Megaprosthesis for Metastatic Bone Disease—A Comparative Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Context
2.2. Inclusion
2.3. Variables
2.4. Statistics
3. Results
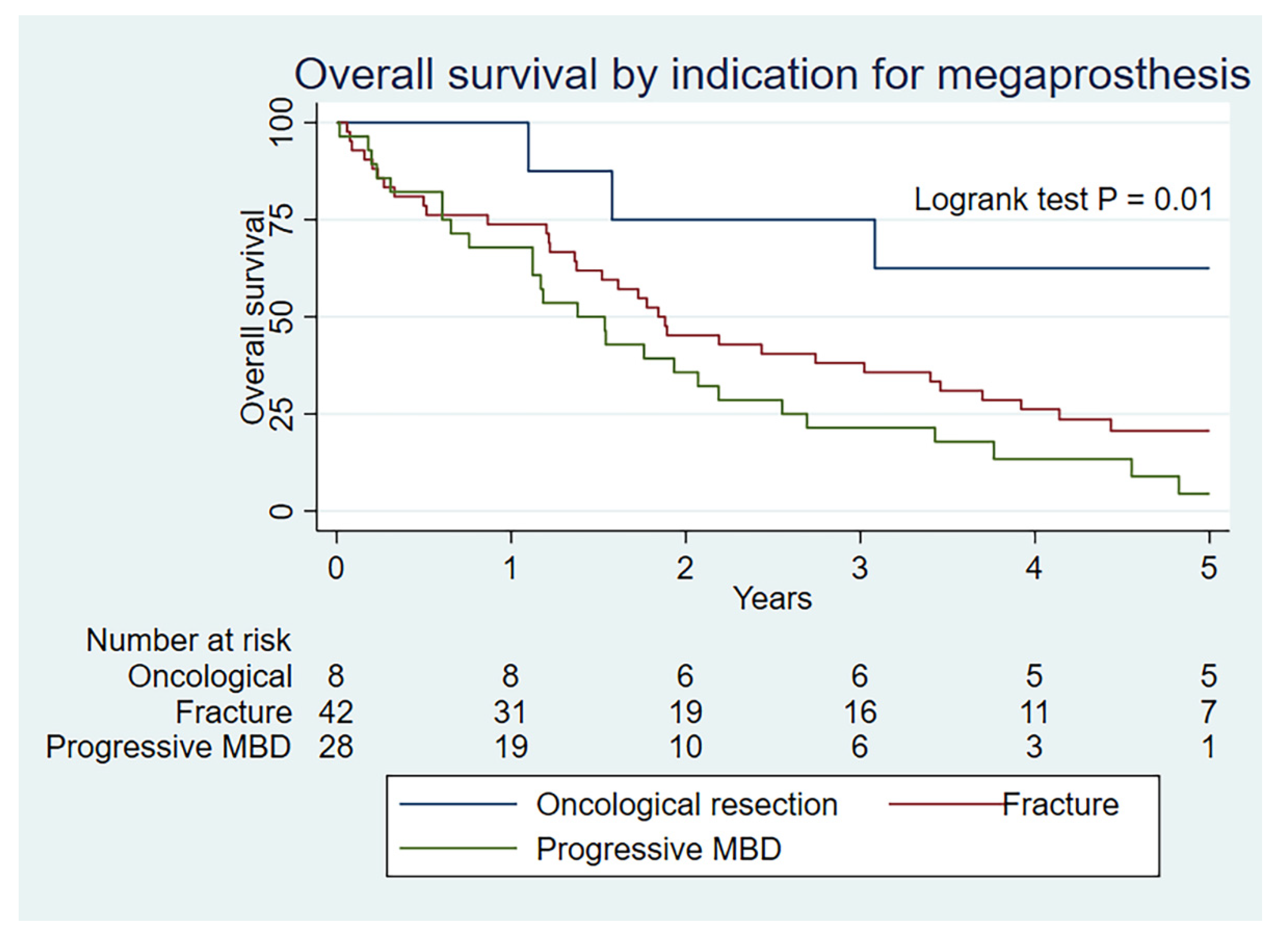
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coleman, R.E. Clinical Features of Metastatic Bone Disease and Risk of Skeletal Morbidity. Clin. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Errani, C.; Mavrogenis, A.F.; Cevolani, L.; Spinelli, S.; Piccioli, A.; Maccauro, G.; Baldini, N.; Donati, D.M. Treatment for long bone metastases based on a systematic literature review. Eur. J. Orthop. Surg. Traumatol. 2016, 27, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Kinnane, N. Burden of bone disease. Eur. J. Oncol. Nurs. 2007, 11, S28–S31. [Google Scholar] [CrossRef] [PubMed]
- Ashford, R.U.; Hanna, S.A.; Park, D.H.; Pollock, R.C.; Skinner, J.A.; Briggs, T.W.; Cannon, S.R. Proximal femoral replacements for metastatic bone disease: Financial implications for sarcoma units. Int. Orthop. 2010, 34, 709–713. [Google Scholar] [CrossRef] [Green Version]
- Schulman, K.L.; Kohles, J. Economic burden of metastatic bone disease in the U.S. Cancer 2007, 109, 2334–2342. [Google Scholar] [CrossRef]
- Gainor, B.J.; Buchert, P. Fracture healing in metastatic bone disease. Clin. Orthop. Relat. Res. 1983, 178, 297–302. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Kido, A.; Tanaka, Y.; Facchini, G.; Peta, G.; Rossi, G.; Mavrogenis, A.F. Current Overview of Treatment for Metastatic Bone Disease. Curr. Oncol. 2021, 28, 3347–3372. [Google Scholar] [CrossRef]
- Bauer, H.C.F. Controversies in the surgical management of skeletal metastases. J. Bone Jt. Surg. Br. Vol. 2005, 87, 608–617. [Google Scholar] [CrossRef]
- Hansen, B.H.; Keller, J.; Laitinen, M.; Berg, P.; Skjeldal, S.; Trovik, C.; Nilsson, J.; Walloe, A.; Kalén, A.; Wedin, R. The Scandinavian Sarcoma Group skeletal metastasis register Survival after surgery for bone metastases in the pelvis and extremities. Acta Orthop. Scand. 2004, 75, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Van der Linden, Y.M.; Dijkstra, P.D.; Kroon, H.M.; Lok, J.J.; Noordijk, E.M.; Leer, J.W.; Marijnen, C.A.M. Comparative analysis of risk factors for pathological fracture with femoral metastases. J. Bone Jt. Surg. Br. Vol. 2004, 86, 566–573. [Google Scholar] [CrossRef]
- Bindels, B.J.J.; Thio, Q.; Raskin, K.A.; Ferrone, M.L.; Lozano Calderón, S.A.; Schwab, J.H. Thirty-day Postoperative Complications after Surgery for Metastatic Long Bone Disease Are Associated with Higher Mortality at 1 Year. Clin. Orthop. Relat. Res. 2020, 478, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, D.; Gazendam, A.M.; Ghert, M. The Surgical Management of Proximal Femoral Metastases: A Narrative Review. Curr. Oncol. 2021, 28, 3748–3757. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Katagiri, H.; Murata, H.; Wasa, J.; Miyagi, M.; Honda, Y.; Takahashi, M. Surgery for femoral metastases. Bone Jt. J. 2020, 102-B, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Potter, B.K.; Chow, V.E.; Adams, S.C.; Letson, G.D.; Temple, H.T. Endoprosthetic proximal femur replacement: Metastatic versus primary tumors. Surg. Oncol. 2009, 18, 343–349. [Google Scholar] [CrossRef]
- Zacherl, M.; Gruber, G.; Glehr, M.; Ofner-Kopeinig, P.; Radl, R.; Greitbauer, M.; Vecsei, V.; Windhager, R. Surgery for pathological proximal femoral fractures, excluding femoral head and neck fractures: Resection vs. stabilisation. Int. Orthop. 2011, 35, 1537–1543. [Google Scholar] [CrossRef] [Green Version]
- Cannon, C.P.; Mirza, A.N.; Lin, P.P.; Lewis, V.O.; Yasko, A.W. Proximal femoral endoprosthesis for the treatment of metastatic. Orthopedics 2008, 31, 361. [Google Scholar]
- Chandrasekar, C.R.; Grimer, R.J.; Carter, S.R.; Tillman, R.M.; Abudu, A.T. Modular endoprosthetic replacement for metastatic tumours of the proximal femur. J. Orthop. Surg. Res. 2008, 3, 50. [Google Scholar] [CrossRef] [Green Version]
- Benevenia, J.; Kirchner, R.; Patterson, F.; Beebe, K.; Wirtz, D.C.; Rivero, S.; Palma, M.; Friedrich, M.J. Outcomes of a Modular Intercalary Endoprosthesis as Treatment for Segmental Defects of the Femur, Tibia, and Humerus. Clin. Orthop. Relat. Res. 2016, 474, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Bernthal, N.M.; Schwartz, A.J.; Oakes, D.A.; Kabo, J.M.; Eckardt, J.J. How long do endoprosthetic reconstructions for proximal femoral tumors last? Clin. Orthop. Relat. Res. 2010, 468, 2867–2874. [Google Scholar] [CrossRef] [Green Version]
- Bus, M.P.; van de Sande, M.A.; Fiocco, M.; Schaap, G.R.; Bramer, J.A.; Dijkstra, P.D. What Are the Long-term Results of MUTARS(R) Modular Endoprostheses for Reconstruction of Tumor Resection of the Distal Femur and Proximal Tibia? Clin. Orthop. Relat. Res. 2017, 475, 708–718. [Google Scholar] [CrossRef] [Green Version]
- Gkavardina, A.; Tsagozis, P. The Use of Megaprostheses for Reconstruction of Large Skeletal Defects in the Extremities: A Critical Review. Open Orthop. J. 2014, 8, 384–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosheger, G.; Gebert, C.; Ahrens, H.; Streitbuerger, A.; Winkelmann, W.; Hardes, J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin. Orthop. Relat. Res. 2006, 450, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Jeys, L.; Kulkarni, A.; Grimer, R.; Carter, S.; Tillman, R.; Abudu, A. Endoprosthetic Reconstruction for the Treatment of Musculoskeletal Tumors of the Appendicular Skeleton and Pelvis. J. Bone Jt. Surg. 2008, 90, 1265–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shehadeh, A.; Noveau, J.; Malawer, M.; Henshaw, R. Late Complications and Survival of Endoprosthetic Reconstruction after Resection of Bone Tumors. Clin. Orthop. Relat. Res. 2010, 468, 2885–2895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkinis, M.N.; Lyne, C.J.; Wilson, M.D.; Choong, P.F. Metastatic bone disease: A review of survival, prognostic factors and outcomes following surgical treatment of the appendicular skeleton. Eur. J. Surg. Oncol. 2016, 42, 1787–1797. [Google Scholar] [CrossRef]
- Guzik, G. Results of the treatment of bone metastases with modular prosthetic replacement—Analysis of 67 patients. J. Orthop. Surg. Res. 2016, 11, 20. [Google Scholar] [CrossRef] [Green Version]
- Scotti, C.; Camnasio, F.; Peretti, G.M.; Fontana, F.; Fraschini, G. Modular prostheses in the treatment of proximal humerus metastases: Review of 40 cases. J. Orthop. Traumatol. 2008, 9, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Casali, P.G.; Bielack, S.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.; Brennan, B.; et al. Bone sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv79–iv95. [Google Scholar] [CrossRef]
- Gerrand, C.; Athanasou, N.; Brennan, B.; Grimer, R.; Judson, I.; Morland, B.; Peake, D.; Seddon, B.; Whelan, J.; on behalf of the British Sarcoma Group. UK guidelines for the management of bone sarcomas. Clin. Sarcoma Res. 2016, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Henderson, E.R.; Groundland, J.S.; Pala, E.; Dennis, J.A.; Wooten, R.; Cheong, D.; Windhager, R.; Kotz, R.I.; Mercuri, M.; Funovics, P.T.; et al. Failure mode classification for tumor endoprostheses: Retrospective review of five institutions and a literature review. J. Bone Jt. Surg. Am. 2011, 93, 418–429. [Google Scholar] [CrossRef]
- Palumbo, B.T.; Henderson, E.R.; Groundland, J.S.; Cheong, D.; Pala, E.; Letson, G.D.; Ruggieri, P. Advances in segmental endoprosthetic reconstruction for extremity tumors: A review of contemporary designs and techniques. Cancer Control 2011, 18, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Ranstam, J.; Karrholm, J.; Pulkkinen, P.; Makela, K.; Espehaug, B.; Pedersen, A.B.; Mehnert, F.; Furnes, O.; NARA Study Group. Statistical analysis of arthroplasty data. II. Guidelines. Acta Orthop. 2011, 82, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Ranstam, J.; Karrholm, J.; Pulkkinen, P.; Makela, K.; Espehaug, B.; Pedersen, A.B.; Mehnert, F.; Furnes, O.; NARA Study Group. Statistical analysis of arthroplasty data. I. Introduction and background. Acta Orthop. 2011, 82, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Kirkinis, M.N.; Spelman, T.; May, D.; Choong, P.F.M. Metastatic bone disease of the pelvis and extremities: Rationalizing orthopaedic treatment. ANZ J. Surg. 2017, 87, 940–944. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Davis, A.M.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Marshall, D.A. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet 2002, 359, 2235–2241. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457. [Google Scholar] [CrossRef]
- Lacny, S.; Wilson, T.; Clement, F.; Roberts, D.J.; Faris, P.D.; Ghali, W.A. Kaplan-Meier Survival Analysis Overestimates the Risk of Revision Arthroplasty: A Meta-analysis. Clin. Orthop. Relat. Res. 2015, 473, 3431–3442. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.E.; Croucher, P.I.; Padhani, A.R.; Clézardin, P.; Chow, E.; Fallon, M.; Guise, T.; Colangeli, S.; Capanna, R.; Costa, L. Bone metastases. Nat. Rev. Dis. Primers 2020, 6, 83. [Google Scholar] [CrossRef]
- Clayer, M.; Doyle, S.; Sangha, N.; Grimer, R. The toronto extremity salvage score in unoperated controls: An age, gender, and country comparison. Sarcoma 2012, 2012, 717213. [Google Scholar] [CrossRef] [Green Version]
| Variable Name | Sarcoma n = 133 | Metastasis n = 78 | Statistical Test |
|---|---|---|---|
| Mean age at surgery, years of age (std. deviation) | 42 yoa (22.6) | 63 yoa (11.2) | p < 0.01 |
| Sex male /female frequency (%) | 71/62 (53/47) | 35/43 (45/55) | p = 0.23 |
| Upper-/lower extremity frequency (%) | 22/111 (17/83) | 27/51 (35/65) | p = 0.01 |
| Pre-op Hemoglobin g/dl mean (std. deviation) | 12.7 (1.8) | 12.4 (1.6) | p = 0.39 |
| Smoking status yes/no frequency (%) | 22/111 (17/83) | 20/58 (26/74) | p = 0.22 |
| Radiotherapy pre/ post /total frequency | 1/6/7 | 38/26/55 | p < 0.01 |
| Chemotherapy pre/post/total frequency | 60/68/68 | 30/49/54 | p = 0.01 |
| Previous surgery yes/no frequency (%) | 51/82 (38/62) | 17/61(22/78) | p = 0.01 |
| Fixation: frequency (%) | p < 0.01 | ||
| *Hybrid | 18 (14) | 5(6) | |
| *Uncemented | 87(65) | 14(18) | |
| *Cemented | 28(21) | 59(76) | |
| Observation time years (median) | 3.7 years | 1.6 years | p < 0.01 |
| Bone | Type/Site | Sarcoma n = 133 | Metastatic Bone Disease (MBD) n = 78 |
|---|---|---|---|
| Femur | Proximal | 44 | 27 |
| Diaphyseal | 0 | 7 | |
| Distal | 40 | 13 | |
| Total femur | 4 | 1 | |
| Tibia | Proximal | 23 | 3 |
| Humerus | Proximal | 17 | 17 |
| Diaphyseal | 0 | 2 | |
| Distal | 2 | 6 | |
| Total humerus | 3 | 2 |
| Variable Name | Sarcoma Indication n = 133 | Metastatic Indication n = 78 |
|---|---|---|
| Limb salvage yes/ no frequency (%) | 120/12 (90/10) | 73/4 (94/6) |
| Surgical margin frequency (%) | ||
| R0 | 124 (93) | 29 (37) |
| R1 | 9 (7) | 41 (53) |
| R2 | 0 | 8 (10) |
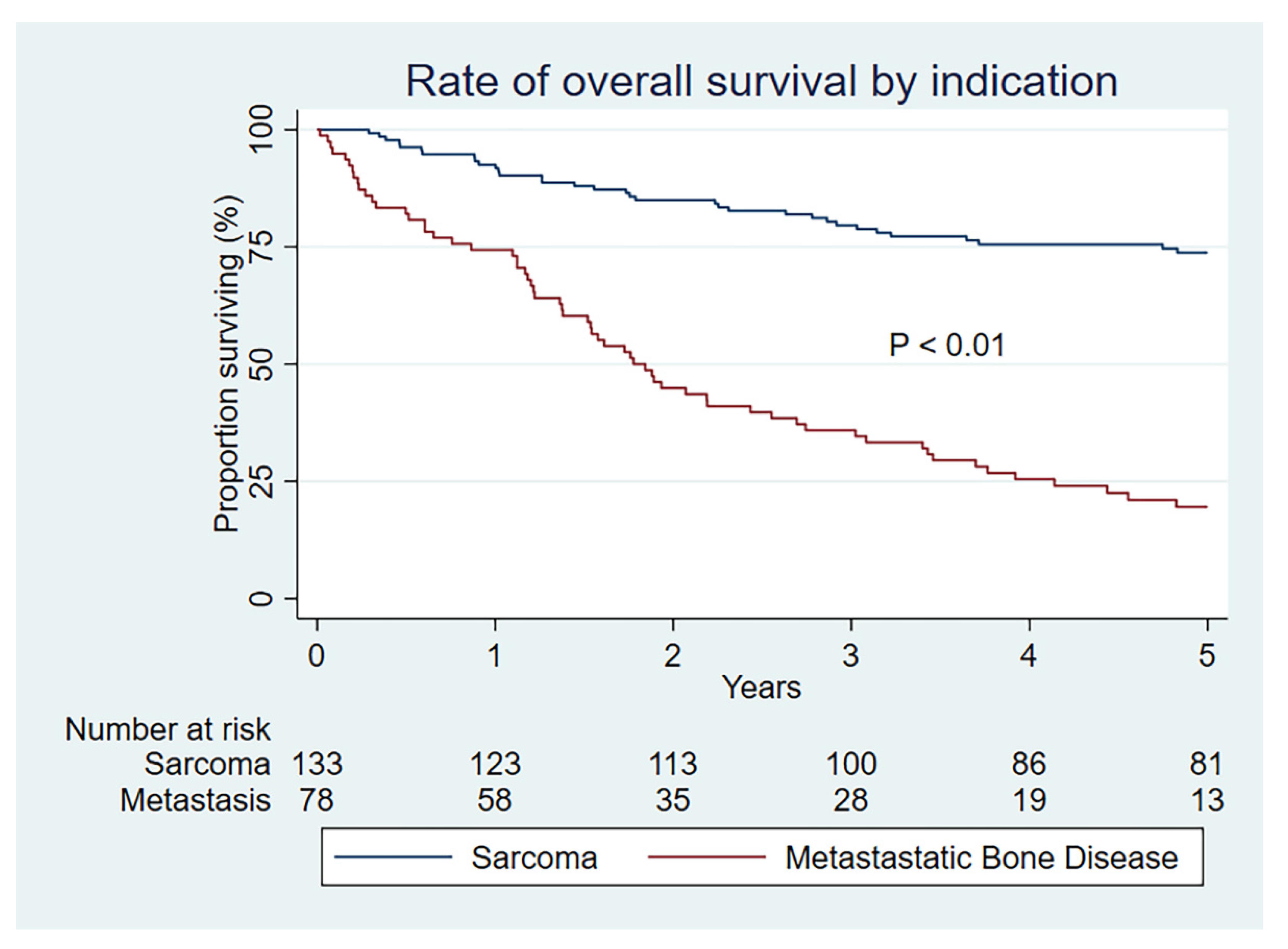
| Overall survival (95% CI) | ||
| 1 year | 92 (86–96) | 74 (63–83) |
| 2 year | 85 (78–90) | 45 (34–56) |
| 5 year | 74 (65–81) | 20 (11–29) |
| Cases undergoing revision frequency (%) | ||
| 47 (35) | 12 (15) | |
| Frequency of revision by type | ||
| (Henderson–Palumbo) | ||
| Type 1—Soft tissue failure | 7 | 0 |
| Type 2—Aseptic loosening | 6 | 5 |
| Type 3—Structural failure | 13 | 1 |
| Type 4—Infection | 18 | 5 |
| Type 5—Tumour progression | 3 | 1 |
| Kaplan–Meier rate of revision (95% CI) | ||
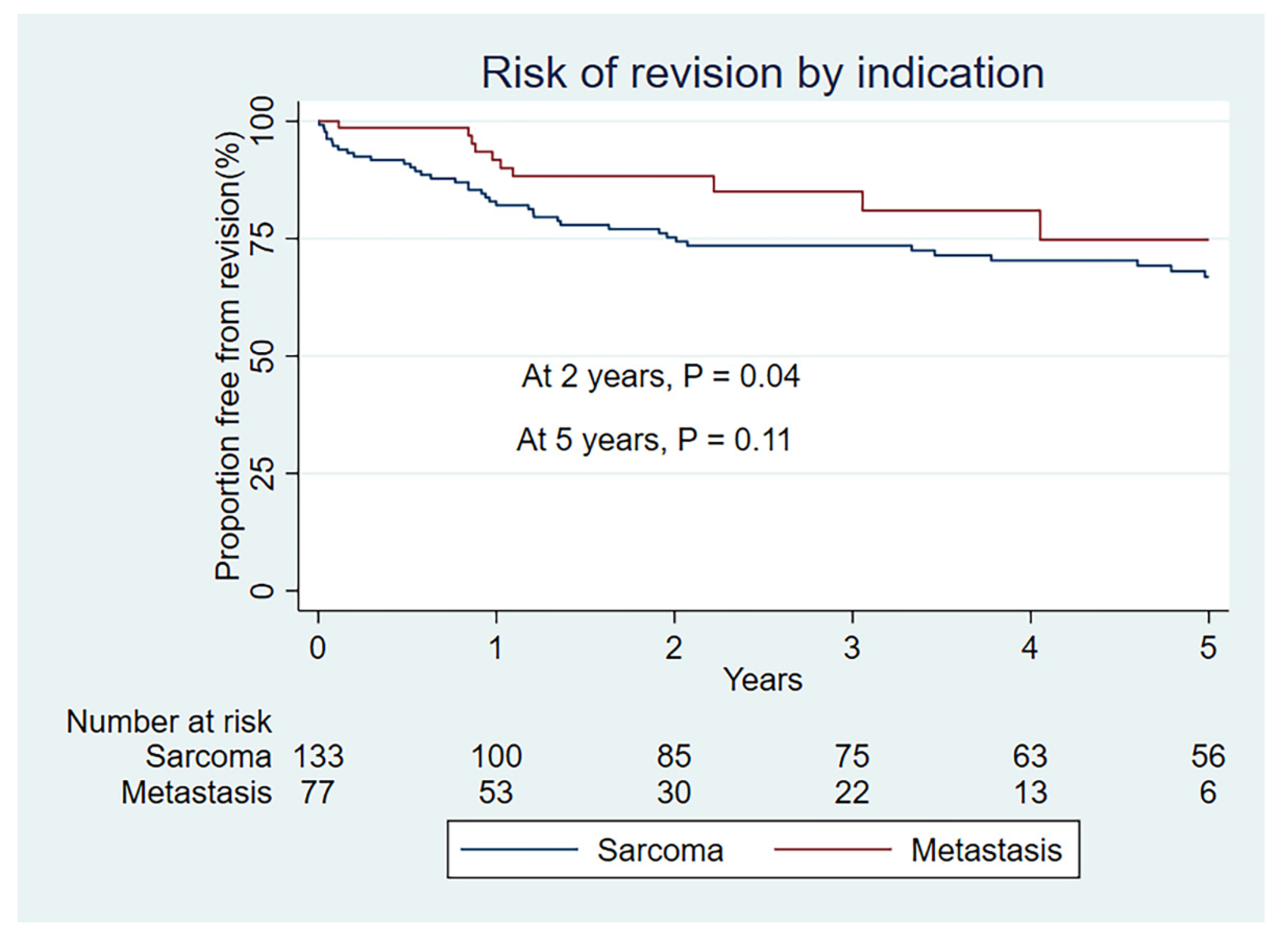
| 1 year | 18 (12–26) | 8 (4–19) |
| 2 year | 24 (18–33) | 12 (6–23) |
| 5 year | 33 (25–43) | 25 (13–46) |
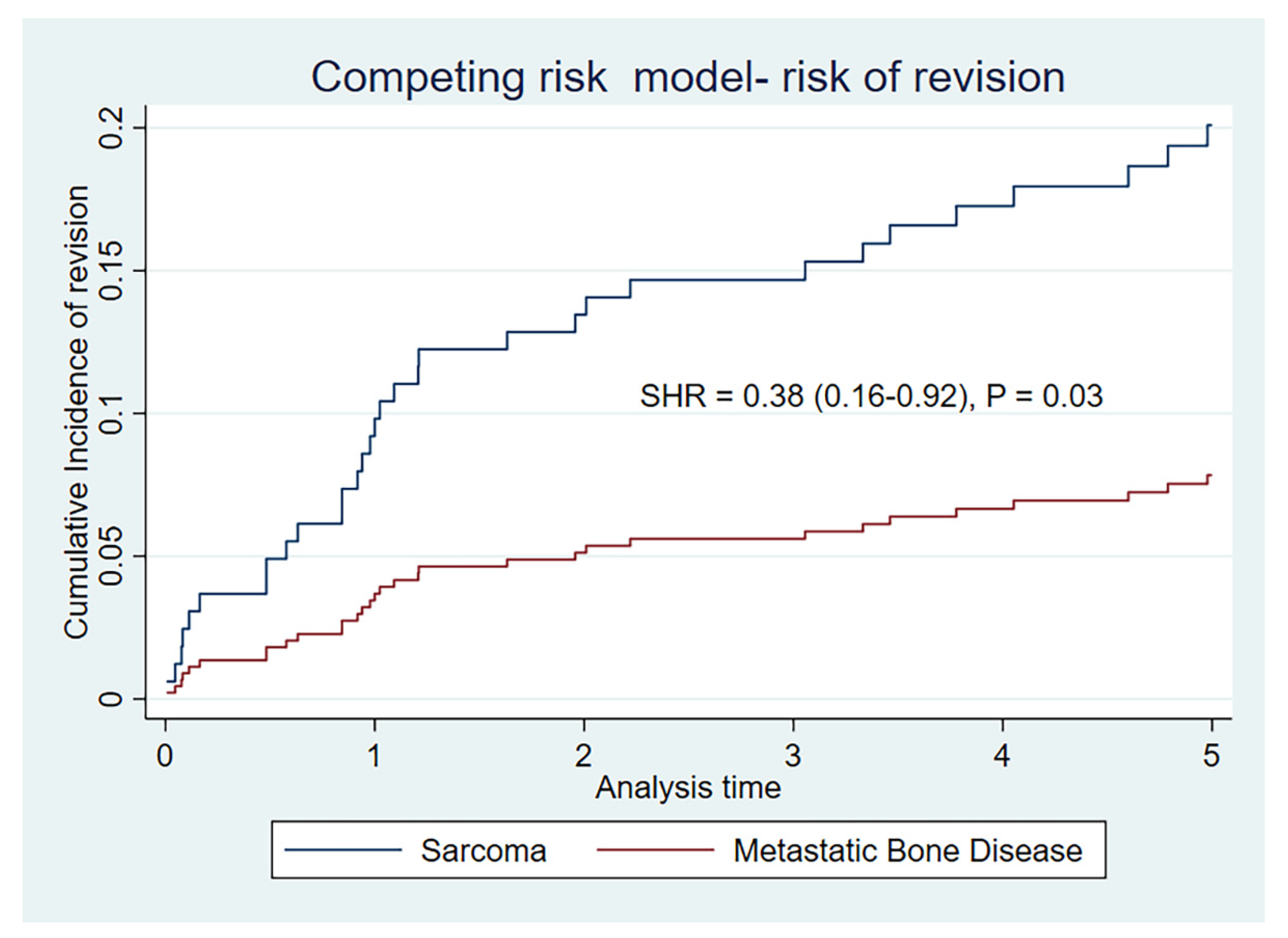
| Cumulative incidence of risk of revision-death as competing event (95% CI) | ||
| 1 year | 11 (6–17) | 1 (0–6) |
| 2 year | 14 (9–20) | 4 (1–10) |
| 5 year | 20 (13–27) | 8 (3–15) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thorkildsen, J.; Strøm, T.A.; Strøm, N.J.; Sellevold, S.; Norum, O.-J. Megaprosthesis for Metastatic Bone Disease—A Comparative Analysis. Curr. Oncol. 2022, 29, 3460-3471. https://doi.org/10.3390/curroncol29050279
Thorkildsen J, Strøm TA, Strøm NJ, Sellevold S, Norum O-J. Megaprosthesis for Metastatic Bone Disease—A Comparative Analysis. Current Oncology. 2022; 29(5):3460-3471. https://doi.org/10.3390/curroncol29050279
Chicago/Turabian StyleThorkildsen, Joachim, Thale Asp Strøm, Nils Jørgen Strøm, Simen Sellevold, and Ole-Jacob Norum. 2022. "Megaprosthesis for Metastatic Bone Disease—A Comparative Analysis" Current Oncology 29, no. 5: 3460-3471. https://doi.org/10.3390/curroncol29050279
APA StyleThorkildsen, J., Strøm, T. A., Strøm, N. J., Sellevold, S., & Norum, O.-J. (2022). Megaprosthesis for Metastatic Bone Disease—A Comparative Analysis. Current Oncology, 29(5), 3460-3471. https://doi.org/10.3390/curroncol29050279





