Understanding the Experience of Canadian Women Living with Ovarian Cancer through the Every Woman StudyTM
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Summary of Participants
3.2. Ovarian Cancer Awareness Prior to Diagnosis
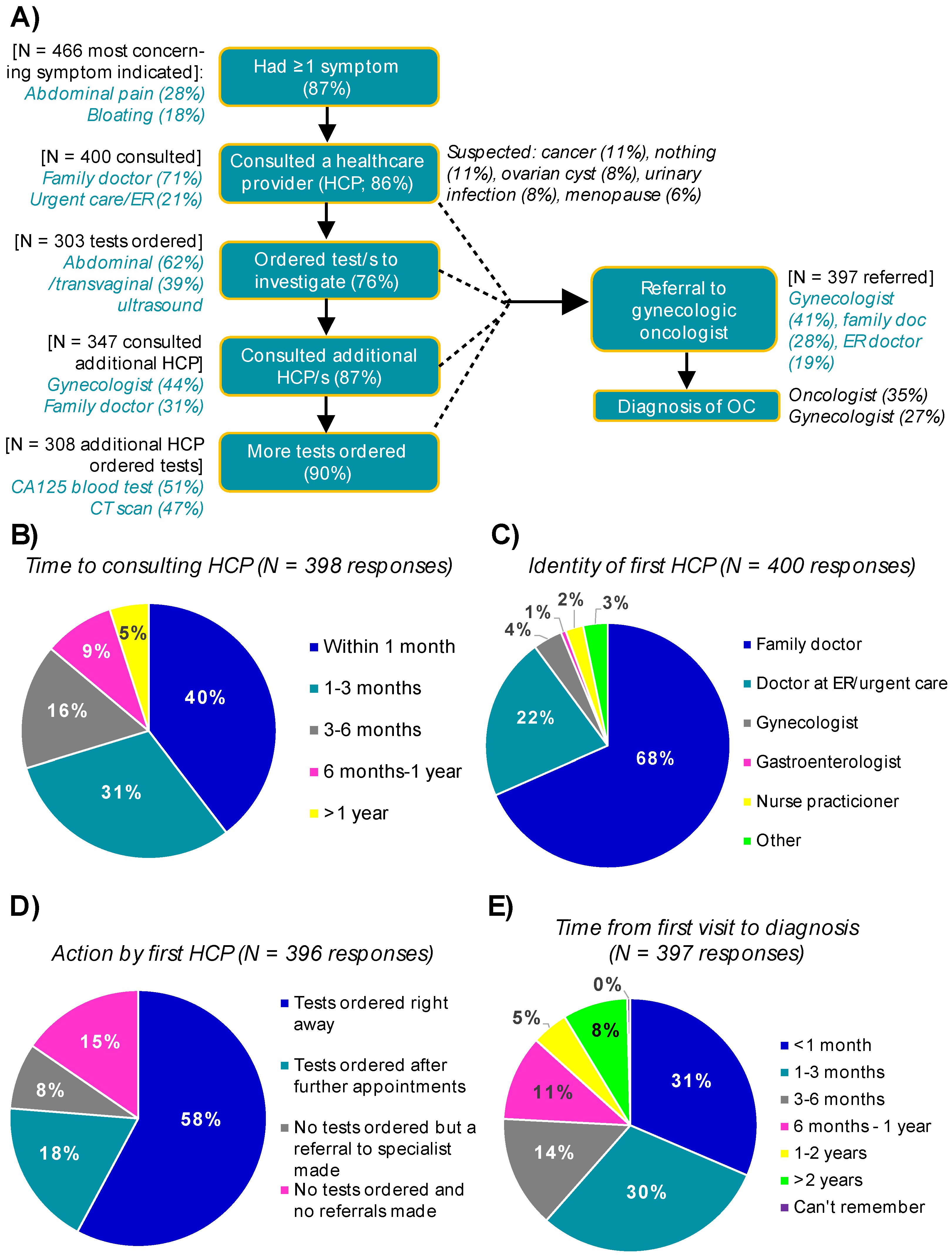
3.3. The Pathway to a Diagnosis of Ovarian Cancer
3.3.1. Symptoms
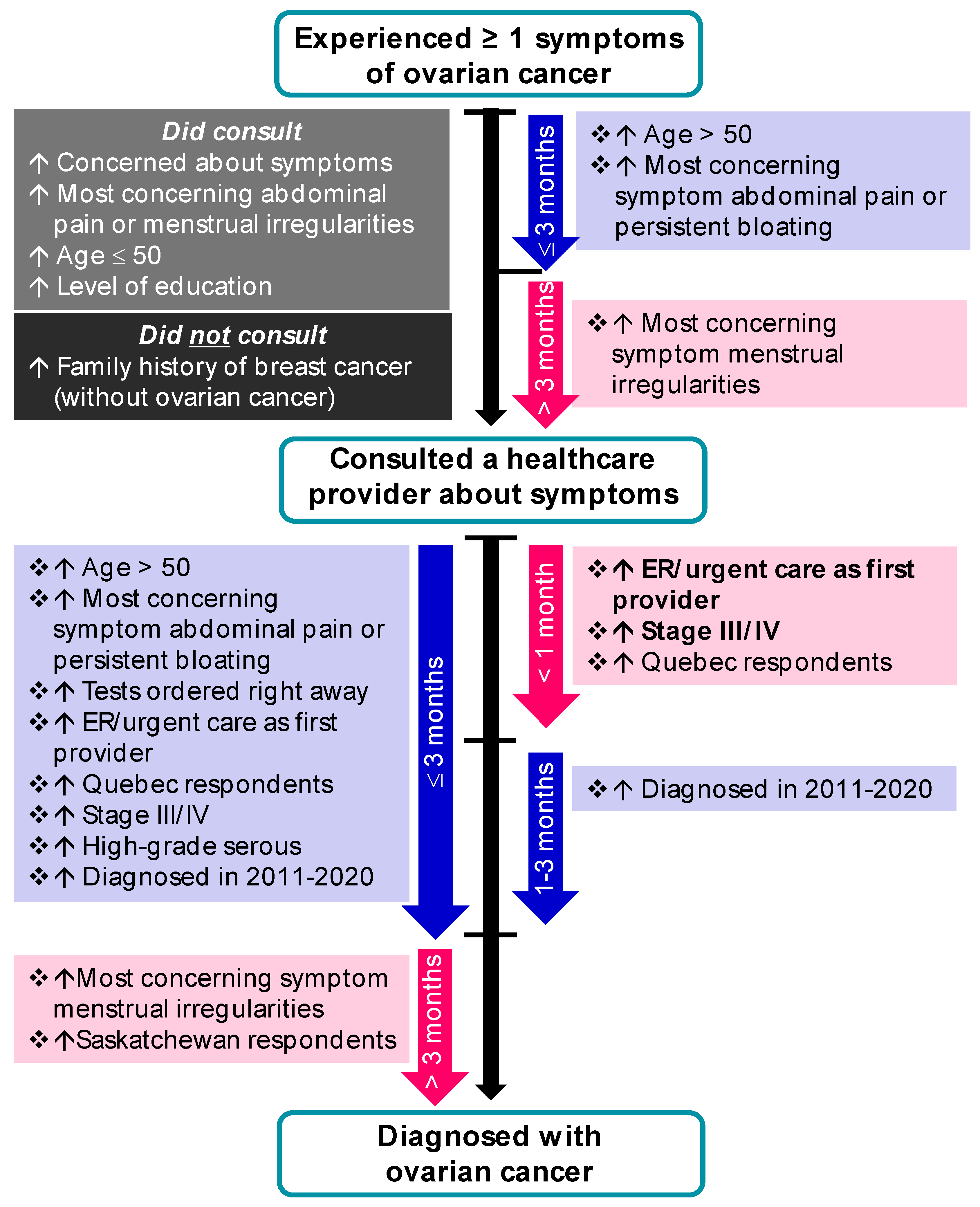
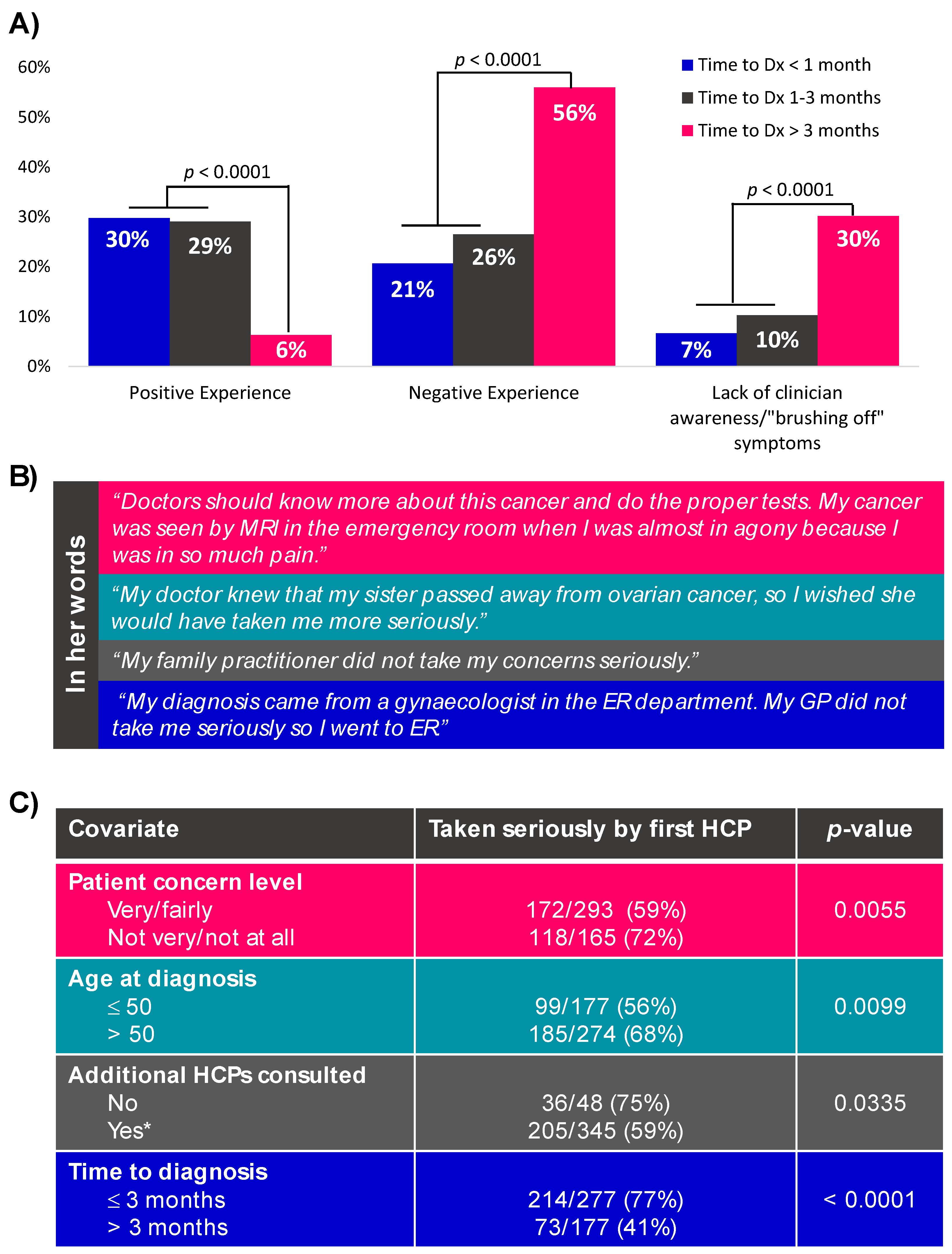
3.3.2. Consulting a Healthcare Provider
3.3.3. From First Consult to Diagnosis in Symptomatic Women
3.3.4. Alternate Pathways to an Ovarian Cancer Diagnosis
3.4. Post-Diagnostic Clinical Care
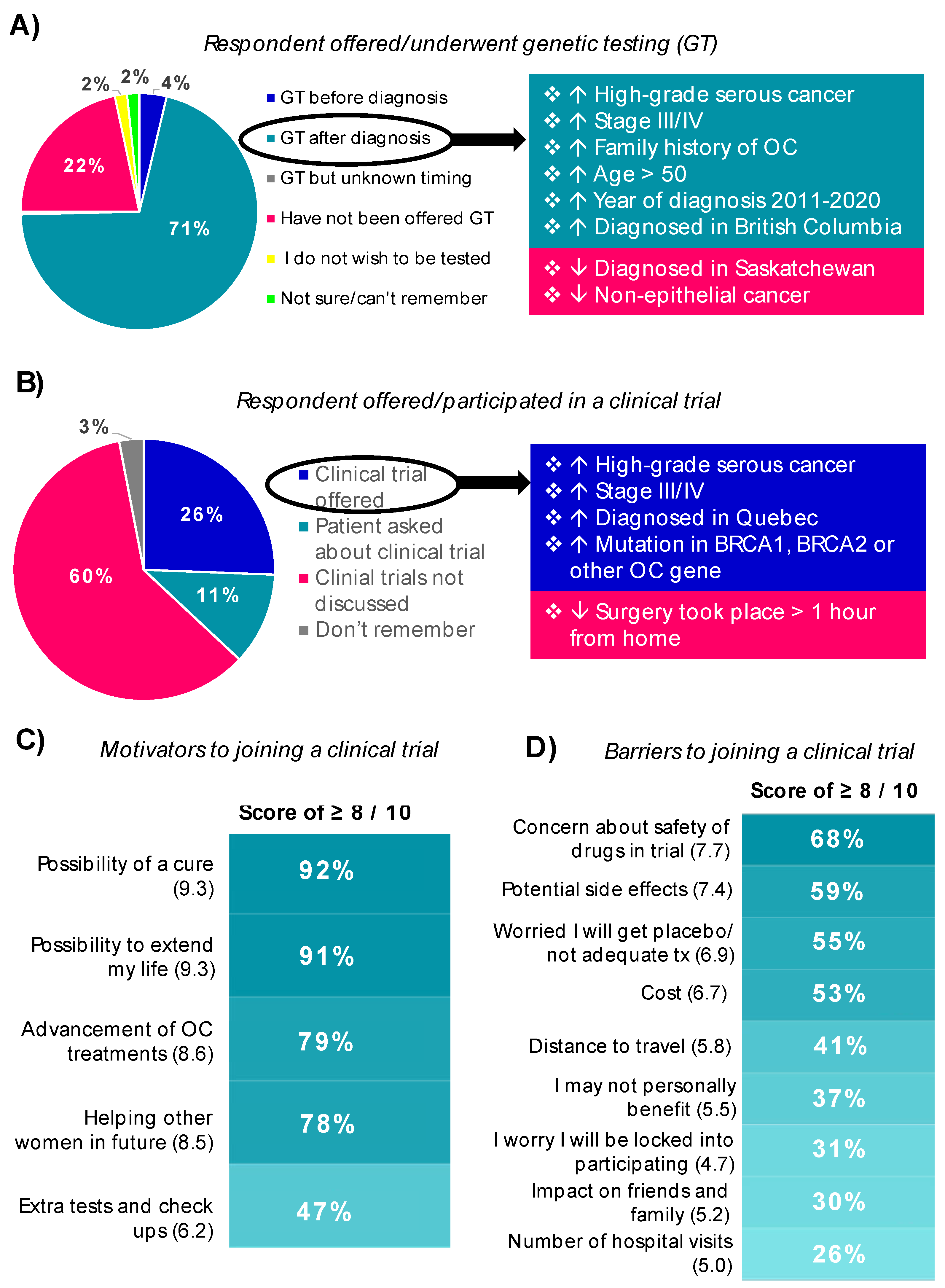
3.4.1. Genetic Testing
3.4.2. Treatments and Clinical Trials
3.4.3. Post-Treatment Follow-Up Care
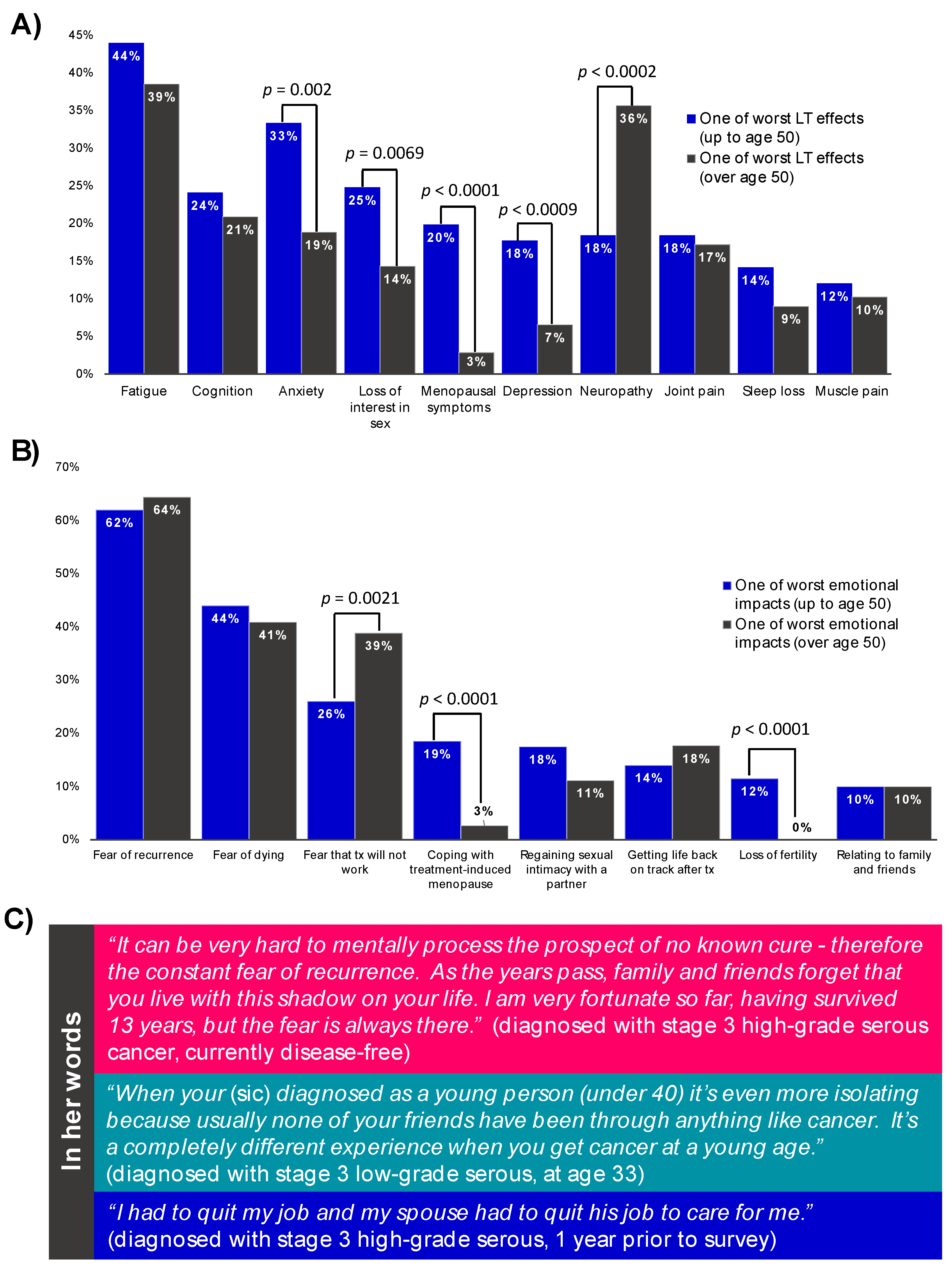
3.5. The Many Impacts of Ovarian Cancer
4. Discussion
5. Conclusions
- (1)
- The vast majority of women with OC—irrespective of age, stage or type—reported symptoms before diagnosis, yet many felt that their concerns were not taken seriously by their healthcare provider and there were wide variations in care before cancer was confirmed. Regional differences in access to care are noted and must be addressed.
- (2)
- A family history of OC or disease/symptom awareness did not impact whether a woman consulted a healthcare provider about her symptoms, or the health-seeking interval. This demonstrates the futility of relying solely on an individuals’ disease knowledge and agency to act upon symptoms appropriately. Primary care physicians also need to be armed with tools that help them recognize and respond to OC symptoms and risk factors in their patients, to ensure these women do not fall through the cracks.
- (3)
- The contribution of hereditary causes to OC is significant, and genetic testing is essential for prevention in at-risk relatives and directed treatment for patients themselves. Despite improvements in genetic testing access for OC patients in recent years, gaps still exist (even in those with a family history of OC) and need to be resolved by systemic processes and health policies.
- (4)
- Women with OC in Canada have a low rate of access to and enrolment in research; participation in OC clinical trials in Canada must be increased to bring new treatment options to patients who may benefit.
- (5)
- Despite the long-lasting physical and emotional burdens of OC, support and survivorship from women after completion of treatment is an underserved area. Programs to address ongoing survivorship should be prioritized, including standardization of follow-up care when possible and strategies to manage patients’ fear of recurrence.
- (6)
- Disparities in OC care across Canada are a concern, and the homogeneity of our study sample is an important indicator that Ovarian Cancer Canada must do more to reach the many different people affected by OC. Therefore, a key strategic priority is to understand, engage, support and represent the diversity of people affected by OC. In doing so, we will access, synthesize and share research that examines the OC experiences of underrepresented groups, recognize and represent the heterogeneity of the OC community and develop resources that reflect the diversity of people we serve. These efforts are essential as we continue to enrich our understanding of OC patient experiences and fulfill our mission of improving outcomes for all Canadians with OC.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brenner, D.R.; Weir, H.K.; Demers, A.A.; Ellison, L.F.; Louzado, C.; Shaw, A.; Turner, D.; Woods, R.R.; Smith, L.M. Projected estimates of cancer in Canada in 2020. Can. Med. Assoc. J. 2020, 192, E199–E205. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Köbel, M.; Kang, E.Y. The Evolution of Ovarian Carcinoma Subclassification. Cancers 2022, 14, 416. [Google Scholar] [CrossRef] [PubMed]
- Peres, L.C.; Cushing-Haugen, K.L.; Köbel, M.; Harris, H.R.; Berchuck, A.; Rossing, M.A.; Schildkraut, J.M.; Doherty, J.A. Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage. JNCI J. Natl. Cancer Inst. 2018, 111, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Cheasley, D.; Wakefield, M.J.; Ryland, G.L.; Allan, P.E.; Alsop, K.; Amarasinghe, K.C.; Ananda, S.; Anglesio, M.S.; Au-Yeung, G.; Böhm, M.; et al. The molecular origin and taxonomy of mucinous ovarian carcinoma. Nat. Commun. 2019, 10, 3935. [Google Scholar] [CrossRef]
- Cochrane, D.R.; Tessier-Cloutier, B.; Lawrence, K.M.; Nazeran, T.; Karnezis, A.N.; Salamanca, C.; Cheng, A.S.; McAlpine, J.N.; Hoang, L.N.; Gilks, C.B.; et al. Clear cell and endometrioid carcinomas: Are their differences attributable to distinct cells of origin? J. Pathol. 2017, 243, 26–36. [Google Scholar] [CrossRef]
- Bowtell, D.D.; Böhm, S.; Ahmed, A.A.; Aspuria, P.-J.; Bast, R.C., Jr.; Beral, V.; Berek, J.S.; Birrer, M.J.; Blagden, S.; Bookman, M.A.; et al. Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer. Nat. Rev. Cancer 2015, 15, 668–679. [Google Scholar] [CrossRef]
- Vaughan, S.; Coward, J.I.; Bast, R.C., Jr.; Berchuck, A.; Berek, J.S.; Brenton, J.D.; Coukos, G.; Crum, C.C.; Drapkin, R.; Etemadmoghadam, D.; et al. Rethinking ovarian cancer: Recommendations for improving outcomes. Nat. Rev. Cancer 2011, 11, 719–725. [Google Scholar] [CrossRef]
- Baugh, E.; Tone, A.; Boghosian, T.; Ross, A.; Crawford, C. Advocacy in Action: Leveraging the Power of Patient Voices to Impact Ovarian Cancer Outcomes in Canada. Curr. Oncol. 2022, 29, 1252–1261. [Google Scholar] [CrossRef]
- Reid, F.; Bhatla, N.; Oza, A.M.; Blank, S.V.; Cohen, R.; Adams, T.; Benites, A.; Gardiner, D.; Gregory, S.; Suzuki, M.; et al. The World Ovarian Cancer Coalition Every Woman Study: Identifying challenges and opportunities to improve survival and quality of life. Int. J. Gynecol. Cancer 2020, 31, 238–244. [Google Scholar] [CrossRef]
- Goff, B.A.; Mandel, L.; Muntz, H.G.; Melancon, C.H. Ovarian carcinoma diagnosis. Cancer 2000, 89, 2068–2075. [Google Scholar] [CrossRef]
- Balasubramaniam, K.; Elnegaard, S.; Rasmussen, S.; Haastrup, P.; Christensen, R.D.; Søndergaard, J.; Jarbøl, D. Lifestyle, socioeconomic status and healthcare seeking among women with gynaecological cancer alarm symptoms: A combined questionnaire-based and register-based population study. BMJ Open 2018, 8, e021815. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.P.; Gelb, C.A.; Trivers, K.F.; Stewart, S.L. Intended care seeking for ovarian cancer symptoms among U.S. women. Prev. Med. Rep. 2016, 3, 234–237. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brain, K.E.; Smits, S.; Simon, A.E.; Forbes, L.J.; Roberts, C.; Robbé, I.J.; Steward, J.; White, C.; Neal, R.D.; Hanson, J. Ovarian cancer symptom awareness and anticipated delayed presentation in a population sample. BMC Cancer 2014, 14, 171. [Google Scholar] [CrossRef]
- Goldstein, C.; Susman, E.; Lockwood, S.; Medlin, E.; Behbakht, K. Awareness of Symptoms and Risk Factors of Ovarian Cancer in a Population of Women and Healthcare Providers. Clin. J. Oncol. Nurs. 2015, 19, 206–212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McCutchan, G.; Wood, F.; Smits, S.; Edwards, A.; Brain, K. Barriers to cancer symptom presentation among people from low socioeconomic groups: A qualitative study. BMC Public Health 2016, 16, 1052. [Google Scholar] [CrossRef] [PubMed]
- Petrova, D.; Okan, Y.; Salamanca-Fernandez, E.; Domínguez-López, S.; Sánchez, M.-J.; Rodríguez-Barranco, M. Psychological factors related to time to help-seeking for cancer symptoms: A meta-analysis across cancer sites. Health Psychol. Rev. 2019, 14, 245–268. [Google Scholar] [CrossRef]
- McCutchan, G.M.; Wood, F.; Edwards, A.; Richards, R.; Brain, K.E. Influences of cancer symptom knowledge, beliefs and barriers on cancer symptom presentation in relation to socioeconomic deprivation: A systematic review. BMC Cancer 2015, 15, 1000. [Google Scholar] [CrossRef]
- Low, E.L.; Waller, J.; Menon, U.; Jones, A.; Reid, F.; Simon, A.E. Ovarian cancer symptom awareness and anticipated time to help-seeking for symptoms among UK women. J. Fam. Plan. Reprod. Health Care 2013, 39, 163–171. [Google Scholar] [CrossRef]
- Boban, S.; Downs, J.; Codde, J.; Cohen, P.A.; Bulsara, C. Women Diagnosed with Ovarian Cancer: Patient and Carer Experiences and Perspectives. Patient Relat. Outcome Meas. 2021, ume 12, 33–43. [Google Scholar] [CrossRef]
- Primary Health Care Providers, 2019. 2020. Available online: https://www150.statcan.gc.ca/n1/pub/82-625-x/2020001/article/00004-eng.htm (accessed on 17 March 2022).
- Altman, A.D.; Lambert, P.; Love, A.J.; Turner, D.; Lotocki, R.; Dean, E.; Popowich, S.; Nachtigal, M.W. Examining the Effects of Time to Diagnosis, Income, Symptoms, and Incidental Detection on Overall Survival in Epithelial Ovarian Cancer. Int. J. Gynecol. Cancer 2017, 27, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Præstegaard, C.; Kjaer, S.K.; Nielsen, T.S.; Jensen, S.M.; Webb, P.M.; Nagle, C.M.; Høgdall, E.; Risch, H.A.; Rossing, M.A.; Doherty, J.A.; et al. The association between socioeconomic status and tumour stage at diagnosis of ovarian cancer: A pooled analysis of 18 case-control studies. Cancer Epidemiol. 2016, 41, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Ziebland, S.; McPherson, A. Minimizing delays in ovarian cancer diagnosis: An expansion of Andersen’s model of ‘total patient delay’. Fam. Pract. 2006, 24, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.M.; Christensen, K.B.; Ottesen, B.; Krasnik, A. Diagnostic delay, quality of life and patient satisfaction among women diagnosed with endometrial or ovarian cancer: A nationwide Danish study. Qual. Life Res. 2011, 21, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- GOC. No Woman Left Behind: The Society of Gynecologic Oncology of Canada (GOC) Statement Regarding BRCA Testing. 2016. Available online: https://gyneoncology.ca (accessed on 17 March 2022).
- Jacobson, M.; Bernardini, M.; Sobel, M.L.; Kim, R.H.; McCuaig, J.; Allen, L. No. 366-Gynaecologic Management of Hereditary Breast and Ovarian Cancer. J. Obstet. Gynaecol. Can. 2018, 40, 1497–1510. [Google Scholar] [CrossRef]
- Kwon, J.S.; Tinker, A.V.; Hanley, G.; Pansegrau, G.; Sun, S.; Carey, M.S.; Schrader, I. BRCA mutation testing for first-degree relatives of women with high-grade serous ovarian cancer. Gynecol. Oncol. 2019, 152, 459–464. [Google Scholar] [CrossRef]
- Domchek, S.M. Association of Risk-Reducing Surgery in BRCA1 or BRCA2 Mutation Carriers with Cancer Risk and Mortality. JAMA 2010, 304, 967–975. [Google Scholar] [CrossRef]
- Finch, A.P.; Lubinski, J.; Møller, P.; Singer, C.F.; Karlan, B.; Senter, L.; Rosen, B.; Maehle, L.; Ghadirian, P.; Cybulski, C.; et al. Impact of Oophorectomy on Cancer Incidence and Mortality in Women With a BRCA1 or BRCA2 Mutation. J. Clin. Oncol. 2014, 32, 1547–1553. [Google Scholar] [CrossRef]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.L.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014, 15, 852–861. [Google Scholar] [CrossRef]
- Armel, S.R.; Volenik, A.; Demsky, R.; Malcolmson, J.; Maganti, M.; McCuaig, J. Setting a baseline: A 7-year review of referral rates and outcomes for serous ovarian cancer prior to implementation of oncologist mediated genetic testing. Gynecol. Oncol. 2020, 158, 440–445. [Google Scholar] [CrossRef]
- Powell, C.B.; Laurent, C.; Garcia, C.; Hoodfar, E.; Karlea, A.; Kobelka, C.; Lee, J.; Roh, J.; Kushi, L.H. Factors influencing genetic counseling and testing for hereditary breast and ovarian cancer syndrome in a large US health care system. Clin. Genet. 2021, 101, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Muessig, K.R.; Zepp, J.M.; Keast, E.; Shuster, E.E.; Reyes, A.A.; Arnold, B.; Ingphakorn, C.; Gilmore, M.J.; Kauffman, T.L.; Hunter, J.E.; et al. Retrospective assessment of barriers and access to genetic services for hereditary cancer syndromes in an integrated health care delivery system. Hered. Cancer Clin. Pract. 2022, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- McBride, C.M.; Pathak, S.; Ms, C.E.J.; Alberg, A.J.; Bandera, E.V.; Barnholtz-Sloan, J.S.; Bondy, M.L.; Cote, M.L.; Moorman, P.G.; Peres, L.C.; et al. Psychosocial factors associated with genetic testing status among African American women with ovarian cancer: Results from the African American Cancer Epidemiology Study. Cancer 2021, 128, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Chapman-Davis, E.; Ni Zhou, Z.; Fields, J.C.; Frey, M.K.; Jordan, B.; Sapra, K.J.; Chatterjee-Paer, S.; Carlson, A.D.; Holcomb, K.M. Racial and Ethnic Disparities in Genetic Testing at a Hereditary Breast and Ovarian Cancer Center. J. Gen. Intern. Med. 2020, 36, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Hinchcliff, E.M.; Bednar, E.; Lu, K.H.; Rauh-Hain, J.A. Disparities in gynecologic cancer genetics evaluation. Gynecol. Oncol. 2019, 153, 184–191. [Google Scholar] [CrossRef]
- Hildebrand, J.S.; Wallace, K.; Graybill, W.S.; Kelemen, L.E. Racial disparities in treatment and survival from ovarian cancer. Cancer Epidemiol. 2018, 58, 77–82. [Google Scholar] [CrossRef]
- Weeks, K.; Lynch, C.F.; West, M.; Carnahan, R.; O’Rorke, M.; Oleson, J.; McDonald, M.; Stewart, S.L.; Charlton, M. Rural disparities in surgical care from gynecologic oncologists among Midwestern ovarian cancer patients. Gynecol. Oncol. 2020, 160, 477–484. [Google Scholar] [CrossRef]
- Lutgendorf, S.K.; Ramirez, E.; Schrepf, A.; Valentine, M.C.; Charlton, M.; Zimmerman, M.B.; Goodheart, M.J.; Zia, S.; Sood, A.K.; Thaker, P.H. Rural residence is related to shorter survival in epithelial ovarian cancer patients. Gynecol. Oncol. 2021, 163, 22–28. [Google Scholar] [CrossRef]
- Armstrong, K. Racial Differences in the Use of BRCA1/2 Testing Among Women with a Family History of Breast or Ovarian Cancer. JAMA 2005, 293, 1729–1736. [Google Scholar] [CrossRef]
- Cragun, D.; Weidner, A.; Lewis, C.; Bonner, D.; Kim, J.; Vadaparampil, S.T.; Pal, T. Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer 2017, 123, 2497–2505. [Google Scholar] [CrossRef]
- Randall, T.C.; Armstrong, K. Health Care Disparities in Hereditary Ovarian Cancer: Are We Reaching the Underserved Population? Curr. Treat. Options Oncol. 2016, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, C.; Saad, N.; Köbel, M.; Mattatall, F.; Nelson, G.; Glaze, S. Implications for management of ovarian cancer in a transgender man: Impact of androgens and androgen receptor status. Gynecol. Oncol. 2021, 161, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Joint, R.; Chen, Z.E.; Cameron, S. Breast and reproductive cancers in the transgender population: A systematic review. BJOG: Int. J. Obstet. Gynaecol. 2018, 125, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- News, P.O. University of Saskatchewan to Advance Ovarian Cancer Tumor Bank, Genetic Test with Government Grant. 2021. Available online: https://www.precisiononcologynews.com/cancer/university-saskatchewan-advance-ovarian-cancer-tumor-bank-genetic-test-government-grant#.YnNP6WhByUk (accessed on 24 February 2022).
- Ozga, M.; Aghajanian, C.; Myers-Virtue, S.; McDonnell, G.; Jhanwar, S.; Hichenberg, S.; Sulimanoff, I. A systematic review of ovarian cancer and fear of recurrence. Palliat. Support. Care 2015, 13, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, J.; Black, A.; Drummond, N.; Power, J.; Maheu, C. Fear of cancer recurrence: A study of the experience of survivors of ovarian cancer. Can. Oncol. Nurs. J. 2017, 27, 236–242. [Google Scholar] [CrossRef]
- Domenici, L.; Palaia, I.; Giorgini, M.; Piscitelli, V.P.; Tomao, F.; Marchetti, C.; Di Donato, V.; Perniola, G.; Musella, A.; Monti, M.; et al. Sexual Health and Quality of Life Assessment among Ovarian Cancer Patients during Chemotherapy. Oncology 2016, 91, 205–210. [Google Scholar] [CrossRef]
- Stafford, L.; Russell, H.; Knoetze, E.; Wilson, V.; Little, R. Sexual functioning after ovarian cancer: Are women receiving the information and support they need? Support. Care Cancer 2022, 30, 4583–4586. [Google Scholar] [CrossRef]
- Rizzuto, I.; Oehler, M.; Lalondrelle, S. Sexual and Psychosexual Consequences of Treatment for Gynaecological Cancers. Clin. Oncol. 2021, 33, 602–607. [Google Scholar] [CrossRef]
| Variable | n (%) |
|---|---|
| Province or territory (n = 553) 1 | |
| British Columbia | 83 (15%) |
| Alberta | 70 (13%) |
| Saskatchewan | 33 (6%) |
| Ontario | 221 (40%) |
| Quebec | 100 (18%) |
| Other 2 | 46 (8%) |
| Urban vs. rural (n = 553) 1 | |
| Urban | 447 (86%) |
| Rural | 75 (14%) |
| Age at diagnosis (n = 540) | |
| ≤40 | 75 (14%) |
| 41–50 | 125 (23%) |
| >50 | 340 (63%) |
| Year of diagnosis (n = 545) | |
| ≤2010 | 80 (14%) |
| 2011–2015 | 131 (24%) |
| 2016–2020 | 334 (61%) |
| Time to diagnosis (n = 532) ** | |
| <1 month | 177 (33%) |
| 1–3 months | 160 (30%) |
| 3 months–1 year | 129 (24%) |
| >1 year | 66 (12%) |
| Type of ovarian cancer (n = 542) | |
| High-grade serous | 248 (46%) |
| Endometrioid | 42 (8%) |
| Clear cell | 36 (7%) |
| Low-grade serous | 36 (7%) |
| Mucinous | 11 (2%) |
| Non-epithelial cancer | 43 (8%) |
| Mixed | 6 (1%) |
| Borderline tumor | 24 (4%) |
| Other | 21 (4%) |
| Do not know or do not remember | 75 (14%) |
| Stage at diagnosis (n = 543) 3 | |
| I | 123 (23%) |
| II | 82 (15%) |
| III | 267 (49%) |
| IV | 54 (10%) |
| Unsure | 17 (3%) |
| Disease recurrences (n = 544) | |
| No | 324 (60%) |
| Has recurred at least once | 162 (30%) |
| Disease never went away | 60 (11%) |
| Current status (n = 545) | |
| In active treatment (newly diagnosed or recurrent) | 188 (34%) |
| In remission | 287 (53%) |
| Other | 70 (13%) |
| Self-reported ethnicity (n = 552) | |
| Caucasian only | 410 (74%) |
| French Canadian only | 69 (13%) |
| Multiple | 22 (4%) |
| Others combined 4 | 47 (9%) |
| Indigenous ancestry (n = 556) | |
| No | 550 (99%) |
| Yes, First Nations | 3 (0.5%) |
| Yes, Métis | 3 (0.5%) |
| First language (n = 556) | |
| English | 431 (78%) |
| French | 97 (17%) |
| Other | 28 (5%) |
| Total household income (n = 553) | |
| >CAD 100,000 | 193 (35%) |
| CAD 75–99.9 K | 97 (18%) |
| <CAD 75 K | 167 (30%) |
| Prefer not to say | 96 (17%) |
| Family history of ovarian or breast cancer (n = 527) | |
| No ovarian or breast cancer | 174 (33%) |
| Ovarian cancer only | 32 (6%) |
| Ovarian and breast cancer | 88 (17%) |
| Breast cancer only | 233 (44%) |
| Self-reported genetic testing results (n = 405) 5 | |
| Mutation in BRCA1 or BRCA2 | 77 (19%) |
| Mutation in other gene | 34 (8%) |
| Inconclusive (variant of unknown significance) | 48 (12%) |
| Negative | 226 (56%) |
| Not sure/cannot remember/awaiting results | 20 (5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tone, A.; Boghosian, T.; Ross, A.; Baugh, E.; Altman, A.D.; Dawson, L.; Reid, F.; Crawford, C. Understanding the Experience of Canadian Women Living with Ovarian Cancer through the Every Woman StudyTM. Curr. Oncol. 2022, 29, 3318-3340. https://doi.org/10.3390/curroncol29050271
Tone A, Boghosian T, Ross A, Baugh E, Altman AD, Dawson L, Reid F, Crawford C. Understanding the Experience of Canadian Women Living with Ovarian Cancer through the Every Woman StudyTM. Current Oncology. 2022; 29(5):3318-3340. https://doi.org/10.3390/curroncol29050271
Chicago/Turabian StyleTone, Alicia, Talin Boghosian, Alison Ross, Elisabeth Baugh, Alon D. Altman, Lesa Dawson, Frances Reid, and Cailey Crawford. 2022. "Understanding the Experience of Canadian Women Living with Ovarian Cancer through the Every Woman StudyTM" Current Oncology 29, no. 5: 3318-3340. https://doi.org/10.3390/curroncol29050271
APA StyleTone, A., Boghosian, T., Ross, A., Baugh, E., Altman, A. D., Dawson, L., Reid, F., & Crawford, C. (2022). Understanding the Experience of Canadian Women Living with Ovarian Cancer through the Every Woman StudyTM. Current Oncology, 29(5), 3318-3340. https://doi.org/10.3390/curroncol29050271






