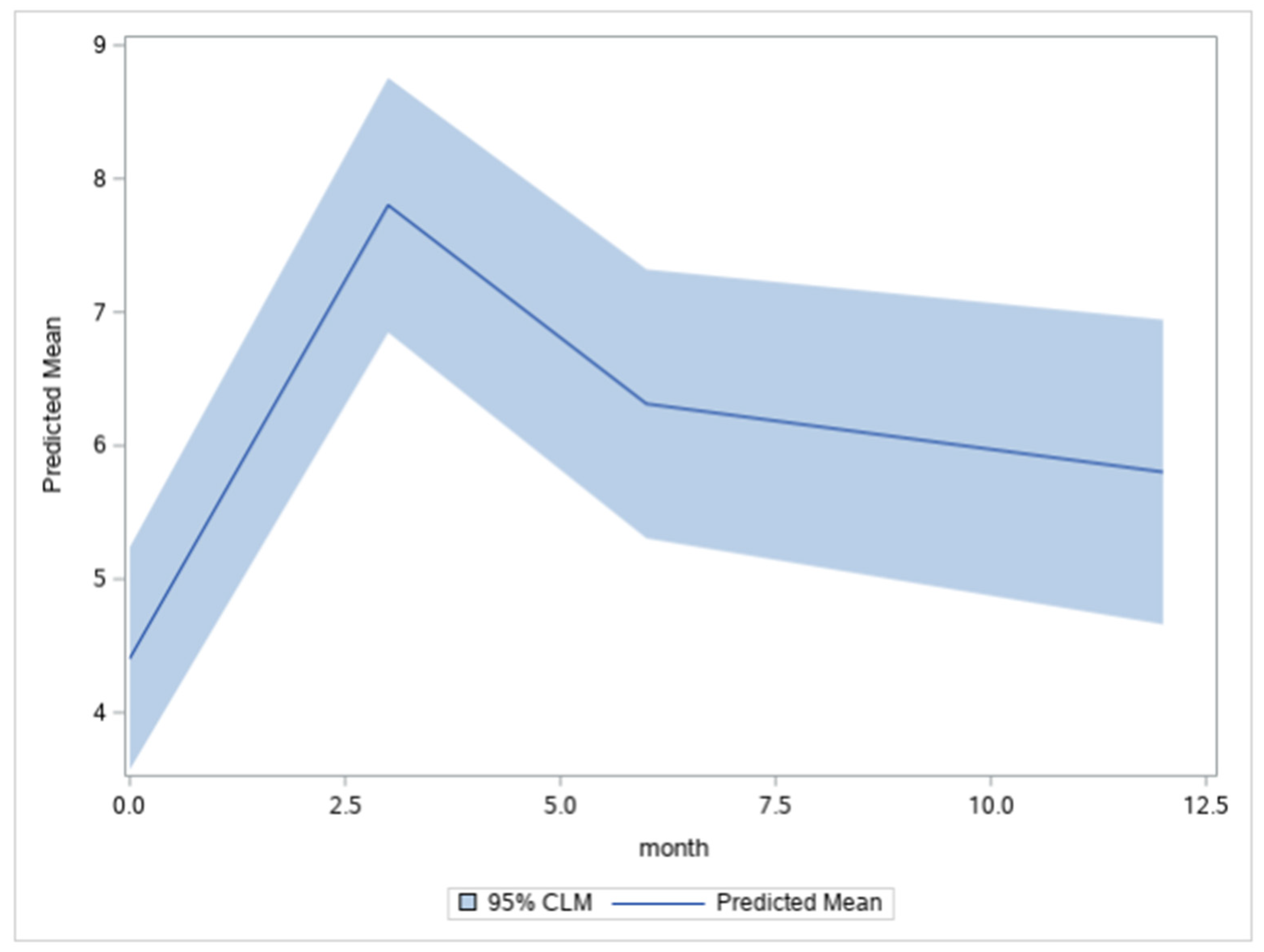
| BIS T0 | 218 | 4.46 | 5.75 |
| BIS T1 | 148 | 7.54 | 7.34 |
| BIS T2 | 141 | 5.83 | 6.22 |
| BIS T3 | 117 | 5.57 | 6.84 |
| HADS ANXIETY T0 | 219 | 6.02 | 4.65 |
| HADS ANXIETY T1 | 146 | 4.67 | 3.97 |
| HADS ANXIETY T2 | 140 | 4.11 | 3.71 |
| HADS ANXIETY T3 | 119 | 3.60 | 3.43 |
| HADS DEPRESSION T0 | 219 | 3.72 | 3.99 |
| HADS DEPRESSION T1 | 146 | 4.15 | 3.80 |
| HADS DEPRESSION T2 | 141 | 3.46 | 4.42 |
| HADS DEPRESSION T3 | 119 | 2.54 | 3.23 |
| FACT PHYSICAL T0 | 222 | 23.15 | 5.52 |
| FACT PHYSICAL T1 | 151 | 19.80 | 6.40 |
| FACT PHYSICAL T2 | 144 | 23.17 | 4.67 |
| FACT PHYSICAL T3 | 123 | 23.71 | 5.08 |
| FACT HEAD NECK T0 | 217 | 35.89 | 7.80 |
| FACT HEAD NECK T1 | 149 | 29.15 | 7.64 |
| FACT HEAD NECK T2 | 143 | 33.88 | 7.03 |
| FACT HEAD NECK T3 | 118 | 35.64 | 7.94 |
| DISFIGUREMENT T0 | 222 | 1.40 | 1.18 |
| DISFIGUREMENT T1 | 181 | 2.04 | 1.70 |
| DISFIGUREMENT T2 | 126 | 1.85 | 1.28 |
| DISFIGUREMENT T3 | 103 | 1.82 | 1.32 |
| N Total Sample | Frequency | Percent | |
| Treatment—3 months | 215 | ||
| -Surgery alone | 37 | 17 | |
| -Radiotherapy alone | 21 | 10 | |
| -Chemotherapy alone | 5 | 2 | |
| -Surgery and radiotherapy | 23 | 11 | |
| -Radiation and chemotherapy | 99 | 46 | |
| -Surgery and radiotherapy and chemotherapy | 30 | 14 | |
| Treatment—6 months | 6 | ||
| -Surgery alone | 3 | 50 | |
| -Radiotherapy alone | 1 | 17 | |
| -Chemotherapy alone | 1 | 17 | |
| -Surgery and radiotherapy | 0 | 0 | |
| -Radiation and chemotherapy | 1 | 17 | |
| -Surgery and radiotherapy and chemotherapy | 0 | 0 | |
| Treatment—12 months | 11 | ||
| -Surgery alone | 5 | 39 | |
| -Radiotherapy alone | 2 | 15 | |
| -Chemotherapy alone | 2 | 15 | |
| -Surgery and radiotherapy | 0 | 0 | |
| -Radiation and chemotherapy | 2 | 15 | |
| -Surgery and radiotherapy and chemotherapy | 2 | 15 | |
| Free flap | 208 | 30 | 14 |
| BIS T0 ≥ 10 | 218 | 31 | 14 |
| BIS T1 ≥ 10 | 148 | 42 | 28 |
| BIS T2 ≥ 10 | 141 | 28 | 20 |
| BIS T3 ≥ 10 | 117 | 25 | 21 |
| Time-Invariant Variables | N | Mean | SD |
| Age | 224 | 62.94 | 11.70 |
| COPE DENIAL | 219 | 3.07 | 1.65 |
| N | Frequency | Percent | |
| Female | 224 | 72 | 32 |
| French Language | 224 | 115 | 51 |
| Marital Status | 224 | ||
| Married | 142 | 63 | |
| Divorced | 47 | 21 | |
| Single | 23 | 10 | |
| Widowed | 12 | 5 | |
| Stage III/IV | 222 | 161 | 73 |
| Tumour site | 223 | ||
| Oropharynx | 82 | 37 | |
| Oral | 46 | 21 | |
| Larynx | 37 | 17 | |
| Skin | 15 | 7 | |
| Nasopharynx | 11 | 5 | |
| Unknown Primary | 12 | 5 | |
| Other | 20 | 9 | |
| Cancer type—HPV+ | 223 | 94 | 42 |
| Physical function—ECOG 2+ | 221 | 25 | 10 |
| Model 1 (Unconditional) | Model 2 (Time Only) | Model 3 (Explanatory) | |
|---|---|---|---|
| Fixed Effects | |||
| Level 1: Time-Dependent Factors | |||
| Time (T0, T1, T2, T3) | - | 1.19 *** (0.19) | 0.60 ** (0.21) |
| Time *Time | - | −0.81 *** (0.18) | −0.37 * (0.19) |
| Time *Time *Time | - | 0.15 ** (0.039) | 0.068 (0.041) |
| FACT PHYSICAL | - | - | −0.12 ** (0.044) |
| FACT HEAD NECK | - | - | −0.15 ** (0.043) |
| HADS ANXIETY | - | - | 0.13 ** (0.046) |
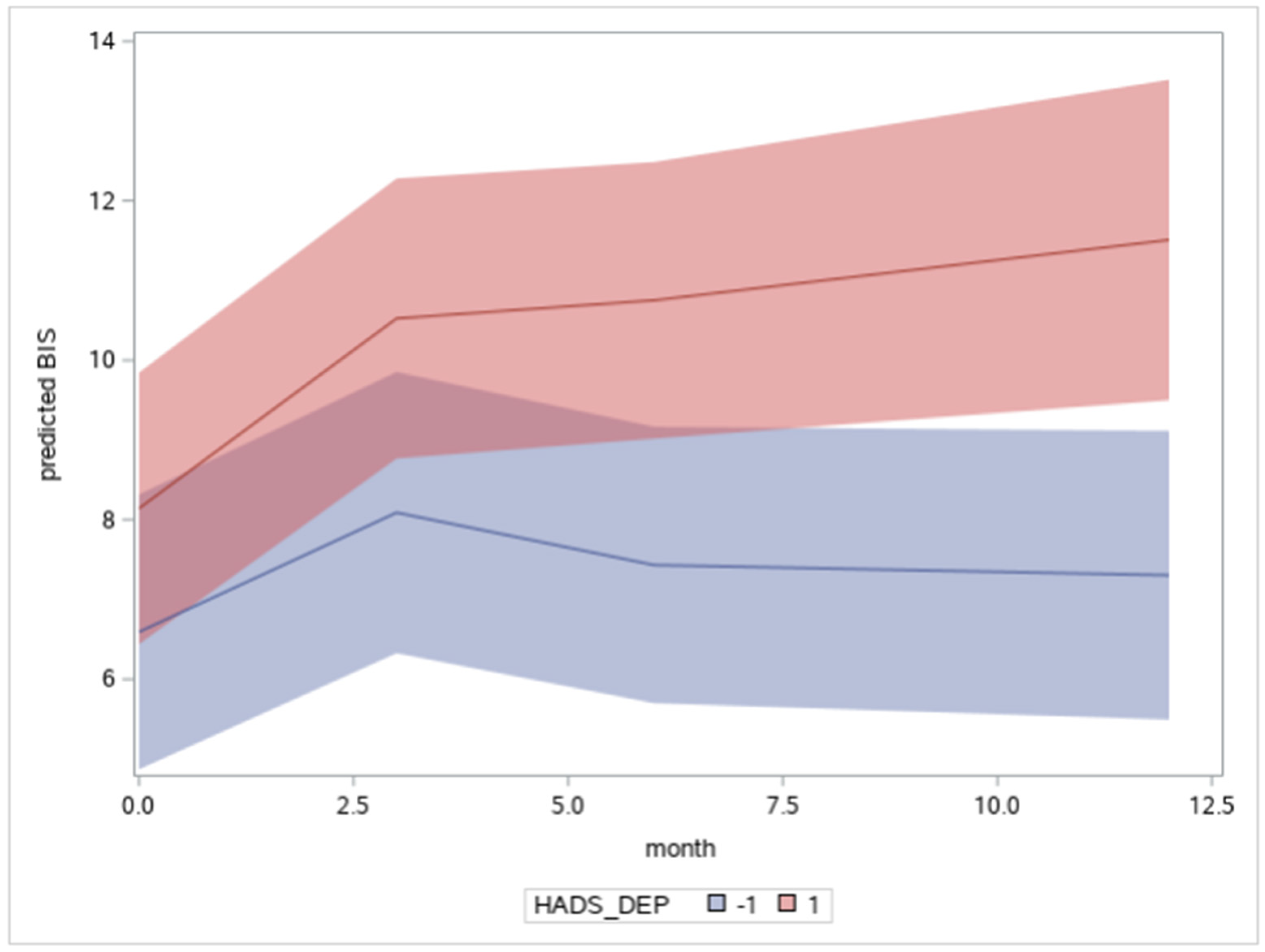
| HADS DEPRESSION | - | - | 0.12 * (0.056) |
| Time*HADS DEPRESSION | - | - | 0.067 * (0.028) |
| DISFIGUREMENT | - | - | 0.079 * (0.037) |
| Level 2: Time-Invariant Factors | |||
| Age at Baseline | - | - | −0.13 ** (0.049) |
| Female | - | - | 0.22 * (0.095) |
| Stage III/IV | - | - | 0.24 * (0.10) |
| COPE DENIAL | - | - | 0.12 ** (0.044) |
| Married † | - | - | −0.36 (0.21) |
| Divorced † | - | - | −0.14 (0.23) |
| Single † | - | - | −0.59 * (0.25) |
| French Language | - | - | 0.19 * (0.086) |
| Error Variance | |||
| Intercept | 0.551 *** (0.072) | 0.547 *** (0.072) | 0.203 *** (0.037) |
| Time | - | 0.025 * (0.012) | 0.014 (0.009) |
| Residual | 0.463 *** (0.033) | 0.371 *** (0.030) | 0.314 *** (0.026) |
| Model Fit | |||
| AIC | 1611.4 | 1572.3 | 1181.3 |
| BIC | 1618.2 | 1582.6 | 1251.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albert, J.G.; Lo, C.; Rosberger, Z.; Frenkiel, S.; Hier, M.; Zeitouni, A.; Kost, K.; Mlynarek, A.; Black, M.; MacDonald, C.; et al. Biopsychosocial Markers of Body Image Concerns in Patients with Head and Neck Cancer: A Prospective Longitudinal Study. Curr. Oncol. 2022, 29, 4438-4454. https://doi.org/10.3390/curroncol29070353
Albert JG, Lo C, Rosberger Z, Frenkiel S, Hier M, Zeitouni A, Kost K, Mlynarek A, Black M, MacDonald C, et al. Biopsychosocial Markers of Body Image Concerns in Patients with Head and Neck Cancer: A Prospective Longitudinal Study. Current Oncology. 2022; 29(7):4438-4454. https://doi.org/10.3390/curroncol29070353
Chicago/Turabian StyleAlbert, Justine G., Christopher Lo, Zeev Rosberger, Saul Frenkiel, Michael Hier, Anthony Zeitouni, Karen Kost, Alex Mlynarek, Martin Black, Christina MacDonald, and et al. 2022. "Biopsychosocial Markers of Body Image Concerns in Patients with Head and Neck Cancer: A Prospective Longitudinal Study" Current Oncology 29, no. 7: 4438-4454. https://doi.org/10.3390/curroncol29070353
APA StyleAlbert, J. G., Lo, C., Rosberger, Z., Frenkiel, S., Hier, M., Zeitouni, A., Kost, K., Mlynarek, A., Black, M., MacDonald, C., Richardson, K., Mascarella, M., Morand, G. B., Chartier, G., Sadeghi, N., Sultanem, K., Shenouda, G., Cury, F. L., & Henry, M. (2022). Biopsychosocial Markers of Body Image Concerns in Patients with Head and Neck Cancer: A Prospective Longitudinal Study. Current Oncology, 29(7), 4438-4454. https://doi.org/10.3390/curroncol29070353







