Real-World Management and Outcomes of Crizotinib-Treated ROS1-Rearranged NSCLC: A Retrospective Canadian Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Clinical Response and Outcome
2.3. Adverse Event Definitions and Capture
2.4. Statistical Methods
3. Results
3.1. Patient Characteristics
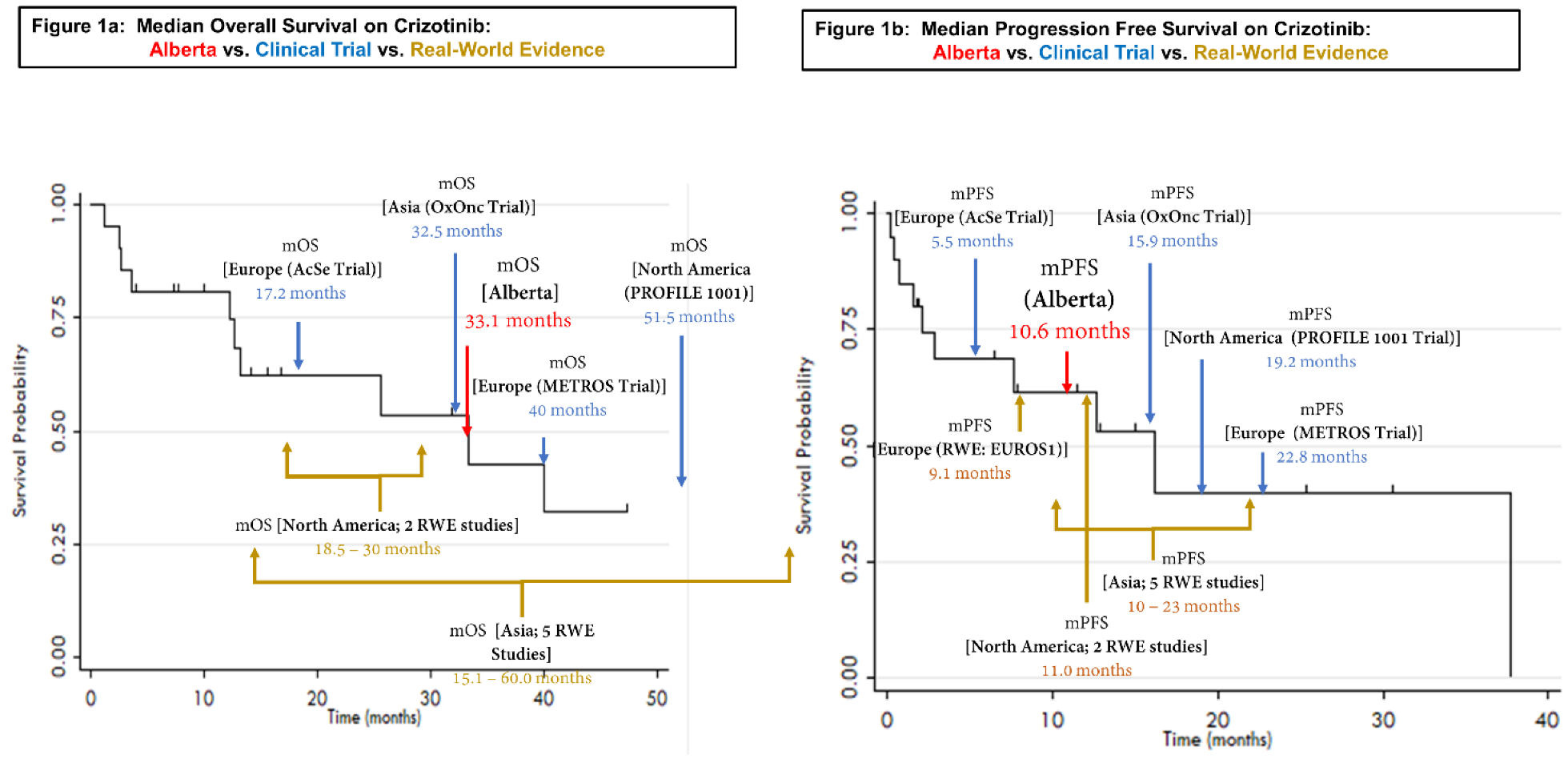
3.2. Crizotinib Treatment Outcomes
3.3. Adverse Events
3.4. Non-Crizotinib Systemic Therapies
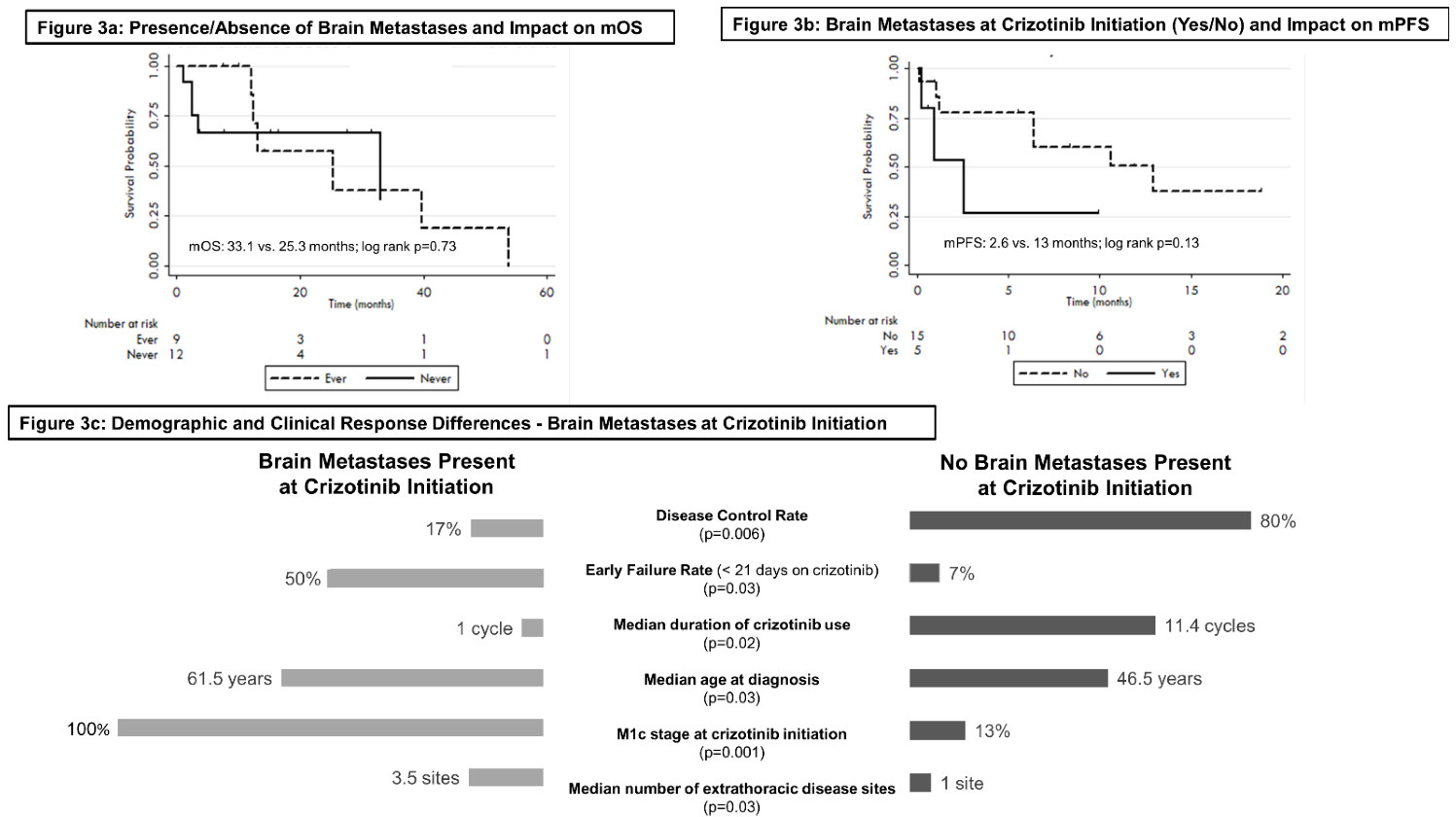
3.5. Brain Metastases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Study | Study Type | Median Progression-Free Survival [95% CI] | Median Overall Survival [95%CI] | ORR | DCR |
|---|---|---|---|---|---|
| Li et al., 2018 | Real-World Evidence (China) | 12.6 months [IQR: 7.7–19.3] | 32.7 months [IQR: 18.8–NR] | 83.3% | 97.2% |
| Liu et al., 2019 | Real-World Evidence (China) | 11.0 months [7.8–14.2] | 41 months [22.5–59.5] | 71.5% | 94.3% |
| Masuda et al., 2019 | Real-World Evidence (Japan) | 10 months [5.1–27.0] | 28.7 months [6.7–NR] | 90% | |
| Park et al., 2018 | Real-World Evidence (Korea) | 13.1 months [4.4–NR] | 15.1 months [5.4–NR] | 73.3% | 80.1% |
| Zheng et al., 2020 | Real-World Evidence (China) | 23.0 months [22.4–33.6] | 60.0 months [40.7–79.3] | 64.7% | 94.1% |
| Doebele et al., 2019 | Real-World Evidence (USA Flatiron Health Dataset) | 8.8 months [8.2–9.9] (time to treatment discontinuation) | 18.5 months [15.1–19.9] | - | - |
| Gainor et al., 2017 | Real-World Evidence (USA) | 11.0 months | 30 months [12–NR] | - | - |
| Mazieres et al., 2015 | Real-World Evidence (Europe: EUROS1) | 9.1 months | - | 80% | 86.7% |
| Patil et al., 2018 | Real-World Evidence (USA) | 11.0 months [8.0–23.0] | - | - | - |
| PROFILE 1001 (as reported by Shaw et al., 2019) | Phase I (NCT00585195) | 19.3 months [15.2–39.1] | 51.5 months [29.3–NR] | 72% | 91% |
| OxOnc (as reported by Wu et al., 2018) | Phase II (NCT01945021) | 15.8 months [12.9–24.0] | 32.5 months [32.5–NR] | 71.7% | - |
| EUCROSS (as reported by Michels et al., 2019) | Phase II (NCT02183870) | 20.0 months [10.1–NR] | Not reached (data immature) [17.7 months–NR] | 70% | 90% |
| AcSé (as reported by Moro-Silbot et al., 2019) | Phase II (NCT02034981) | 5.5 months [4.2–9.1] | 17.2 months [6.8–32.8] | 47.2% | - |
| METROS (as reported by Landi et al., 2019) | Phase II (NCT02499614) | 22.8 months [15.2–30.3] | 40.0 | 65% | 85% |
Appendix B
| Treatment Details, Response and Outcome | Total Cohort (n = 21) | |
|---|---|---|
| n (%) | ||
| One Year Survival Rate [95% CI] | ||
| After Diagnosis | 74% [48.9–88.4] | |
| After Crizotinib Initiation | 47% [24.4–67.2] | |
| Five Year Survival Rate [95% CI] | ||
| After Diagnosis | 11% [0.7–37.1] | |
| After Crizotinib Initiation | 0% | |
| Median time on crizotinib (net duration of use) | ||
| Cycles, (IQR) | 6.9 (1.3–17.1) | |
| Systemic Treatment Line in which crizotinib was received | ||
| 1st Palliative Line | 11 | |
| 2nd Palliative Line | 8 | |
| 3rd Palliative Line | 0 | |
| 4th Palliative Line | 2 | |
| Median, (IQR) | 1 (1–2) | |
| Best Response Metrics | ||
| Complete response | 0 | |
| Partial response | 6 | |
| Stable disease | 7 | |
| Progressive disease | 3 | |
| Non-evaluable | 2 | |
| Time to best response (days), (IQR) | 48.5 (32–65.5) | |
| Duration of best response (months), (IQR) | 5.0 (0.3–10.8) | |
| Disease Control Rate | 62% | |
| Objective Response Rate | 29% | |
| Adverse Event(s) Occurred | ||
| No | 10 | |
| Yes | 11 | |
| Adverse Event Management (n = 19 adverse events) | % of all AE | % of cohort |
| No treatment modifications | 11 | 15 |
| Terminate crizotinib | 5 | 3 |
| Treatment break only | 1 | 1 |
| Dose modification only | 0 | 0 |
| Treatment break and dose modification | 2 | 2 |
| Median duration of treatment break, (IQR) | 14 days (14–153) | |
| Dose modification | 250 mg twice daily reduced to 200 mg twice daily | |
| Highest Grade of Reported AE, per patient | ||
| None | 10 | |
| Low (grade 1 or 2) | 7 | |
| Serious (grade 3 or 4) | 4 | |
| Number of Adverse Events per Patient | ||
| Median, (IQR) | 1 (0–2) | |
| Time to First Reported Adverse Event (days) | ||
| Median, (IQR) | 24 (7–35) | |
| Adverse Events Reported (CTCAE 5.0 Category) | ||
| Eye Disorders: Floaters | ||
| Yes | 2 | |
| No | 19 | |
| General Disorders: Edema | ||
| Yes | 1 | |
| No | 20 | |
| Gastrointestinal Disorders: | ||
| Nausea, Vomiting, Diarrhea, Constipation, Abdominal pain | ||
| Yes | 6 | |
| No | 15 | |
| Investigational Disorders: | ||
| Increased ALT, AST or liver function test | ||
| Yes | 3 | |
| No | 18 | |
| Nervous System Disorders: Dizziness | ||
| Yes | 1 | |
| No | 20 | |
| Respiratory System Disorders: Pneumonitis | ||
| Yes | 1 | |
| No | 20 | |
| Reasons for crizotinib termination | % of terminations | |
| Progressive Disease | 6 | |
| Adverse Events | 3 | |
| Death | 5 | |
| Different Treatment Option Identified | 1 | |
| Additional Systemic Therapy post-crizotinib | ||
| Yes | 5 | |
| No | 10 | |
| Crizotinib ongoing | 6 | |
References
- Bebb, D.G.; Agulnik, J.; Albadine, R.; Banerji, S.; Bigras, G.; Butts, C.; Couture, C.; Cutz, J.C.; Desmeules, P.; Ionescu, D.N.; et al. Crizotinib Inhibition of ROS1-Positive Tumours in Advanced Non-Small-Cell Lung Cancer: A Canadian Perspective. Curr. Oncol. 2019, 26, 551–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gainor, J.F.; Tseng, D.; Yoda, S.; Dagogo-Jack, I.; Friboulet, L.; Lin, J.J.; Hubbeling, H.G.; Dardaei, L.; Farago, A.F.; Schultz, K.R.; et al. Patterns of Metastatic Spread and Mechanisms of Resistance to Crizotinib in ROS1 -Positive Non–Small-Cell Lung Cancer. JCO Precis. Oncol. 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Expanding the Roster of ROS1 Inhibitors. J. Clin. Oncol. 2017, 35, 2595–2597. [Google Scholar] [CrossRef] [PubMed]
- Juan, O.; Popat, S. Crizotinib for ROS1 Patients: One Small Step in Biomarker Testing, One Giant Leap for Advanced NSCLC Patients. Lung Cancer 2017, 104, 131–133. [Google Scholar] [CrossRef]
- Shaw, A.T.; Ou, S.-H.I.; Bang, Y.-J.; Camidge, D.R.; Solomon, B.J.; Salgia, R.; Riely, G.J.; Varella-Garcia, M.; Shapiro, G.I.; Costa, D.B.; et al. Crizotinib in ROS1 -Rearranged Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2014, 371, 1963–1971. [Google Scholar] [CrossRef] [Green Version]
- Bubendorf, L.; Büttner, R.; Al-Dayel, F.; Dietel, M.; Elmberger, G.; Kerr, K.; López-Ríos, F.; Marchetti, A.; Öz, B.; Pauwels, P.; et al. Testing for ROS1 in Non-Small Cell Lung Cancer: A Review with Recommendations. Virchows Arch. 2016, 469, 489–503. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)—A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap Consortium: Building an International Community of Software Platform Partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med. 2018, 142, 321–346. [Google Scholar] [CrossRef] [Green Version]
- Pan-Canadian Oncology Drug Review Final Clinical Guidance Report Crizotinib (Xalkori) for ROS1-Positive Non-Small Cell Lung Cancer. 2019. Available online: www.cadth.ca/pcodr (accessed on 4 May 2021).
- Griffith, S.D.; Tucker, M.; Bowser, B.; Calkins, G.; Chang, C. (Joe); Guardino, E.; Khozin, S.; Kraut, J.; You, P.; Schrag, D.; et al. Generating Real-World Tumor Burden Endpoints from Electronic Health Record Data: Comparison of RECIST, Radiology-Anchored, and Clinician-Anchored Approaches for Abstracting Real-World Progression in Non-Small Cell Lung Cancer. Adv. Ther. 2019, 36, 2122–2136. [Google Scholar] [CrossRef] [Green Version]
- StataCorp. Stata Statistical Software: Release 12; StataCorp LP: College Station, TX, USA, 2011. [Google Scholar]
- Hong, J.C. Strategies to Turn Real-World Data into Real-World Knowledge. JAMA Netw. Open 2021, 4, e2128045. [Google Scholar] [CrossRef] [PubMed]
- Code U.S. 21 U.S.C. 335c—Authority to Withdraw Approval of Abbreviated Drug Applications—Content Details—USCODE-2016-title21-chap9-subchapIII-sec335c. Available online: https://www.govinfo.gov/app/details/USCODE-2016-title21/USCODE-2016-title21-chap9-subchapIII-sec335c (accessed on 19 January 2022).
- Hernán, M.A.; Robins, J.M. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am. J. Epidemiol. 2016, 183, 758–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doebele, R.; Perez, L.; Trinh, H.; Martinec, M.; Martina, R.; Riehl, T.; Krebs, M.; Meropol, N.; Wong, W.; Crane, G. P1.01-83 Comparative Efficacy Analysis Between Entrectinib Trial and Crizotinib Real-World ROS1 Fusion-Positive (ROS1+) NSCLC Patients. J. Thorac. Oncol. 2019, 14, S392. [Google Scholar] [CrossRef]
- Waterhouse, D.; Iadeluca, L.; Sura, S.; Wilner, K.; Emir, B.; Krulewicz, S.; Espirito, J.; Bartolome, L. Real-World Outcomes Among Crizotinib-Treated Patients with ROS1-Positive Advanced Non-Small-Cell Lung Cancer: A Community Oncology-Based Observational Study. Target. Oncol. 2022, 17, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shen, L.; Ding, D.; Huang, J.; Zhang, J.; Chen, Z.; Lu, S. Efficacy of Crizotinib among Different Types of ROS1 Fusion Partners in Patients with ROS1-Rearranged Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 987–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Yu, H.; Chang, J.; Chen, H.; Li, Y.; Zhao, W.; Zhao, K.; Zhu, Z.; Sun, S.; Fan, M.; et al. Crizotinib in Chinese Patients with ROS1-Rearranged Advanced Non-Small-Cell Lung Cancer in Routine Clinical Practice. Target. Oncol. 2019, 14, 315–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, K.; Fujiwara, Y.; Shinno, Y.; Mizuno, T.; Sato, J.; Morita, R.; Matsumoto, Y.; Murakami, S.; Goto, Y.; Kanda, S.; et al. Efficacy and Safety of Crizotinib in Patients with ROS1 Rearranged Non-Small Cell Lung Cancer: A Retrospective Analysis. J. Thorac. Dis. 2019, 11, 2965–2972. [Google Scholar] [CrossRef]
- Park, S.; Ahn, B.-C.; Lim, S.W.; Sun, J.-M.; Kim, H.R.; Hong, M.H.; Lee, S.-H.; Ahn, J.S.; Park, K.; Choi, Y.L.; et al. Characteristics and Outcome of ROS1-Positive Non–Small Cell Lung Cancer Patients in Routine Clinical Practice. J. Thorac. Oncol. 2018, 13, 1373–1382. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Cao, H.; Li, Y.; Rao, C.; Zhang, T.; Luo, J.; Lv, D.; Zhu, Y.; Zhou, J.; Zhou, J. Effectiveness and Prognostic Factors of First-Line Crizotinib Treatment in Patients with ROS1-Rearranged Non-Small Cell Lung Cancer: A Multicenter Retrospective Study. Lung Cancer 2020, 147, 130–136. [Google Scholar] [CrossRef]
- Shaw, A.T.; Riely, G.J.; Bang, Y.J.; Kim, D.W.; Camidge, D.R.; Solomon, B.J.; Varella-Garcia, M.; Iafrate, A.J.; Shapiro, G.I.; Usari, T.; et al. Crizotinib in ROS1-Rearranged Advanced Non-Small-Cell Lung Cancer (NSCLC): Updated Results, Including Overall Survival, from PROFILE 1001. Ann. Oncol. 2019, 30, 1121–1126. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Yang, J.C.-H.; Kim, D.-W.; Lu, S.; Zhou, J.; Seto, T.; Yang, J.-J.; Yamamoto, N.; Ahn, M.-J.; Takahashi, T.; et al. Phase II Study of Crizotinib in East Asian Patients With ROS1-Positive Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 1405–1411. [Google Scholar] [CrossRef]
- Michels, S.; Massutí, B.; Schildhaus, H.-U.; Franklin, J.; Sebastian, M.; Felip, E.; Grohé, C.; Rodriguez-Abreu, D.; Abdulla, D.S.Y.; Bischoff, H.; et al. Safety and Efficacy of Crizotinib in Patients With Advanced or Metastatic ROS1-Rearranged Lung Cancer (EUCROSS): A European Phase II Clinical Trial. J. Thorac. Oncol. 2019, 14, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Moro-Sibilot, D.; Cozic, N.; Pérol, M.; Mazières, J.; Otto, J.; Souquet, P.J.; Bahleda, R.; Wislez, M.; Zalcman, G.; Guibert, S.D.; et al. Crizotinib in C-MET- or ROS1-Positive NSCLC: Results of the AcSé Phase II Trial. Ann. Oncol. 2019, 30, 1985–1991. [Google Scholar] [CrossRef]
- Landi, L.; Chiari, R.; Tiseo, M.; D’Incà, F.; Dazzi, C.; Chella, A.; Delmonte, A.; Bonanno, L.; Giannarelli, D.; Cortinovis, D.L.; et al. Crizotinib in MET -Deregulated or ROS1 -Rearranged Pretreated Non–Small Cell Lung Cancer (METROS): A Phase II, Prospective, Multicenter, Two-Arms Trial. Clin. Cancer Res. 2019, 25, 7312–7319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-Based to a More “Personalized” Approach to Cancer Staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, K.; Wei, S.; Liu, C.; Zhou, J.; Gou, Q.; Wu, X.; Yang, Z.; Yang, Y.; Peng, Y.; et al. ROS1-Fusion Protein Induces PD-L1 Expression via MEK-ERK Activation in Non-Small Cell Lung Cancer. Oncoimmunology 2020, 9, 1758003. [Google Scholar] [CrossRef]
- Wagner, G.; Stollenwerk, H.K.; Klerings, I.; Pecherstorfer, M.; Gartlehner, G.; Singer, J. Efficacy and Safety of Immune Checkpoint Inhibitors in Patients with Advanced Non–Small Cell Lung Cancer (NSCLC): A Systematic Literature Review. Oncoimmunology 2020, 9, 1774314. [Google Scholar] [CrossRef]
- Alessi, J.V.; Ricciuti, B.; Jiménez-Aguilar, E.; Hong, F.; Wei, Z.; Nishino, M.; Plodkowski, A.J.; Sawan, P.; Luo, J.; Rizvi, H.; et al. Outcomes to First-Line Pembrolizumab in Patients with PD-L1-High (≥50%) Non–Small Cell Lung Cancer and a Poor Performance Status. J. Immunother. Cancer 2020, 8, e001007. [Google Scholar] [CrossRef]
- Choudhury, N.J.; Schneider, J.L.; Patil, T.; Zhu, V.W.; Goldman, D.A.; Yang, S.-R.; Falcon, C.J.; Do, A.; Nie, Y.; Plodkowski, A.J.; et al. Response to Immune Checkpoint Inhibition as Monotherapy or in Combination with Chemotherapy in Metastatic ROS1-Rearranged Lung Cancers. JTO Clin. Res. Rep. 2021, 2, 100187. [Google Scholar] [CrossRef]
- Mazieres, J.; Drilon, A.E.; Mhanna, L.; Milia, J.; Lusque, A.; Cortot, A.B.; Mezquita, L.; Thai, A.; Couraud, S.; Veillon, R.; et al. Efficacy of Immune-Checkpoint Inhibitors (ICI) in Non-Small Cell Lung Cancer (NSCLC) Patients Harboring Activating Molecular Alterations (ImmunoTarget). J. Clin. Oncol. 2018, 36 (Suppl. S15), 9010. [Google Scholar] [CrossRef]
- Remon, J.; Hendriks, L.E.; Cabrera, C.; Reguart, N.; Besse, B. Immunotherapy for Oncogenic-Driven Advanced Non-Small Cell Lung Cancers: Is the Time Ripe for a Change? Cancer Treat. Rev. 2018, 71, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, M.; Schultheis, A.; Teixido, C.; Michels, S.; Morales-Espinosa, D.; Viteri, S.; Hartmann, W.; Merkelbach-Bruse, S.; Fischer, R.; Schildhaus, H.-U.; et al. ROS1 Rearrangements in Lung Adenocarcinoma: Prognostic Impact, Therapeutic Options and Genetic Variability. Oncotarget 2015, 6, 10577–10585. [Google Scholar] [CrossRef] [Green Version]
- Takezawa, K.; Okamoto, I.; Okamoto, W.; Takeda, M.; Sakai, K.; Tsukioka, S.; Kuwata, K.; Yamaguchi, H.; Nishio, K.; Nakagawa, K. Thymidylate Synthase as a Determinant of Pemetrexed Sensitivity in Non-Small Cell Lung Cancer. Br. J. Cancer 2011, 104, 1594–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, V.W.; Lin, Y.-T.; Kim, D.-W.; Loong, H.H.; Nagasaka, M.; To, H.; Ang, Y.L.-E.; Ock, C.-Y.; Tchekmedyian, N.; Ou, S.-H.I.; et al. An International Real-World Analysis of the Efficacy and Safety of Lorlatinib through Early or Expanded Access Programs in Patients With Tyrosine Kinase Inhibitor–Refractory ALK-Positive or ROS1-Positive NSCLC. J. Thorac. Oncol. 2020, 15, 1484–1496. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Felip, E.; Bauer, T.M.; Besse, B.; Navarro, A.; Postel-Vinay, S.; Gainor, J.F.; Johnson, M.; Dietrich, J.; James, L.P.; et al. Lorlatinib in Non-Small-Cell Lung Cancer with ALK or ROS1 Rearrangement: An International, Multicentre, Open-Label, Single-Arm First-in-Man Phase 1 Trial. Lancet Oncol. 2017, 18, 1590–1599. [Google Scholar] [CrossRef]
- Dodson, C.; Richards, T.J.; Smith, D.A.; Ramaiya, N.H. Tyrosine Kinase Inhibitor Therapy for Brain Metastases in Non-Small-Cell Lung Cancer: A Primer for Radiologists. Am. J. Neuroradiol. 2020, 41, 738–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, J.-Y.; Inoue, A.; Cheng, R.; Varea, R.; Kim, S.-W. Does Pemetrexed Work in Targetable, Nonsquamous Non-Small-Cell Lung Cancer? A Narrative Review. Cancers 2020, 12, 2658. [Google Scholar] [CrossRef]
- Mazières, J.; Zalcman, G.; Crinò, L.; Biondani, P.; Barlesi, F.; Filleron, T.; Dingemans, A.-M.C.; Léna, H.; Monnet, I.; Rothschild, S.I.; et al. Crizotinib Therapy for Advanced Lung Adenocarcinoma and a ROS1 Rearrangement: Results from the EUROS1 Cohort. J. Clin. Oncol. 2015, 33, 992–999. [Google Scholar] [CrossRef]
- Isozaki, H.; Ichihara, E.; Takigawa, N.; Ohashi, K.; Ochi, N.; Yasugi, M.; Ninomiya, T.; Yamane, H.; Hotta, K.; Sakai, K.; et al. Non–Small Cell Lung Cancer Cells Acquire Resistance to the ALK Inhibitor Alectinib by Activating Alternative Receptor Tyrosine Kinases. Cancer Res. 2016, 76, 1506–1516. [Google Scholar] [CrossRef] [Green Version]
- Katayama, R.; Shaw, A.T.; Khan, T.M.; Mino-Kenudson, M.; Solomon, B.J.; Halmos, B.; Jessop, N.A.; Wain, J.C.; Yeo, A.T.; Benes, C.; et al. Mechanisms of Acquired Crizotinib Resistance in ALK-Rearranged Lung Cancers. Sci. Transl. Med. 2012, 4, ra17–ra120. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Jiang, T.; Li, X.; Wang, Y.; Zhao, C.; Zhao, S.; Xi, L.; Zhang, S.; Liu, X.; Jia, Y.; et al. Clinical Features of Bim Deletion Polymorphism and Its Relation with Crizotinib Primary Resistance in Chinese Patients with ALK / ROS1 Fusion-Positive Non-Small Cell Lung Cancer. Cancer 2017, 123, 2927–2935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperduto, P.W.; Kased, N.; Roberge, D.; Xu, Z.; Shanley, R.; Luo, X.; Sneed, P.K.; Chao, S.T.; Weil, R.J.; Suh, J.; et al. Summary Report on the Graded Prognostic Assessment: An Accurate and Facile Diagnosis-Specific Tool to Estimate Survival for Patients With Brain Metastases. J. Clin. Oncol. 2012, 30, 419–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerdan, L.; Segedin, B.; Nagy, V.; Khoa, M.; NT, T.; Schild, S. Brain Metastases from Non-Small Cell Lung Cancer (NSCLC): Prognostic Imporance of the Number of Involved Extracranial Organs. Strahlenther. Onkol. 2014, 190, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.J.W.; Li, H.; D’Silva, A.; Tudor, R.A.; Elegbede, A.A.; Otsuka, S.M.; Bebb, D.G.; Cheung, W.Y. Impact of Number versus Location of Metastases on Survival in Stage IV M1b Non-Small Cell Lung Cancer. Med. Oncol. 2018, 35, 117. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, A.; Sobhani, N.; Chapman, R.; Bagby, S.; Bortoletti, C.; Traversini, M.; Ferrari, K.; Voltolini, L.; Darlow, J.; Roviello, G. Focus on ROS1-Positive Non-Small Cell Lung Cancer (NSCLC): Crizotinib, Resistance Mechanisms and the Newer Generation of Targeted Therapies. Cancers 2020, 12, 3293. [Google Scholar] [CrossRef]
- Greenspoon, J.N.; Ellis, P.M.; Pond, G.; Caetano, S.; Broomfield, J.; Swaminath, A. Comparative Survival in Patients with Brain Metastases from Non-Small-Cell Lung Cancer Treated before and after Implementation of Radiosurgery. Curr. Oncol. 2017, 24, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, S.; Dziadziuszko, R.; Peters, S. Indications and Limitations of Chemotherapy and Targeted Agents in Non-Small Cell Lung Cancer Brain Metastases. Cancer Treat. Rev. 2014, 40, 716–722. [Google Scholar] [CrossRef]
- Patil, T.; Smith, D.E.; Bunn, P.A.; Aisner, D.L.; Le, A.T.; Hancock, M.; Purcell, W.T.; Bowles, D.W.; Camidge, D.R.; Doebele, R.C. The Incidence of Brain Metastases in Stage IV ROS1-Rearranged Non–Small Cell Lung Cancer and Rate of Central Nervous System Progression on Crizotinib. J. Thorac. Oncol. 2018, 13, 1717–1726. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.S.; Roy, U.B.; Ersek, J.L.; King, J.; Smith, R.A.; Martin, N.; Martins, R.; Moore, A.; Silvestri, G.A.; Jett, J. Updates Regarding Biomarker Testing for Non–Small Cell Lung Cancer: Considerations from the National Lung Cancer Roundtable. J. Thorac. Oncol. 2019, 14, 338–342. [Google Scholar] [CrossRef] [Green Version]
- Pennell, N.A.; Arcila, M.E.; Gandara, D.R.; West, H. Biomarker Testing for Patients With Advanced Non–Small Cell Lung Cancer: Real-World Issues and Tough Choices. Am. Soc. Clin. Oncol. Educ B. 2019, 39, 531–542. [Google Scholar] [CrossRef]
- Vinod, S.K.; Chandra, A.; Berthelsen, A.; Descallar, J. Does Timeliness of Care in Non-Small Cell Lung Cancer Impact on Survival? Lung Cancer 2017, 112, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Kasymjanova, G.; Small, D.; Cohen, V.; Jagoe, R.T.; Batist, G.; Sateren, W.; Ernst, P.; Pepe, C.; Sakr, L.; Agulnik, J. Lung Cancer Care Trajectory at a Canadian Centre: An Evaluation of How Wait Times Affect Clinical Outcomes. Curr. Oncol. 2017, 24, 302–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Statistics Canada. Population Estimates, Quarterly. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000901 (accessed on 7 May 2021).

| Demographic or Clinical Feature | Total Cohort (n = 21) | |
|---|---|---|
| n (%) | ||
| Age at treatment initiation | ||
| Median (years), (IQR) | 51.6 (43.9–59.7) | |
| <50 years | 10 | |
| ≥50 years | 11 | |
| Sex | ||
| Male | 7 | |
| Female | 14 | |
| Smoking Status | ||
| Never Smoker | 18 | |
| Ever Smoker | 3 | |
| Body Mass Index (kg/m2) | ||
| Median, (IQR) | 23.6 (22.6–26.7) | |
| <18.5 (underweight) | 0 | |
| 18.5–24.8 (normal) | 10 | |
| 24.9–29.9 (overweight) | 6 | |
| >29.9 (obese) | 1 | |
| Missing data | 4 | |
| Race | ||
| Asian | 8 | |
| Caucasian | 12 | |
| Non-Asian/Non-Caucasian | 1 | |
| Geographic Location of Residence | ||
| Urban | 21 | |
| Rural | 0 | |
| Cancer Treatment Centre Type | ||
| Academic | 20 | |
| Community/Regional | 1 | |
| ROS1 Testing | ||
| Testing Location: | ||
| Within Canada | 5 | |
| Outside Canada (USA; Germany) | 16 | |
| Testing Funding: | ||
| Provincial Health System Funding | 7 | |
| Patient Funded | 7 | |
| Unknown | 7 | |
| Time from Diagnosis to Crizotinib Initiation | ||
| Median (IQR) | 58 days (29–359) | |
| Eastern Cooperative Oncology Group Performance Status | At Diagnosis | At Crizotinib Initiation |
| Good (0 or 1) | 16 | 14 |
| Poor (2 or 3) | 4 | 6 |
| Unknown | 1 | 1 |
| Histological Subtype | ||
| Adenocarcinoma | 21 | |
| PD-L1 Status (at diagnosis) | ||
| Negative (<1%) | 3 | |
| Low (1–49%) | 2 | |
| High (≥50%) | 10 | |
| Not tested/insufficient sample | 6 | |
| Metastatic Disease Presentation | ||
| Upon Relapse | 2 | |
| (following resection for early stage disease) | ||
| Advanced/Non-resectable Disease at diagnosis | 1 | |
| De novo Stage IV | 18 | |
| (metastatic disease present at diagnosis) | ||
| Previous Systemic Therapy Exposure | ||
| Curative-intent (adjuvant cytotoxic chemotherapy) | 2 | |
| Palliative-intent | 10 | |
| (cytotoxic chemotherapy or immune checkpoint inhibitors) | ||
| None (treatment naive) | 9 | |
| Previous Thoracic Radiation Therapy Exposure | ||
| None | 10 | |
| Curative to thorax (>4500 cGY) | 2 | |
| Palliative to Thorax | 9 | |
| Brain Metastases Development | ||
| None (to date) | 12 | |
| At baseline | 6 | |
| During crizotinib therapy | 3 | |
| Platin-Pemetrexed (+/−Maintenance Pemetrexed Therapy) (n = 7) | Lorlatinib (n = 5) | Immune Checkpoint Inhibitors (n = 6) | ||||
|---|---|---|---|---|---|---|
| Treatment Sequence | ||||||
| Prior to Crizotinib | 6 (86%) | 0 (0%) | 6 (100%) | |||
| After Crizotinib | 1 (14%) | 5 (100%) | 0 (0%) | |||
| Duration of Treatment | ||||||
| Median | 7.8 months | 118 days | 33.5 days | |||
| IQR | 21 days–14.3 months | 16–140 days | 21–231 days | |||
| Range | 18 days–5.5 years | 11–845 days | 1–294 days | |||
| Best Response | ||||||
| Complete Response | 0 (0%) | 0 (0%) | 0 (0%) | |||
| Partial Response | 0 (0%) | ORR: 0% | 1 (20%) | ORR: 20% | 1 (17%) | ORR: 17% |
| Stable Disease | 4 (57%) | DCR: 57% | 2 (40%) | DCR: 60% | 1 (17%) | DCR: 33% |
| Progressive Disease | 1 (14%) | 0 (0%) | 2 (33%) | |||
| Non-evaluable | 2 (29%) | 2 (40%) | 2 (33%) | |||
| Progression-Free Survival | ||||||
| Median [95% CI] | 9.8 months [0.2–NR] | NR [16 days–NR] | 10.1 weeks [23 days–NR] | |||
| Overall Survival | ||||||
| (post-metastatic disease discovery) | ||||||
| Median [95% CI] | 39.6 months [12.1–NR] | 39.6 months [13.1–NR] | 12.5 months [2.6–NR] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibson, A.J.W.; Box, A.; Cheung, W.Y.; Dean, M.L.; Elegbede, A.A.; Hao, D.; Pabani, A.; Sangha, R.; Bebb, D.G. Real-World Management and Outcomes of Crizotinib-Treated ROS1-Rearranged NSCLC: A Retrospective Canadian Cohort. Curr. Oncol. 2022, 29, 1967-1982. https://doi.org/10.3390/curroncol29030160
Gibson AJW, Box A, Cheung WY, Dean ML, Elegbede AA, Hao D, Pabani A, Sangha R, Bebb DG. Real-World Management and Outcomes of Crizotinib-Treated ROS1-Rearranged NSCLC: A Retrospective Canadian Cohort. Current Oncology. 2022; 29(3):1967-1982. https://doi.org/10.3390/curroncol29030160
Chicago/Turabian StyleGibson, Amanda J. W., Adrian Box, Winson Y. Cheung, Michelle L. Dean, Anifat A. Elegbede, Desiree Hao, Aliyah Pabani, Randeep Sangha, and Dafydd Gwyn Bebb. 2022. "Real-World Management and Outcomes of Crizotinib-Treated ROS1-Rearranged NSCLC: A Retrospective Canadian Cohort" Current Oncology 29, no. 3: 1967-1982. https://doi.org/10.3390/curroncol29030160
APA StyleGibson, A. J. W., Box, A., Cheung, W. Y., Dean, M. L., Elegbede, A. A., Hao, D., Pabani, A., Sangha, R., & Bebb, D. G. (2022). Real-World Management and Outcomes of Crizotinib-Treated ROS1-Rearranged NSCLC: A Retrospective Canadian Cohort. Current Oncology, 29(3), 1967-1982. https://doi.org/10.3390/curroncol29030160







