Impact of Postoperative Chemotherapy in Patients with Gastric/Gastroesophageal Adenocarcinoma Treated with Perioperative Chemotherapy
Abstract
:1. Introduction
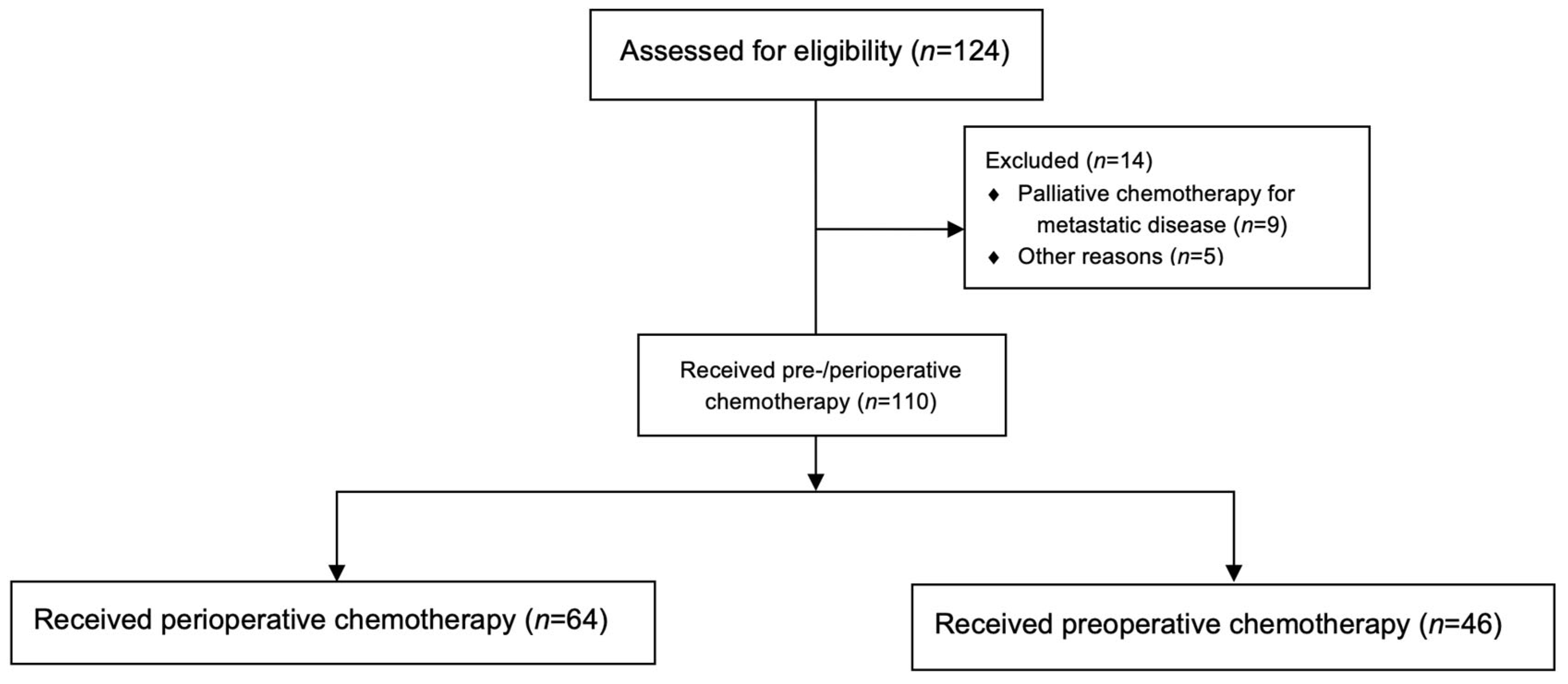
2. Patients and Methods
2.1. Study Design and Objectives
2.2. Statistical Analyses
2.3. Review of the Literature
3. Results
3.1. Patient Characteristics
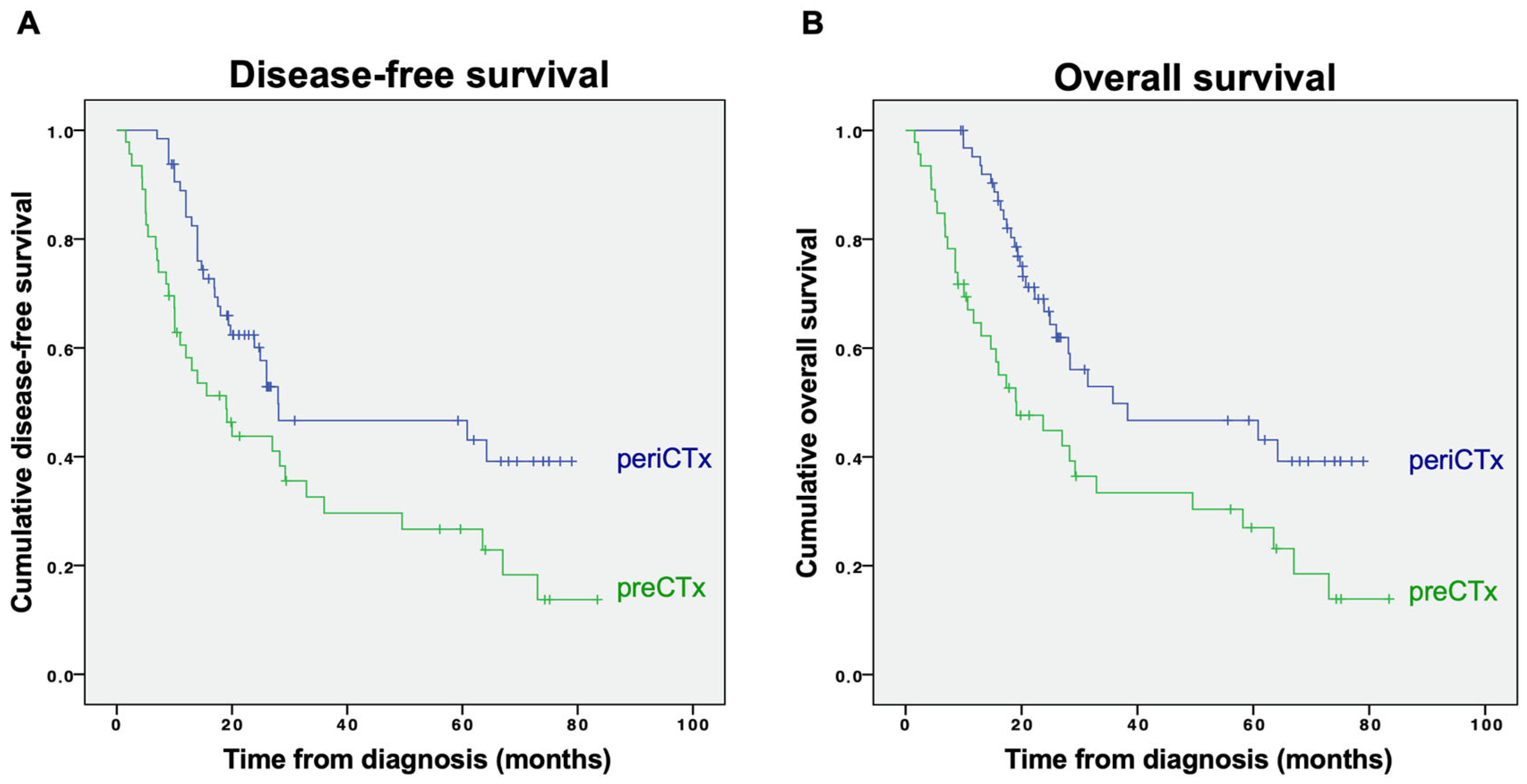
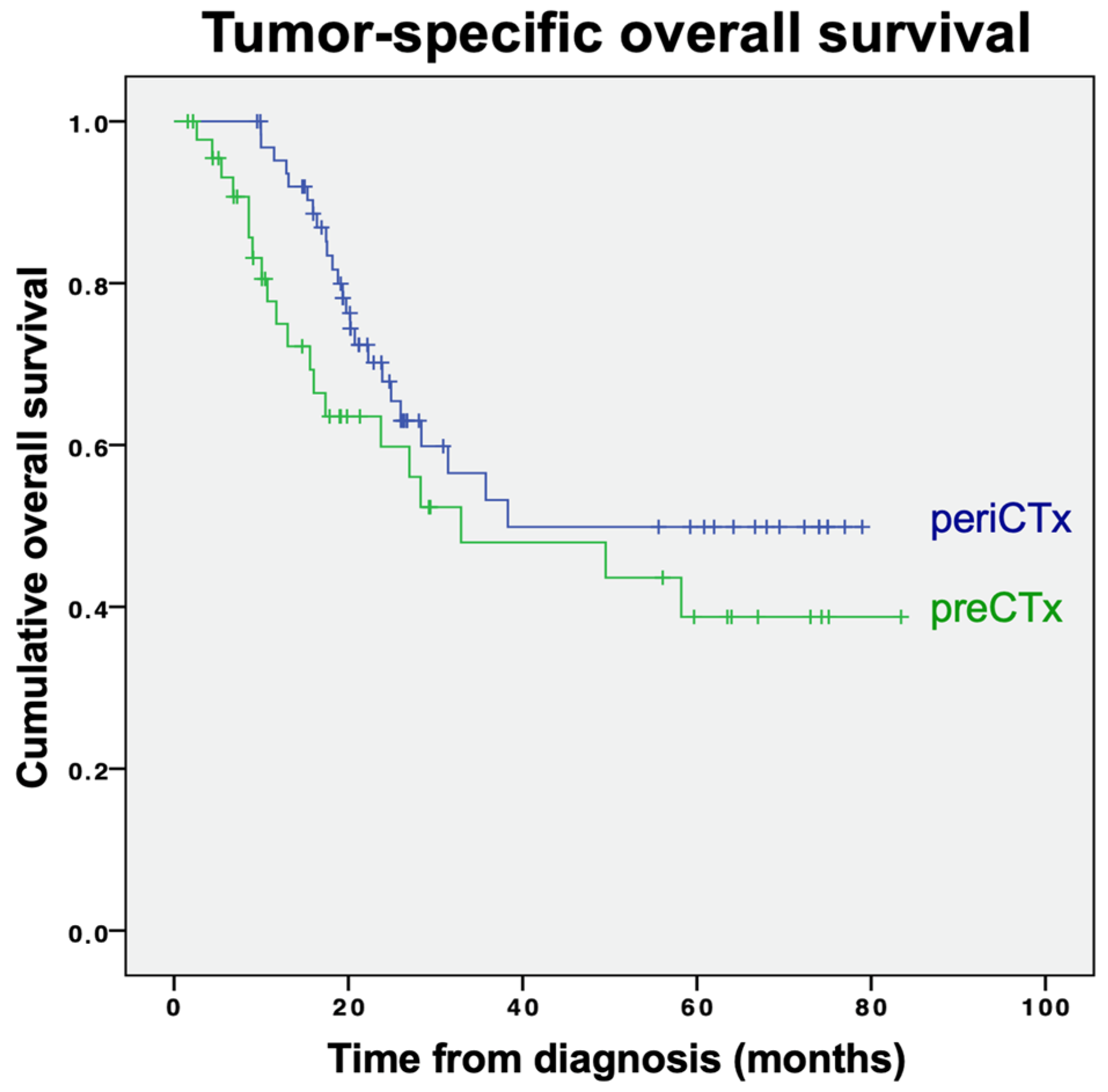
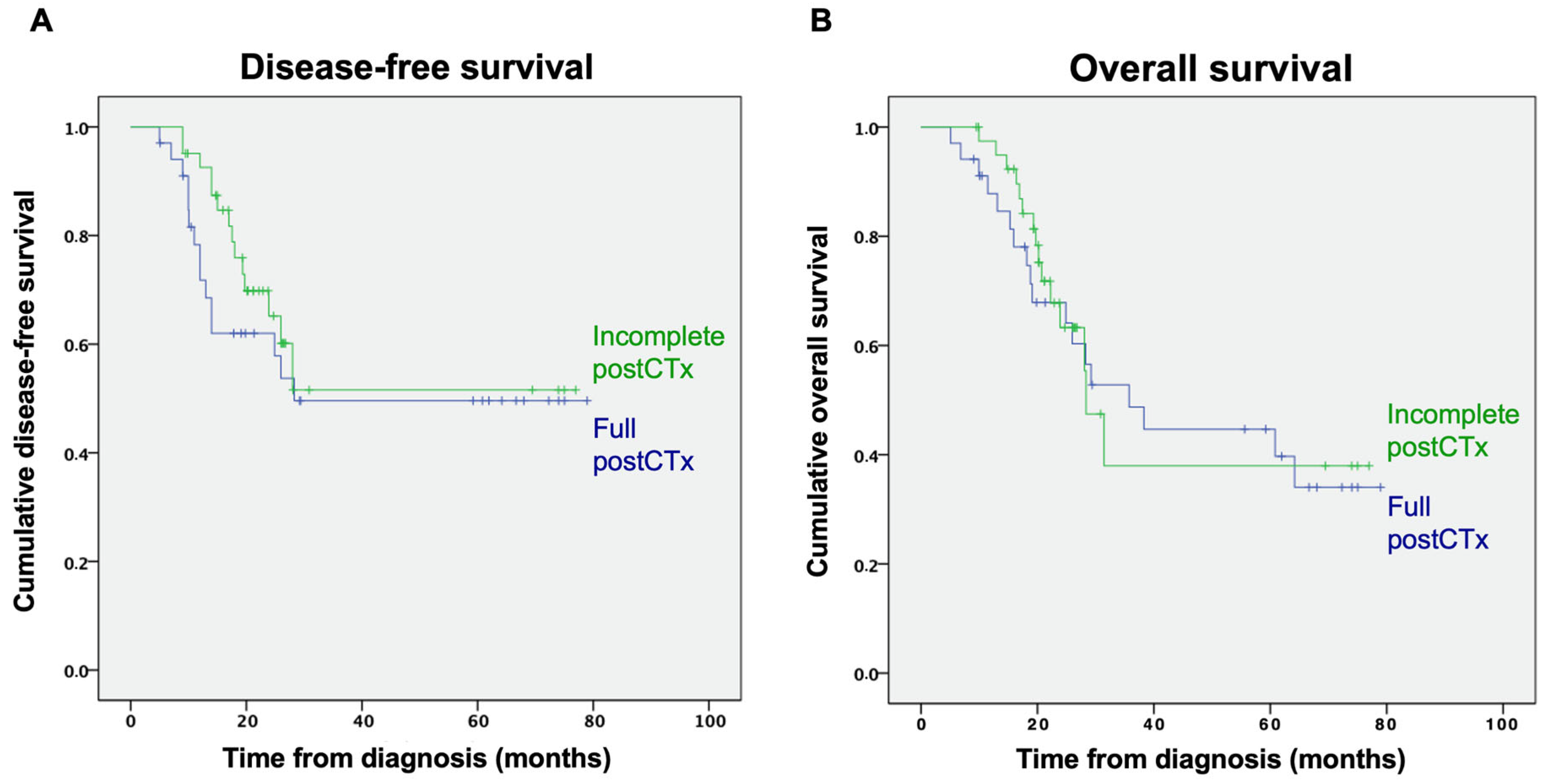
3.2. Outcomes
3.2.1. Disease-Free and Overall Survival
3.2.2. Tumor-Specific Survival
3.2.3. Completeness of Postoperative Chemotherapy
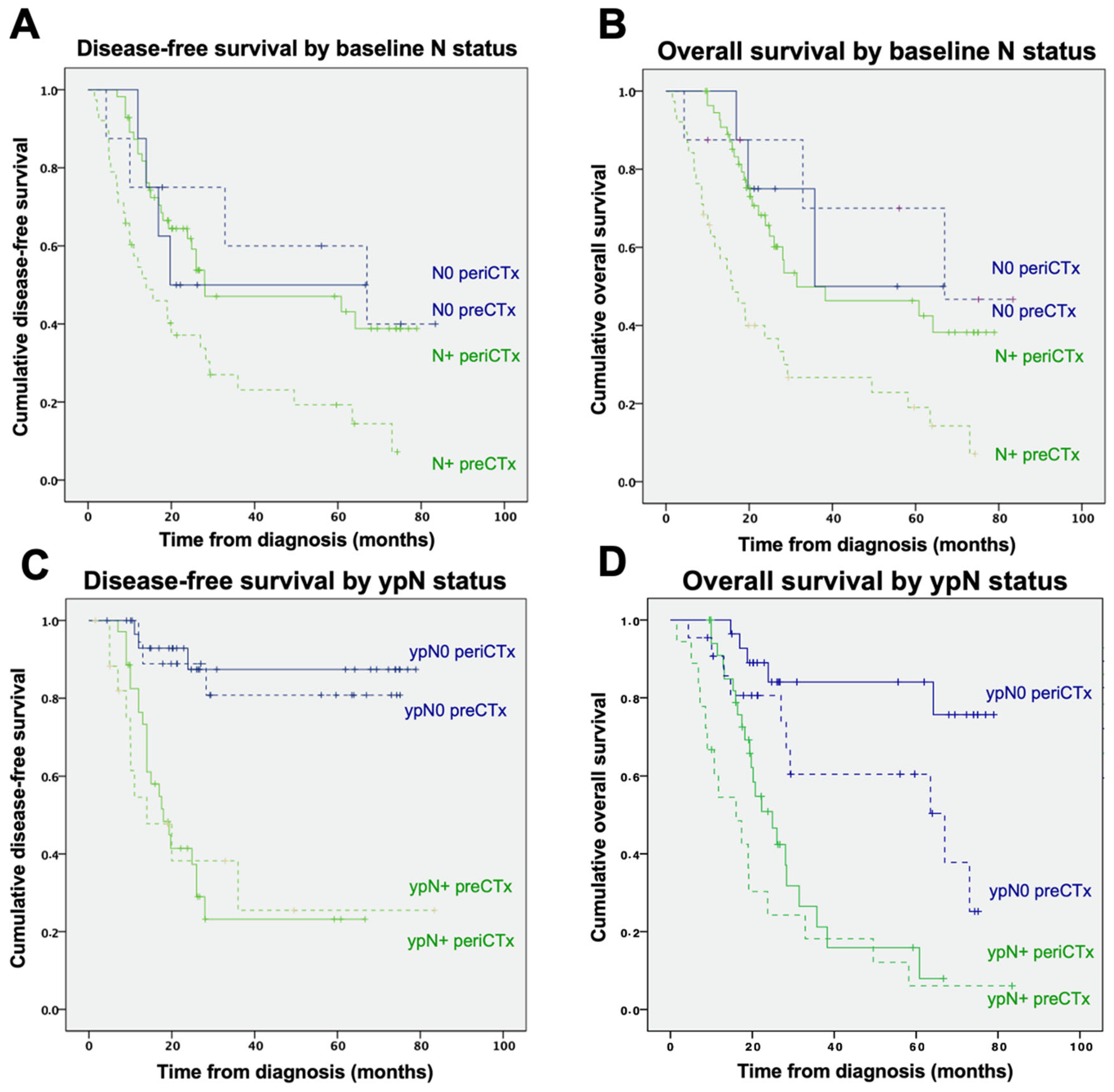
3.2.4. Lymph Node Involvement at Baseline and at Time of Resection
3.3. Review of the Literature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ychou, M.; Boige, V.; Pignon, J.P.; Conroy, T.; Bouche, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.M.; Saint-Aubert, B.; et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Schuhmacher, C.; Gretschel, S.; Lordick, F.; Reichardt, P.; Hohenberger, W.; Eisenberger, C.F.; Haag, C.; Mauer, M.E.; Hasan, B.; Welch, J.; et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J. Clin. Oncol. 2010, 28, 5210–5218. [Google Scholar] [CrossRef] [PubMed]
- Allum, W.H.; Stenning, S.P.; Bancewicz, J.; Clark, P.I.; Langley, R.E. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J. Clin. Oncol. 2009, 27, 5062–5067. [Google Scholar] [CrossRef] [PubMed]
- Thuss-Patience, P.C.; Hofheinz, R.D.; Arnold, D.; Florschutz, A.; Daum, S.; Kretzschmar, A.; Mantovani-Loffler, L.; Bichev, D.; Breithaupt, K.; Kneba, M.; et al. Perioperative chemotherapy with docetaxel, cisplatin and capecitabine (DCX) in gastro-oesophageal adenocarcinoma: A phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Ann. Oncol. 2012, 23, 2827–2834. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Groman, A.; Jiang, C.; Perimbeti, S.; Gabriel, E.; Kukar, M.; Mukherjee, S. Association of Preoperative Chemosensitivity With Postoperative Survival in Patients With Resected Gastric Adenocarcinoma. JAMA Netw. Open 2021, 4, e2135340. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.A.; Stiles, Z.E.; Tsao, M.W.; Deneve, J.L.; Glazer, E.S.; Yakoub, D.; Grothey, A.; Somer, B.G.; Dickson, P.V. Analysis of the Survival Impact of Postoperative Chemotherapy After Preoperative Chemotherapy and Resection for Gastric Cancer. Ann. Surg. Oncol. 2021, 28, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Papaxoinis, G.; Kamposioras, K.; Weaver, J.M.J.; Kordatou, Z.; Stamatopoulou, S.; Germetaki, T.; Nasralla, M.; Owen-Holt, V.; Anthoney, A.; Mansoor, W. The Role of Continuing Perioperative Chemotherapy Post Surgery in Patients with Esophageal or Gastroesophageal Junction Adenocarcinoma: A Multicenter Cohort Study. J. Gastrointest. Surg. 2019, 23, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, F.J.F.; de Jesus, V.H.F.; Ribeiro, H.S.C.; Diniz, A.L.; de Godoy, A.L.; de Farias, I.C.; Felismino, T.; Mello, C.A.L.; Almeida, M.F.; Begnami, M.; et al. Impact of ypT, ypN, and Adjuvant Therapy on Survival in Gastric Cancer Patients Treated with Perioperative Chemotherapy and Radical Surgery. Ann. Surg. Oncol. 2019, 26, 3618–3626. [Google Scholar] [CrossRef] [PubMed]
- van Putten, M.; Lemmens, V.; van Laarhoven, H.W.M.; Pruijt, H.F.M.; Nieuwenhuijzen, G.A.P.; Verhoeven, R.H.A. Poor compliance with perioperative chemotherapy for resectable gastric cancer and its impact on survival. Eur. J. Surg. Oncol. 2019, 45, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Sisic, L.; Blank, S.; Nienhuser, H.; Haag, G.M.; Jager, D.; Bruckner, T.; Ott, K.; Schmidt, T.; Ulrich, A. The postoperative part of perioperative chemotherapy fails to provide a survival benefit in completely resected esophagogastric adenocarcinoma. Surg. Oncol. 2020, 33, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.H.; Bowman, C.R.; Reece-Smith, A.M.; Pang, V.; Dorrington, M.S.; Mumtaz, E.; Soomro, I.; Kaye, P.; Madhusudan, S.; Parsons, S.L. The role of adjuvant platinum-based chemotherapy in esophagogastric cancer patients who received neoadjuvant chemotherapy prior to definitive surgery. J. Surg. Oncol. 2017, 115, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Karagkounis, G.; Squires, M.H., 3rd; Melis, M.; Poultsides, G.A.; Worhunsky, D.; Jin, L.X.; Fields, R.C.; Spolverato, G.; Pawlik, T.M.; Votanopoulos, K.I.; et al. Predictors and Prognostic Implications of Perioperative Chemotherapy Completion in Gastric Cancer. J. Gastrointest. Surg. 2017, 21, 1984–1992. [Google Scholar] [CrossRef] [PubMed]
- Lichthardt, S.; Kerscher, A.; Dietz, U.A.; Jurowich, C.; Kunzmann, V.; von Rahden, B.H.; Germer, C.T.; Wiegering, A. Original article: Role of adjuvant chemotherapy in a perioperative chemotherapy regimen for gastric cancer. BMC Cancer 2016, 16, 650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glatz, T.; Bronsert, P.; Schafer, M.; Kulemann, B.; Marjanovic, G.; Sick, O.; Hopt, U.T.; Zirlik, K.; Makowiec, F.; Hoeppner, J. Perioperative platin-based chemotherapy for locally advanced esophagogastric adenocarcinoma: Postoperative chemotherapy has a substantial impact on outcome. Eur. J. Surg. Oncol. 2015, 41, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Luc, G.; Gersen-Cherdieu, H.; Degrandi, O.; Terrebonne, E.; Chiche, L.; Collet, D. Impact of postoperative chemotherapy in patients with locally advanced gastroesophageal adenocarcinoma treated with perioperative chemotherapy strategy. Am. J. Surg. 2015, 210, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.; Pritchard, S.; Welch, I. The postoperative component of MAGIC chemotherapy is associated with improved prognosis following surgical resection in gastric and gastrooesophageal junction adenocarcinomas. Int. J. Surg. Oncol. 2013, 2013, 781742. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Fassan, M.; Cunningham, D.; Allum, W.H.; Okines, A.F.; Lampis, A.; Hahne, J.C.; Rugge, M.; Peckitt, C.; Nankivell, M.; et al. Effect of Pathologic Tumor Response and Nodal Status on Survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. J. Clin. Oncol. 2016, 34, 2721–2727. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Kullmann, F.; Kunzmann, V.; Fuchs, M.; Geissler, M.; Vehling-Kaiser, U.; Stauder, H.; Wein, A.; Al-Batran, S.E.; Kubin, T.; et al. NeoFLOT: Multicenter phase II study of perioperative chemotherapy in resectable adenocarcinoma of the gastroesophageal junction or gastric adenocarcinoma-Very good response predominantly in patients with intestinal type tumors. Int. J. Cancer 2015, 137, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.; Knodler, M.; Giraut, A.; Mauer, M.; Nilsson, M.; Van Grieken, N.; Wagner, A.D.; Moehler, M.; Lordick, F. VESTIGE: Adjuvant Immunotherapy in Patients With Resected Esophageal, Gastroesophageal Junction and Gastric Cancer Following Preoperative Chemotherapy With High Risk for Recurrence (N+ and/or R1): An Open Label Randomized Controlled Phase-2-Study. Front. Oncol. 2019, 9, 1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | All Patients (n = 110) | Perioperative Chemotherapy ° (n = 64) | Preoperative Chemotherapy (n = 46) | p-Value * (Perioperative vs. Preoperative Chemotherapy) | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age at initial diagnosis in years | 0.325 § | ||||||
| Mean (standard deviation) | 62.76 (10.12) | 61.44 (10.01) | 64.61 (10.10) | ||||
| Median (minimum, maximum) | 65 (32, 79) | 64 (32, 79) | 67 (36, 77) | ||||
| Gender | 0.005 | ||||||
| Male | 90 | 81.8% | 58 | 90.6% | 32 | 69.6% | |
| Female | 20 | 18.2% | 6 | 9.4% | 14 | 30.4% | |
| ECOG performance status | 0.621 | ||||||
| 0 | 105 | 95.5% | 60 | 93.8% | 45 | 97.8% | |
| 1 | 5 | 4.5% | 4 | 6.3% | 1 | 2.2% | |
| Primary tumor location | 0.104 | ||||||
| Esophagus | 11 | 10.0% | 6 | 9.4% | 5 | 10.9% | |
| Gastroesophageal junction | 50 | 45.5% | 34 | 53.1% | 16 | 34.8% | |
| Stomach | 49 | 44.5% | 24 | 37.6% | 25 | 54.4% | |
| Histology | 0.609 | ||||||
| Intestinal type | 51 | 46.4% | 28 | 43.8% | 23 | 50.0% | |
| Diffuse type | 36 | 32.7% | 20 | 31.3% | 16 | 34.8% | |
| Mixed type | 10 | 9.1% | 6 | 9.4% | 4 | 8.7% | |
| Not specified | 6 | 5.5% | 4 | 6.3% | 2 | 4.3% | |
| Chemotherapy | <0.001 | ||||||
| ECF/ECX | 61 | 55.5% | 27 | 42.2% | 34 | 73.9% | |
| DCF/DCX | 49 | 44.5% | 37 | 57.8% | 12 | 26.1% | |
| T and N status at baseline | 0.691 | ||||||
| uT2 | 10 | 9.1% | 7 | 10.9% | 3 | 6.5% | 0.218 |
| uT3 | 92 | 83.6% | 52 | 81.3% | 40 | 87.0% | |
| uT4 | 8 | 7.3% | 5 | 7.8% | 3 | 6.5% | |
| uN0 | 16 | 14.5% | 8 | 12.5% | 8 | 17.4% | |
| uN1 | 72 | 65.5% | 39 | 60.9% | 33 | 71.7% | |
| uN2 | 1 | 0.9% | 1 | 1.6% | 0 | 0% | |
| uN+ | 21 | 19.1% | 16 | 25.0% | 5 | 10.9% | |
| T and N status at time of resection | <0.001 | ||||||
| ypT0 | 15 | 13.6% | 8 | 12.5% | 7 | 15.2% | <0.001 |
| ypT1 | 10 | 9.1% | 7 | 10.9% | 3 | 6.5% | |
| ypT2 | 51 | 46.4% | 33 | 51.6% | 18 | 39.1% | |
| ypT3 | 23 | 20.9% | 16 | 25.0% | 7 | 15.2% | |
| ypT4 | 4 | 3.6% | 0 | 0% | 4 | 8.7% | |
| ypN0 | 50 | 45.5% | 29 | 45.3% | 21 | 45.7% | |
| ypN1 | 34 | 30.9% | 26 | 40.6% | 8 | 17.4% | |
| ypN2 | 11 | 10.0% | 8 | 12.5% | 3 | 6.5% | |
| ypN3 | 8 | 7.3% | 1 | 1.6% | 7 | 15.2% | |
| Becker tumor regression grading | 0.001 | ||||||
| 1a (complete response) | 15 | 13.6% | 8 | 12.5% | 7 | 15.2% | |
| 1b (<10% residual tumor) | 8 | 7.3% | 4 | 6.3% | 4 | 8.7% | |
| 2 (10–50% residual tumor) | 33 | 30.0% | 20 | 31.3% | 13 | 28.3% | |
| 3 (>50% residual tumor) | 47 | 42.7% | 32 | 50.0% | 15 | 32.6% | |
| Not available | 7 | 6.3% | 0 | 0% | 7 | 15.2% | |
| Study | Country | Multi-Center, n | Recruitment Period | Primary Tumor Location | Chemotherapy Regimen | Patients | Perioperative vs. Preoperative Chemotherapy Alone | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Esophagus | GEJ | Stomach | Epirubicin Based | Docetaxel Based | Total n | periCTx * | Favors periCTx (OS)? | Reported Statistical Analysis | General Remarks | ||||
| Deng et al., 2021 [6] | USA | yes, n.a. (NCDB) | 2006–2017 | - | - | 100% | n.a. | n.a. | 2382 | 36% | no | HR 0.88 (95%CI 0.75–1.02), p = 0.37 | |
| Drake et al., 2020 [7] | USA | yes, n.a. (NCDB) | 2006–2014 | - | - | 100% | n.a. | n.a. | 3449 | 32% | no | median OS: 56.8 vs. 52.5 mo, p = 0.131; 5-year survival 48.9% vs. 47.3% | PSM applied |
| Papaxoinis et al., 2019 [8] | UK | yes, n = 3 | 2009–2017 | 33% | 67% | - | 99% ECX(like) | - | 312 | 72% | no | median OS: 46.1 vs. 36.7 mo, p = 0.199 | PSM applied; no difference in DFS (22.2 vs. 25.7 mo, p = 0.627) and postrelapse survival (15.3 vs. 8.7 mo, p = 0.122) |
| Coimbra et al., 2019 [9] | Brazil | no | 2006–2016 | - | - | 100% | 30% ECX(like), 59% PF | 11% DCF/ DCX | 225 | 65% | yes | 5-year survival 70.3% vs. 59.9%; HR 0.55 (95%CI 0.33–0.91, p = 0.019) | after exclusion of patients with postoperative death, postoperative treatment did not remain as an independent predictor of survival |
| van Putten et al., 2019 [10] | Netherlands | yes, n.a. (NCR) | 2006–2014 | - | - | 100% | n.a. | n.a. | 1686 | 57% | yes | HR 0.80 (95%CI 0.70–0.93); PSM analysis: HR 0.84 (95%CI 0.71–0.99) | some of the patients received postoperative chemoradiotherapy, proportion not reported |
| Sisic et al., 2017 [11] | Germany | no | 2006–2015 | - | 62% | 38% | 46% ECF(like), 17% others (PF/FLO/OX) | 36% FLOT | 299 | 57% | no | median OS: 78.2 mo vs. n.r., p = 0.331 | no difference in DFS (43.3 vs. 41.1 mo, p = 0.118) |
| Saunders et al., 2017 [12] | UK | no | 2006–2013 | 35% | 47% | 17% | 100% ECX(like) | - | 333 | 57% | n.a. | n.a. | statistical analysis only for subgroups reported, see Table 3 |
| Karagkounis et al., 2017 [13] | USA | yes, n = 8 | 2000–2012 | - | 23% | 73% | 79% ECX(like) | - | 163 | 69% | yes | HR 0.33 (95%CI 0.14–0.82), p = 0.01 | improved DFS (HR 0.52, 95%CI 0.27–0.96) |
| Lichthardt et al., 2016 [14] | Germany | no | 2006–2013 | - | 42% | 57% | ECX/ECF (% n.a.) | FLO(T) (% n.a.) | 72 | 72% | no | trend for shorter survival for periCTx, but not statistically significant (p = 0.101) | after exclusion of two patients with perioperative death (corresponding to all other study protocols), statistically significant shorter 3-year-survival for patients with periCTx: 71.2% vs. 100%, p = 0.038 |
| Glatz et al., 2015 [15] | Germany | no | 2006–2013 | - | 72% | 28% | 43% ECF/EOX | 57% FLOT | 134 | 64% | yes | med. OS: n.r. vs. 44 mo; 5-year survival 75.8% vs. 40.3%, p < 0.001 | |
| Luc et al., 2015 [16] | France | no | 2000–2012 | 18% | 43% | 39% | ECF (% n.a.) | DCF (% n.a.) | 110 | 67% | no | median OS: 43 vs. 20 mo, p = 0.59 | no difference in DFS (35 vs. 11 mo, p = 0.098); additional analysis identified two cycles of postCTx necessary to improve survival (HR 5.13, 95%CI 1.55–16.97, p = 0.007) |
| Mirza et al., 2013 [17] | UK | no | 1996–2010 | - | 64% | 36% | 100% ECF | - | 66 | 47% | yes | significant difference (p = 0.02); HR 0.26, p = 0.008 | |
| Study | Subgroup with Benefit from periCTx | Number of Patients | Subgroup Analysis: periCTx vs. preCTx Alone | |
|---|---|---|---|---|
| n | periCTx * vs. preCTx Alone | |||
| Deng et al., 2021 [6] | good HPR (pTNM < cTNM stage, excluding ypT0N0) | 727 | 255 vs. 472 | improved 5-year survival in periCTx patients with preCTx sensitive disease (73.8% vs. 65.0%; HR 0.64, 95%CI 0.46–0.91, p = 0.02); no benefit from periCTx in subgroups with (i) very sensitive disease (ypT0N0) and (ii) refractory disease (pTNM ≥ cTNM) |
| Drake et al., 2020 [7] | ypN1 (AJCC 8th) | 678 | 222 vs. 456 | improved OS in periCTx patients with ypN1 disease (79.6 vs. 41.3 mo; p = 0.025) |
| Papaxoinis et al., 2019 [8] | R1 | 104 | 69 vs. 35 | improved OS (HR 0.53, 95%CI 0.31–0.90, p=0.018) and DFS (HR 0.56, 95%CI 0.33–0.94, p = 0.027) in periCTx patients with R1 resection |
| ypN0 | 129 | 94 vs. 35 | improved DFS in periCTx patients with tumor-free lymph nodes (HR 0.35, 95%CI 0.13–0.95, p = 0.038); trend for improved OS (HR 0.44; 95%CI 0.19–1.0, p = 0.051) | |
| Sisic et al., 2017 [11] | FLOT | 108 | 74 vs. 34 | improved DFS in periCTx patients receiving FLOT regimen (n.r. vs. 37.7 mo, p = 0.038) |
| nonintestinal tumors | 111 | 65 vs. 46 | improved DFS in periCTx patients with nonintestinal tumors (56.2 vs. 20.3 mo, p = 0.023) | |
| Saunders et al., 2017 [12] | good HPR (TRG 1–3) | 129 | 70 vs. 59 | improved OS in periCTx patients with preCTx responsive disease (HR 0.51, 95%CI 0.28–0.93, p = 0.028) |
| Karagkounis et al., 2017 [13] | stage II (AJCC 7th) | 43 | 26 vs. 17 | improved DFS in periCTx patients with stage II tumors (20% vs. 64.7%, p = 0.003) |
| Glatz et al., 2015 [15] | ypN+ | 56 | 33 vs. 23 | improved 5-year survival in periCTx patients with ypN+ stages (64.5% vs. 9.7%, p = 0.002) |
| poor HPR (>50% vital tumor cells) | 64 | 36 vs. 28 | improved 5-year survival in periCTx patients with poor HPR (55.5% vs. 19.3%, p = 0.015) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballhausen, A.; Bartels, P.; Iacovella, I.; Hoegner, A.; Lorusso, A.; Bichev, D.; Daum, S.; Thuss-Patience, P. Impact of Postoperative Chemotherapy in Patients with Gastric/Gastroesophageal Adenocarcinoma Treated with Perioperative Chemotherapy. Curr. Oncol. 2022, 29, 1983-1996. https://doi.org/10.3390/curroncol29030161
Ballhausen A, Bartels P, Iacovella I, Hoegner A, Lorusso A, Bichev D, Daum S, Thuss-Patience P. Impact of Postoperative Chemotherapy in Patients with Gastric/Gastroesophageal Adenocarcinoma Treated with Perioperative Chemotherapy. Current Oncology. 2022; 29(3):1983-1996. https://doi.org/10.3390/curroncol29030161
Chicago/Turabian StyleBallhausen, Alexej, Prisca Bartels, Ines Iacovella, Anica Hoegner, Alessandro Lorusso, Dmitry Bichev, Severin Daum, and Peter Thuss-Patience. 2022. "Impact of Postoperative Chemotherapy in Patients with Gastric/Gastroesophageal Adenocarcinoma Treated with Perioperative Chemotherapy" Current Oncology 29, no. 3: 1983-1996. https://doi.org/10.3390/curroncol29030161
APA StyleBallhausen, A., Bartels, P., Iacovella, I., Hoegner, A., Lorusso, A., Bichev, D., Daum, S., & Thuss-Patience, P. (2022). Impact of Postoperative Chemotherapy in Patients with Gastric/Gastroesophageal Adenocarcinoma Treated with Perioperative Chemotherapy. Current Oncology, 29(3), 1983-1996. https://doi.org/10.3390/curroncol29030161





