COVID-19 Impact on Diagnosis and Staging of Colorectal Cancer: A Single Tertiary Canadian Oncology Center Experience
Abstract
1. Introduction
2. Materials and Methods
3. Results
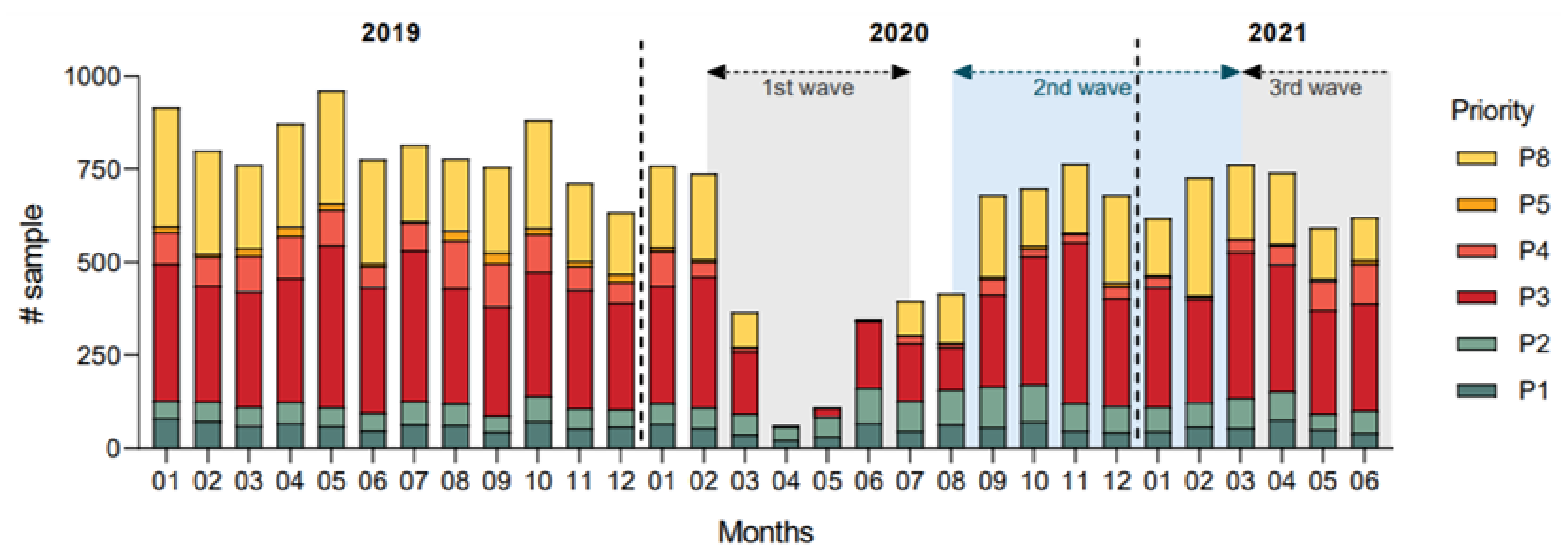
3.1. Colorectal Cancer Diagnosis Rates
3.2. Colonoscopy Priority and Delays
3.3. Surgery and Delays
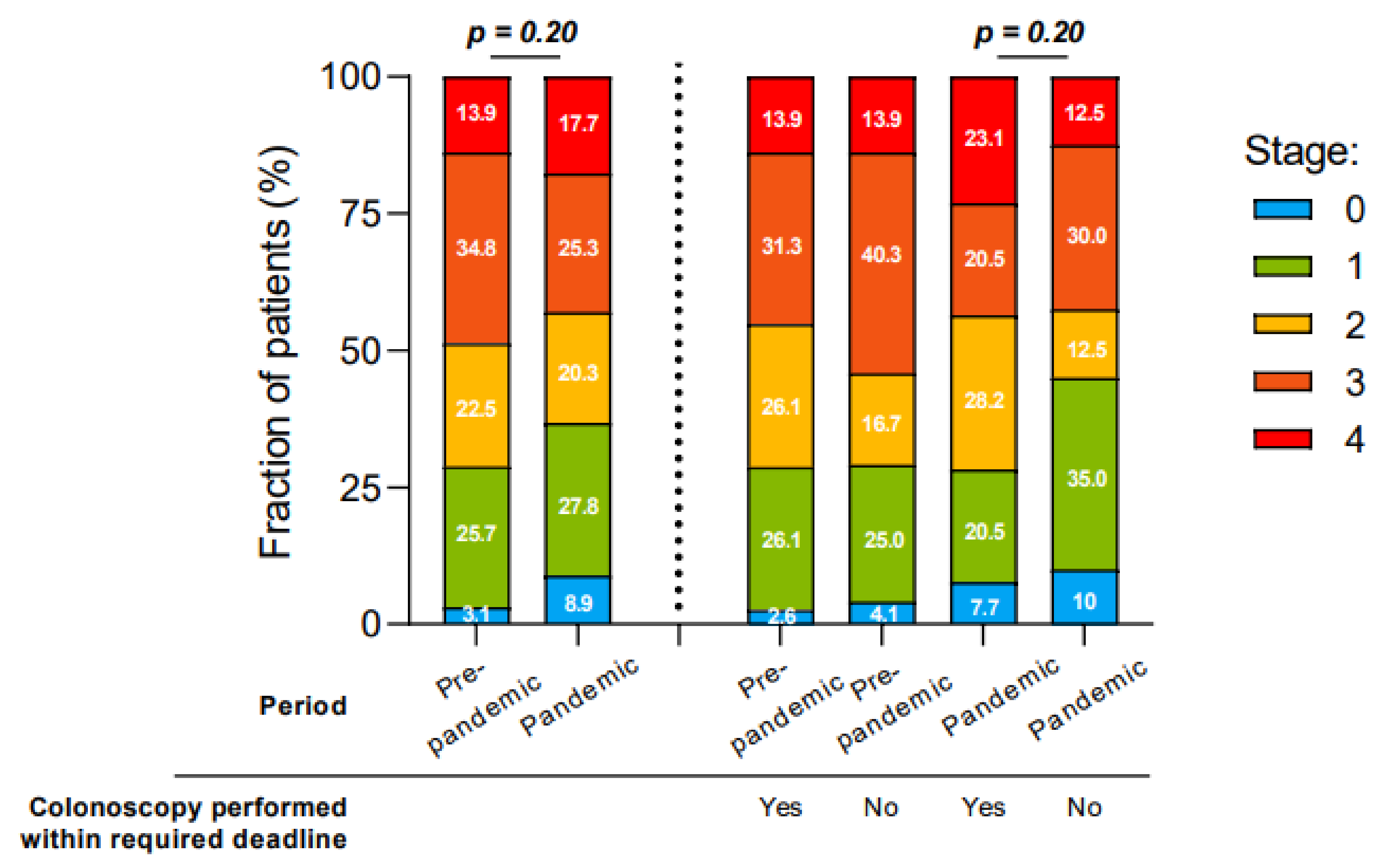
3.4. Colorectal Cancer Staging at Diagnosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- COVID-19 Daily Epidemiology Update (Government of Canada, Ottawa). Available online: https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html (accessed on 5 April 2022).
- Institut National de Santé Publique du Quebec (INSPQ), Données COVID-19 au Québec. Available online: https://www.inspq.qc.ca/covid-19/donnees (accessed on 5 April 2022).
- Ministère de la Santé et des Services Sociaux du Québec. Analyse des Répercussions de la Pandémie de la COVID-19 sur les Soins et les Services en Cancérologie au Québec, Résultat Couvrant les Premiers Mois de Pandémie: Printemps 2020. Available online: https://publications.msss.gouv.qc.ca/msss/fichiers/2020/20-210-378W.pdf (accessed on 1 January 2021).
- Ministère de la Santé et des Services Sociaux du Québec. Analyse des Répercussions de la Pandémie de la COVID-19 sur les Soins et les Services en Cancérologie au Québec, Résultats Couvrant la Première Année de Pandémie: 1 Avril 2020 au 31 Mars 2021. Available online: https://publications.msss.gouv.qc.ca/msss/document-002878 (accessed on 1 September 2021).
- Mayo, M.; Potugari, B.; Bzeih, R.; Scheidel, C.; Carrera, C.; Shellenberger, R.A. Cancer Screening During the COVID-19 Pandemic: A Systematic Review and Meta-analysis. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Priou, S.; Lamé, G.; Chatellier, G.; Tournigand, C.; Kempf, E. Effect of the COVID-19 pandemic on colorectal cancer care in France. Lancet Gastroenterol. Hepatol. 2021, 6, 342–343. [Google Scholar] [CrossRef]
- CRC COVID Research Collaborative. The impact of the COVID-19 pandemic on colorectal cancer service provision. Br. J. Surg. 2020, 107, e521–e522. [Google Scholar] [CrossRef]
- Maringe, C.; Spicer, J.; Morris, M.; Purushotham, A.; Nolte, E.; Sullivan, R.; Rachet, B.; Aggarwal, A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. Lancet Oncol. 2020, 21, 1023–1034, Erratum in: Lancet Oncol. 2021, 22, e5. [Google Scholar] [CrossRef]
- Chen, R.C.; Haynes, K.; Du, S.; Barron, J.; Katz, A.J. Association of Cancer Screening Deficit in the United States With the COVID-19 Pandemic. JAMA Oncol. 2021, 7, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.H.; Mainprize, J.G.; Yaffe, M.J.; Ruan, Y.; Poirier, A.E.; Coldman, A.; Nadeau, C.; Iragorri, N.; Hilsden, R.J.; Brenner, D.R. The impact of episodic screening interruption: COVID-19 and population-based cancer screening in Canada. J. Med Screen. 2021, 28, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Forse, C.L.; Petkiewicz, S.; Teo, I.; Purgina, B.M.; Klaric, K.A.; Ramsay, T.; Wasserman, J.K. Negative Impact of COVID-19 Associated Health System Shutdown on Patients Diagnosed With Colorectal Cancer: A Retrospective Study From a Large Tertiary Center in Ontario, Canada. J. Can. Assoc. Gastroenterol. 2021, gwab044. [Google Scholar] [CrossRef]
- Morris, E.J.A.; Goldacre, R.; Spata, E.; Mafham, M.; Finan, P.J.; Shelton, J.; Richards, M.; Spencer, K.; Emberson, J.; Hollings, S.; et al. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: A population-based study. Lancet Gastroenterol. Hepatol. 2021, 6, 199–208. [Google Scholar] [CrossRef]
- National Cancer Institute–NIH. In NCI Dictionaries. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/relapse (accessed on 26 March 2022).
- Santé et Services Sociaux Québec. Demande de Coloscopie Longue. Available online: http://msssa4.msss.gouv.qc.ca/intra/formres.nsf/a9b0958ceee59c7685256e2a0052d887/96a8089a669d4f4a8525800900661462/$FILE/AH-702DT9241(2017-12)D.pdf (accessed on 5 April 2022).
- Données COVID-19 par Vague Selon L’âge et le Sexe au Québec, Institut National de Santé Publique du Québec (INSPQ). Available online: https://www.inspq.qc.ca/covid-19/donnees/age-sexe (accessed on 5 April 2022).
- Galdas, P.M.; Cheater, F.; Marshall, P. Men and health help-seeking behaviour: Literature review. J. Adv. Nurs. 2005, 49, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.; Camic, P.M.; Brown, J.S. Masculinity, alexithymia, and fear of intimacy as predictors of UK men’s attitudes towards seeking professional psychological help. Br. J. Health Psychol. 2015, 20, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Day, D.W.; Morson, B.C. The adenoma-carcinoma sequence. Major. Probl. Pathol. 1978, 10, 58–71. [Google Scholar] [PubMed]
- Sun, S.; Klebaner, F.; Tian, T. A new model of time scheme for progression of colorectal cancer. BMC Syst. Biol. 2014, 8, S2. [Google Scholar] [CrossRef] [PubMed]
- Hisabe, T.; Hirai, F.; Matsui, T. Development and progression of colorectal cancer based on follow-up analysis. Dig. Endosc. 2014, 26 (Suppl. 2), 73–77. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.; Fu, Y.; Sadowski, D.C.; Stewart, D.; Tang, P.; Kaposhi, B.; Chappell, H.; Robson, P.; van Zanten, S.V. Delayed Colorectal Cancer Diagnosis during the COVID-19 Pandemic in Alberta: A Framework for Analyzing Barriers to Diagnosis and Generating Evidence to Support Health System Changes Aimed at Reducing Time to Diagnosis. Int. J. Environ. Res. Public Health 2021, 18, 9098. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, R.; Mittal, S.; Madan, K.; Mohan, A.; Hadda, V.; Tiwari, P.; Guleria, R. Assessment of the impact and reorganization of interventional pulmonology services at a tertiary care centre during nationwide lockdown for COVID-19 pandemic. Monaldi Arch. Chest Dis. 2021, 91. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Issaka, R.B.; Chen, E.; Somsouk, M. Colorectal Cancer Screening and COVID-19. Am. J. Gastroenterol. 2021, 116, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Feletto, E.; Grogan, P.; Nickson, C.; Smith, M.; Canfell, K. How has COVID-19 impacted cancer screening? Adaptation of services and the future outlook in Australia. Public Health Res. Pract. 2020, 30, 3042026. [Google Scholar] [CrossRef] [PubMed]
- Gorin, S.N.S.; Jimbo, M.; Heizelman, R.; Harmes, K.M.; Harper, D.M. The future of cancer screening after COVID-19 may be at home. Cancer 2021, 127, 498–503, Erratum in: Cancer 2021, 127, 4315. [Google Scholar] [CrossRef] [PubMed]
- Balzora, S.; Issaka, R.B.; Anyane-Yeboa, A.; Gray, D.M., 2nd; May, F.P. Impact of COVID-19 on colorectal cancer disparities and the way forward. Gastrointest. Endosc. 2020, 92, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Tehfe, M.; Charpentier, D.; Vachon, M.F.; Champagne, M. 390MO Colorectal (CRC) cancer screening and diagnosis during the COVID-19 pandemic in Quebec, Canada. Ann. Oncol. 2021, 32 (Suppl. 5), S534. [Google Scholar] [CrossRef]
| Pre-Pandemic Period (N = 254) | Pandemic Period (N = 125) | ||
|---|---|---|---|
| Median age at diagnosis (years) | 69.4 | 67.5 | αp = 0.17 |
| Sex, n (%) | βp = 0.03 | ||
| Male | 148 (58) | 58 (46) | |
| Female | 106 (42) | 67 (54) | |
| Cancer Localization, n (%) | βp < 0.001 | ||
| Colic | 149 (58.7) | 96 (76.8) | |
| Rectal | 105 (41.3) | 29 (23.3) | |
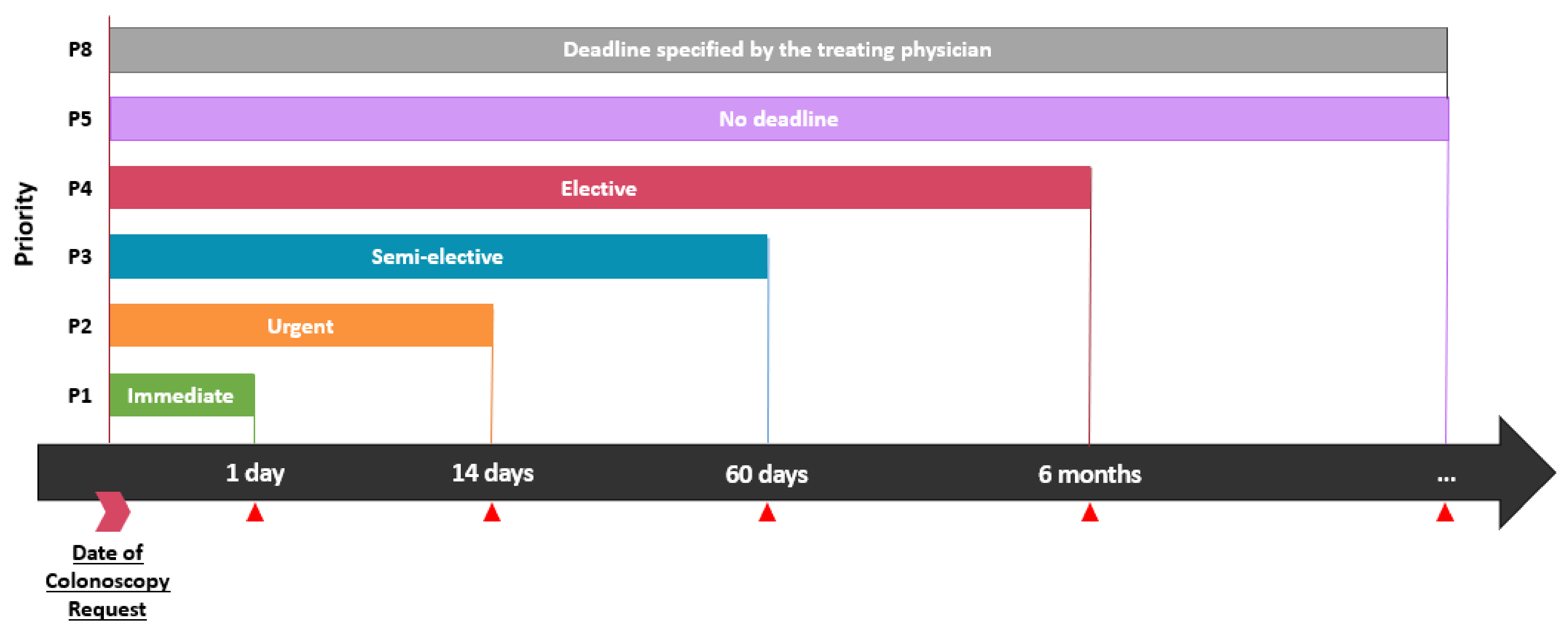
| Elective colonoscopies MSSS priority | βp = 0.07 | ||
| P1 | 11 (4.3) | 1 (0.8) | |
| P2 | 73 (28.7) | 37 (29.6) | |
| P3 | 85 (33.5) | 45 (36.0) | |
| P4 | 9 (3.5) | 0 (0) | |
| P5 | 1 (0.3) | 0 (0) | |
| P8 | 18 (7.1) | 4 (3.2) | |
| Unknown * | 13 (5.1) | 3 (2.4) | |
| Colonoscopy performed during an hospitalization | 44 (17.3) | 35 (28.0) | βp = 0.02 |
| Colonoscopies indicated for a positive FIT * | 39 (15.4) | 20 (16.0) | βp = 0.87 |
| Pre-Pandemic Period (N = 254) | Pandemic Period (N = 125) | ||
|---|---|---|---|
| Diagnosis, per month | 9.8 | 7.8 | αp = 0.048 |
| Diagnosis during hospitalization, n (%) | 43 (25.9) | 35 (37.9) | βp = 0.048 |
| Diagnosis during hospitalization, per month | 1.7 | 2.2 | αp = 0.27 |
| Elective colonoscopies exceeding deadline, n (%) * | 74 (38.3) | 42 (51.7) | βp = 0.049 |
| Delays of elective colonoscopies, days | |||
| P2 | 20.9 | 25.2 | αp = 0.39 |
| P3 | 58.9 | 106.5 | αp < 0.001 |
| Surgeries, per month | 3.5 | 2.9 | αp = 0.39 |
| Delays to surgery, days | 60.4 | 58.6 | αp = 0.77 |
| Stage at diagnosis, n (%) | βp = 0.17 | ||
| 0 | 11 (4.4) | 7 (5.5) | |
| I | 60 (23.6) | 26 (21.0) | |
| II | 56 (22.0) | 24 (19.4) | |
| III | 75 (29.5) | 29 (23.4) | |
| IV | 41 (16.1) | 25 (20.2) | |
| Unknown | 11 (4.4) | 13 (10.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castonguay, M.; El Sayed, R.; Richard, C.; Vachon, M.-F.; Nassabein, R.; Charpentier, D.; Tehfé, M. COVID-19 Impact on Diagnosis and Staging of Colorectal Cancer: A Single Tertiary Canadian Oncology Center Experience. Curr. Oncol. 2022, 29, 3282-3290. https://doi.org/10.3390/curroncol29050268
Castonguay M, El Sayed R, Richard C, Vachon M-F, Nassabein R, Charpentier D, Tehfé M. COVID-19 Impact on Diagnosis and Staging of Colorectal Cancer: A Single Tertiary Canadian Oncology Center Experience. Current Oncology. 2022; 29(5):3282-3290. https://doi.org/10.3390/curroncol29050268
Chicago/Turabian StyleCastonguay, Mathias, Rola El Sayed, Corentin Richard, Marie-France Vachon, Rami Nassabein, Danielle Charpentier, and Mustapha Tehfé. 2022. "COVID-19 Impact on Diagnosis and Staging of Colorectal Cancer: A Single Tertiary Canadian Oncology Center Experience" Current Oncology 29, no. 5: 3282-3290. https://doi.org/10.3390/curroncol29050268
APA StyleCastonguay, M., El Sayed, R., Richard, C., Vachon, M.-F., Nassabein, R., Charpentier, D., & Tehfé, M. (2022). COVID-19 Impact on Diagnosis and Staging of Colorectal Cancer: A Single Tertiary Canadian Oncology Center Experience. Current Oncology, 29(5), 3282-3290. https://doi.org/10.3390/curroncol29050268






