Invasive Fungal Disease in Patients with Chronic Lymphocytic Leukemia in Japan: A Retrospective Database Study
Abstract
1. Introduction
2. Materials and Methods
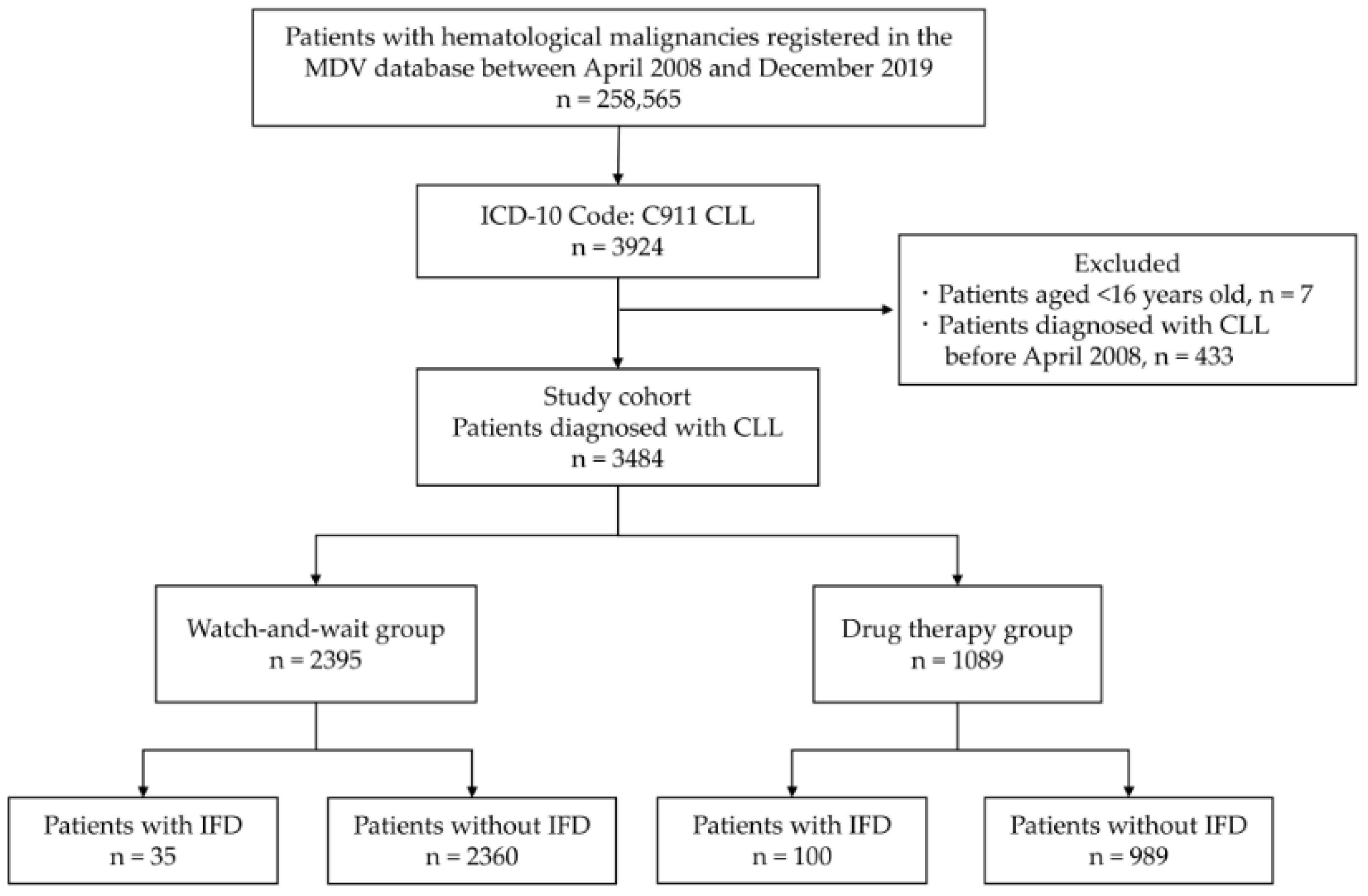
2.1. Study Design and Patients
2.2. Data Collection and Variable Definition
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
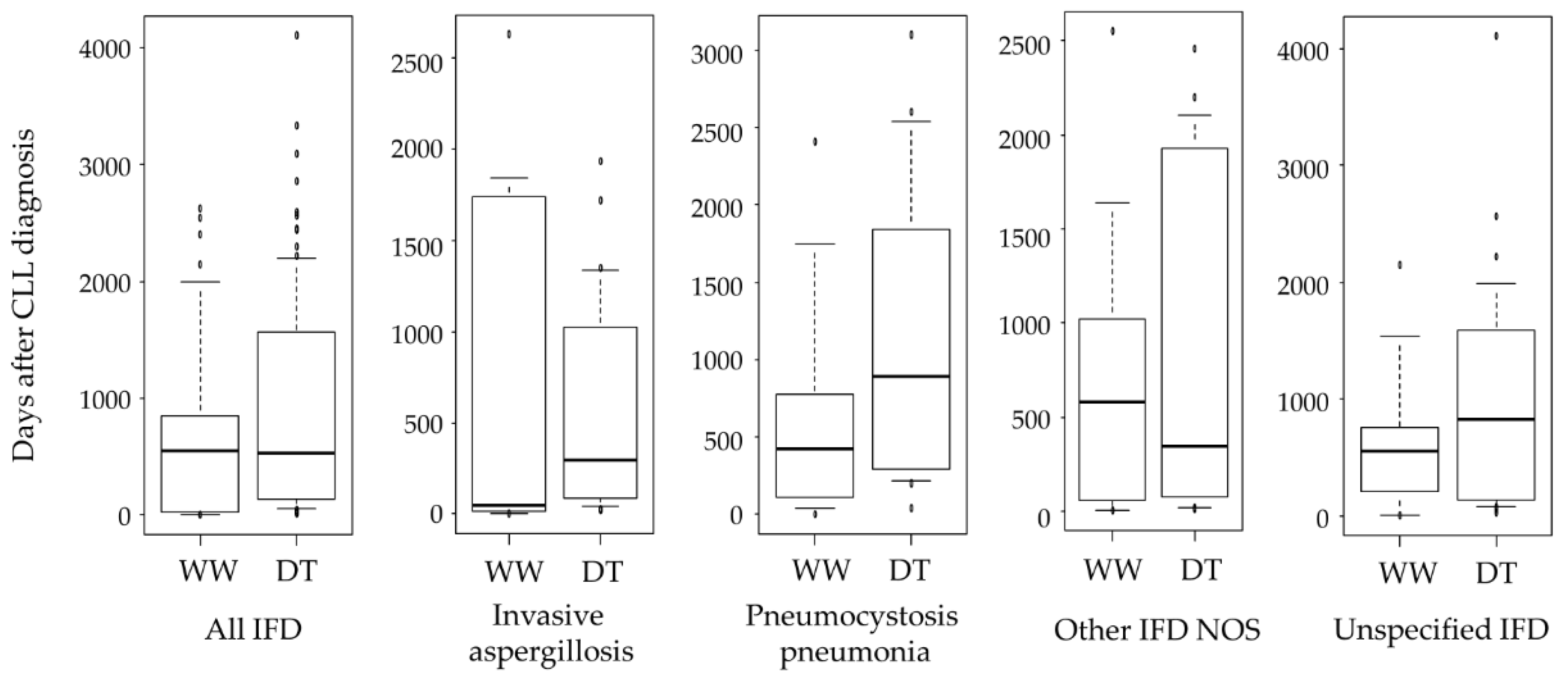
3.2. Incidence and Risk Factors of IFD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slavin, M.; van Hal, S.; Sorrell, T.C.; Lee, A.; Marriott, D.J.; Daveson, K.; Kennedy, K.; Hajkowicz, K.; Halliday, C.; Athan, E.; et al. Invasive infections due to filamentous fungi other than Aspergillus: Epidemiology and determinants of mortality. Clin. Microbiol. Infect. 2015, 21, 490.e1–490.e10. [Google Scholar] [CrossRef]
- Nivoix, Y.; Velten, M.; Letscher-Bru, V.; Moghaddam, A.; Natarajan-Ame, S.; Fohrer, C.; Lioure, B.; Bilger, K.; Lutun, P.; Marcellin, L.; et al. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin. Infect. Dis. 2008, 47, 1176–1184. [Google Scholar] [CrossRef]
- Valentine, J.C.; Morrissey, C.O.; Tacey, M.A.; Liew, D.; Patil, S.; Peleg, A.Y.; Ananda-Rajah, M.R. A population-based analysis of invasive fungal disease in haematology- oncology patients using data linkage of state-wide registries and administrative databases: 2005–2016. BMC Infect. Dis. 2019, 19, 274. [Google Scholar] [CrossRef]
- Kume, H.; Yamazaki, T.; Togano, T.; Abe, M.; Tanuma, H.; Kawana, S.; Okudaira, M. Epidemiology of visceral mycoses in autopsy cases in Japan: Comparison of the data from 1989, 1993, 1997, 2001, 2005 and 2007 in Annual of Pathological Autopsy Cases in Japan. Med. Mycol. J. 2011, 52, 117–127. [Google Scholar] [CrossRef]
- Kurosawa, M.; Yonezumi, M.; Hashino, S.; Tanaka, J.; Nishio, M.; Kaneda, M.; Ota, S.; Koda, K.; Suzuki, N.; Yoshida, M.; et al. Epidemiology and treatment outcome of invasive fungal infections in patients with hematological malignancies. Int. J. Hematol. 2012, 96, 748–757. [Google Scholar] [CrossRef]
- Hsu, L.Y.; Lee, D.G.; Yeh, S.P.; Bhurani, D.; Khanh, B.Q.; Low, C.Y.; Norasetthada, L.; Chan, T.; Kwong, Y.L.; Vaid, A.K.; et al. Epidemiology of invasive fungal diseases among patients with haematological disorders in the Asia-Pacific: A prospective observational study. Clin. Microbiol. Infect. 2015, 21, 594.e7–594.e11. [Google Scholar] [CrossRef][Green Version]
- Chihara, D.; Ito, H.; Matsuda, T.; Shibata, A.; Katsumi, A.; Nakamura, S.; Tomotaka, S.; Morton, L.M.; Weisenburger, D.D.; Matsuo, K. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br. J. Haematol. 2014, 164, 536–545. [Google Scholar] [CrossRef]
- Muto, R.; Miyoshi, H.; Sato, K.; Furuta, T.; Muta, H.; Kawamoto, K.; Yanagida, E.; Yamada, K.; Ohshima, K. Epidemiology and secular trends of malignant lymphoma in Japan: Analysis of 9426 cases according to the World Health Organization classification. Cancer Med. 2018, 7, 5843–5858. [Google Scholar] [CrossRef]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Bairey, O.; Hillmen, P.; Bartlett, N.L.; Li, J.; et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [Google Scholar] [CrossRef] [PubMed]
- Ghez, D.; Calleja, A.; Protin, C.; Baron, M.; Ledoux, M.P.; Damaj, G.; Dupont, M.; Dreyfus, B.; Ferrant, E.; Herbaux, C.; et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood 2018, 131, 1955–1959. [Google Scholar] [CrossRef]
- Ruchlemer, R.; Ben-Ami, R.; Bar-Meir, M.; Brown, J.R.; Malphettes, M.; Mous, R.; Tonino, S.H.; Soussain, C.; Barzic, N.; Messina, J.A.; et al. Ibrutinib-associated invasive fungal diseases in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: An observational study. Mycoses 2019, 62, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Holowka, T.; Cheung, H.; Malinis, M.; Gan, G.; Deng, Y.; Perreault, S.; Isufi, I.; Azar, M.M. Incidence and associated risk factors for invasive fungal infections and other serious infections in patients on ibrutinib. J. Infect. Chemother. 2021, 27, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Takazono, T.; Tashiro, M.; Ota, Y.; Obata, Y.; Wakamura, T.; Miyazaki, T.; Nishino, T.; Izumikawa, K. Factor analysis of acute kidney injury in patients administered liposomal amphotericin B in a real-world clinical setting in Japan. Sci. Rep. 2020, 14, 15033. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Thomas, C.F., Jr.; Limper, A.H. Pneumocystis pneumonia. N. Engl. J. Med. 2004, 350, 2487–2498. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Tong, X.; Liu, T.; Jiang, K.; Wang, D.; Liu, S.; Wang, Y.; Fan, H. Clinical characteristics and prognostic risk factors of patients with proven invasive pulmonary aspergillosis: A single-institution retrospective study. Front. Med. 2021, 8, 756237. [Google Scholar] [CrossRef] [PubMed]
- Mahlich, J.; Okamoto, S.; Tsubota, A. Cost of illness of Japanese patients with chronic lymphocytic leukemia (CLL) and budget impact of the market introduction of ibrutinib. Pharmacoecon Open 2017, 1, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Vallabhaneni, S.; Benedict, K.; Derado, G.; Mody, R.K. Trends in hospitalizations related to invasive aspergillosis and mucormycosis in the United States, 2000–2013. Open Forum. Infect. Dis. 2017, 4, ofw268. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, M.D.; Nathanson, B.H.; Harrington, R.; Spalding, J.R.; Shorr, A.F. Epidemiology and outcomes of hospitalizations with invasive aspergillosis in the United States, 2009–2013. Clin. Infect. Dis. 2018, 67, 727–735. [Google Scholar] [CrossRef]
- Kullberg, B.J.; Arendrup, M.C. Invasive candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar]
- Eichhorst, B.; Fink, A.M.; Bahlo, J.; Busch, R.; Kovacs, G.; Maurer, C.; Lange, E.; Köppler, H.; Kiehl, M.; Sökler, M.; et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): An international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016, 17, 928–942. [Google Scholar] [CrossRef]
- Elter, T.; Vehreschild, J.J.; Gribben, J.; Cornely, O.A.; Engert, A.; Hallek, M. Management of infections in patients with chronic lymphocytic leukemia treated with alemtuzumab. Ann. Hematol. 2009, 88, 121–132. [Google Scholar] [CrossRef]
- Lundin, J.; Porwit-MacDonald, A.; Rossmann, E.D.; Karlsson, C.; Edman, P.; Rezvany, M.R.; Kimby, E.; Osterborg, A.; Mellstedt, H. Cellular immune reconstitution after subcutaneous alemtuzumab (anti-CD52 monoclonal antibody, CAMPATH-1H) treatment as first-line therapy for B-cell chronic lymphocytic leukaemia. Leukemia 2004, 18, 484–490. [Google Scholar] [CrossRef]
- Baden, L.R.; Swaminathan, S.; Angarone, M.; Blouin, G.; Camins, B.C.; Casper, C.; Cooper, B.; Dubberke, E.R.; Engemann, A.M.; Freifeld, A.G.; et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2016, 14, 882–913. [Google Scholar] [CrossRef]
- Ryan, C.E.; Cheng, M.P.; Issa, N.C.; Brown, J.R.; Davids, M.S. Pneumocystis jirovecii pneumonia and institutional prophylaxis practices in CLL patients treated with BTK inhibitors. Blood Adv. 2020, 4, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Stefania Infante, M.; Fernández-Cruz, A.; Núñez, L.; Carpio, C.; Jiménez-Ubieto, A.; López-Jiménez, J.; Vásquez, L.; Del Campo, R.; Romero, S.; Alonso, C.; et al. Severe infections in patients with lymphoproliferative diseases treated with new targeted drugs: A multicentric real-world study. Cancer Med. 2021, 10, 7629–7640. [Google Scholar] [CrossRef] [PubMed]
- Tillman, B.F.; Pauff, J.M.; Satyanarayana, G.; Talbott, M.; Warner, J.L. Systematic review of infectious events with the Bruton tyrosine kinase inhibitor ibrutinib in the treatment of hematologic malignancies. Eur. J. Haematol. 2018, 100, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Varughese, T.; Taur, Y.; Cohen, N.; Palomba, M.L.; Seo, S.K.; Hohl, T.M.; Redelman-Sidi, G. Serious Infections in Patients Receiving Ibrutinib for Treatment of Lymphoid Cancer. Clin. Infect. Dis. 2018, 67, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Hillmen, P.; Ghia, P.; Kater, A.P.; Chanan-Khan, A.; Furman, R.R.; O’Brien, S.; Yenerel, M.N.; Illés, A.; Kay, N.; et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: Results of the first randomized phase III trial. J. Clin. Oncol. 2021, 39, 3441–3452. [Google Scholar] [CrossRef]
- Xu, W.; Yang, S.; Zhou, K.; Pan, L.; Li, Z.; Zhou, J.; Gao, S.; Zhou, D.; Hu, J.; Feng, R.; et al. Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: Phase 2, single-arm, multicenter study. J. Hematol. Oncol. 2020, 13, 48. [Google Scholar] [CrossRef]
- Eichhorst, B.; Robak, T.; Montserrat, E.; Ghia, P.; Hillmen, P.; Hallek, M.; Buske, C.; ESMO Guidelines Committee. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v78–v84. [Google Scholar] [CrossRef]

| Watch-and-Wait Group n = 2395 | Drug Therapy Group n = 1089 | p-Value | |
|---|---|---|---|
| Age (years), median (IQR) | 72 (64–81) | 72 (64–79) | 0.036 |
| ≥75 years, n (%) | 1056 (44.1) | 445 (40.9) | 0.077 |
| Male sex, n (%) | 1341 (56.0) | 693 (63.6) | <0.001 |
| CCI score, median (IQR) | 2 (2–3) | 2 (2–3) | 0.062 |
| CCI score ≥ 5, n (%) | 104 (4.3) | 70 (6.4) | 0.012 |
| Comorbidities | |||
| Diabetes mellitus, n (%) | 728 (30.4) | 347 (31.9) | 0.384 |
| Medication | |||
| Steroid | 289 (12.1) | 349 (32.0) | <0.001 |
| Anticancer drugs for the treatment of CLL | |||
| Cyclophosphamide, n (%) | - | 512 (47.0) | - |
| Rituximab, n (%) | - | 490 (45.0) | - |
| Fludarabine, n (%) | - | 428 (39.3) | - |
| Ibrutinib, n (%) | - | 231 (21.2) | - |
| Bendamustine, n (%) | - | 187 (17.2) | - |
| Ofatumumab, n (%) | - | 43 (3.9) | - |
| Alemtuzumab, n (%) | - | 13 (1.2) | - |
| Overall Group n = 135 | Watch-and-Wait Group n = 35 | Drug Therapy Group n = 100 | p-Value | |
|---|---|---|---|---|
| Invasive aspergillosis, n (%) | 38 (28.1) | 10 (28.6) | 28 (28.0) | 1 |
| Invasive candidiasis, n (%) | 9 (6.7) | 0 | 9 (9.0) | 0.111 |
| Cryptococcosis, n (%) | 2 (1.5) | 1 (2.9) | 1 (1.0) | 0.453 |
| Pneumocystis pneumonia, n (%) | 20 (14.8) | 5 (14.3) | 15 (15.0) | 1 |
| Other IFD NOS, n (%) | 29 (21.5) | 8 (22.9) | 21 (21.0) | 0.814 |
| Unspecified IFD, n (%) | 37 (27.4) | 11 (31.4) | 26 (26.0) | 0.519 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Age ≥ 75 years | 0.928 (0.65–1.32) | 0.677 | 1.03 (0.73–1.47) | 0.855 |
| Male sex | 1.63 (1.13–2.34) | 0.009 | 1.43 (0.99–2.06) | 0.057 |
| CCI score ≥ 5 | 1.60 (0.81–3.15) | 0.175 | 1.24 (0.63–2.45) | 0.537 |
| Diabetes mellitus | 1.25 (0.87–1.79) | 0.232 | 1.20 (0.83–1.72) | 0.334 |
| Steroid | 3.10 (2.20–4.36) | <0.001 | 2.13 (1.50–3.03) | <0.001 |
| Drug therapy | 5.15 (3.50–7.57) | <0.001 | 4.19 (2.82–6.23) | <0.001 |
| Treatment Drugs | Incidence (%) | ||||||
|---|---|---|---|---|---|---|---|
| All IFDs | IA | IC | Cryptococcosis | PCP | Other IFD NOS | Unspecified IFD | |
| Cyclophosphamide | 10.2 | 3.3 | 0.6 | 0 | 0.8 | 2.7 | 2.7 |
| Rituximab | 9.0 | 2.9 | 0.6 | 0 | 1.2 | 1.6 | 2.7 |
| Fludarabine | 12.4 | 3.0 | 1.6 | 0.2 | 1.9 | 3.0 | 2.6 |
| Ibrutinib | 8.2 | 2.2 | 0.9 | 0 | 1.7 | 1.3 | 2.2 |
| Bendamustine | 12.8 | 4.3 | 1.1 | 0 | 1.6 | 2.1 | 3.7 |
| Ofatumumab | 14.0 | 2.3 | 2.3 | 0 | 2.3 | 4.7 | 2.3 |
| Alemtuzumab | 38.5 | 30.8 | 0 | 0 | 0 | 0 | 7.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasu, T.; Sakurai, K.; Akazawa, M. Invasive Fungal Disease in Patients with Chronic Lymphocytic Leukemia in Japan: A Retrospective Database Study. Curr. Oncol. 2022, 29, 3242-3251. https://doi.org/10.3390/curroncol29050264
Yasu T, Sakurai K, Akazawa M. Invasive Fungal Disease in Patients with Chronic Lymphocytic Leukemia in Japan: A Retrospective Database Study. Current Oncology. 2022; 29(5):3242-3251. https://doi.org/10.3390/curroncol29050264
Chicago/Turabian StyleYasu, Takeo, Kotono Sakurai, and Manabu Akazawa. 2022. "Invasive Fungal Disease in Patients with Chronic Lymphocytic Leukemia in Japan: A Retrospective Database Study" Current Oncology 29, no. 5: 3242-3251. https://doi.org/10.3390/curroncol29050264
APA StyleYasu, T., Sakurai, K., & Akazawa, M. (2022). Invasive Fungal Disease in Patients with Chronic Lymphocytic Leukemia in Japan: A Retrospective Database Study. Current Oncology, 29(5), 3242-3251. https://doi.org/10.3390/curroncol29050264





