Novel, First-in-Human, Oral PCLX-001 Treatment in a Patient with Relapsed Diffuse Large B-Cell Lymphoma
Abstract
:1. Introduction
2. Materials and Methods
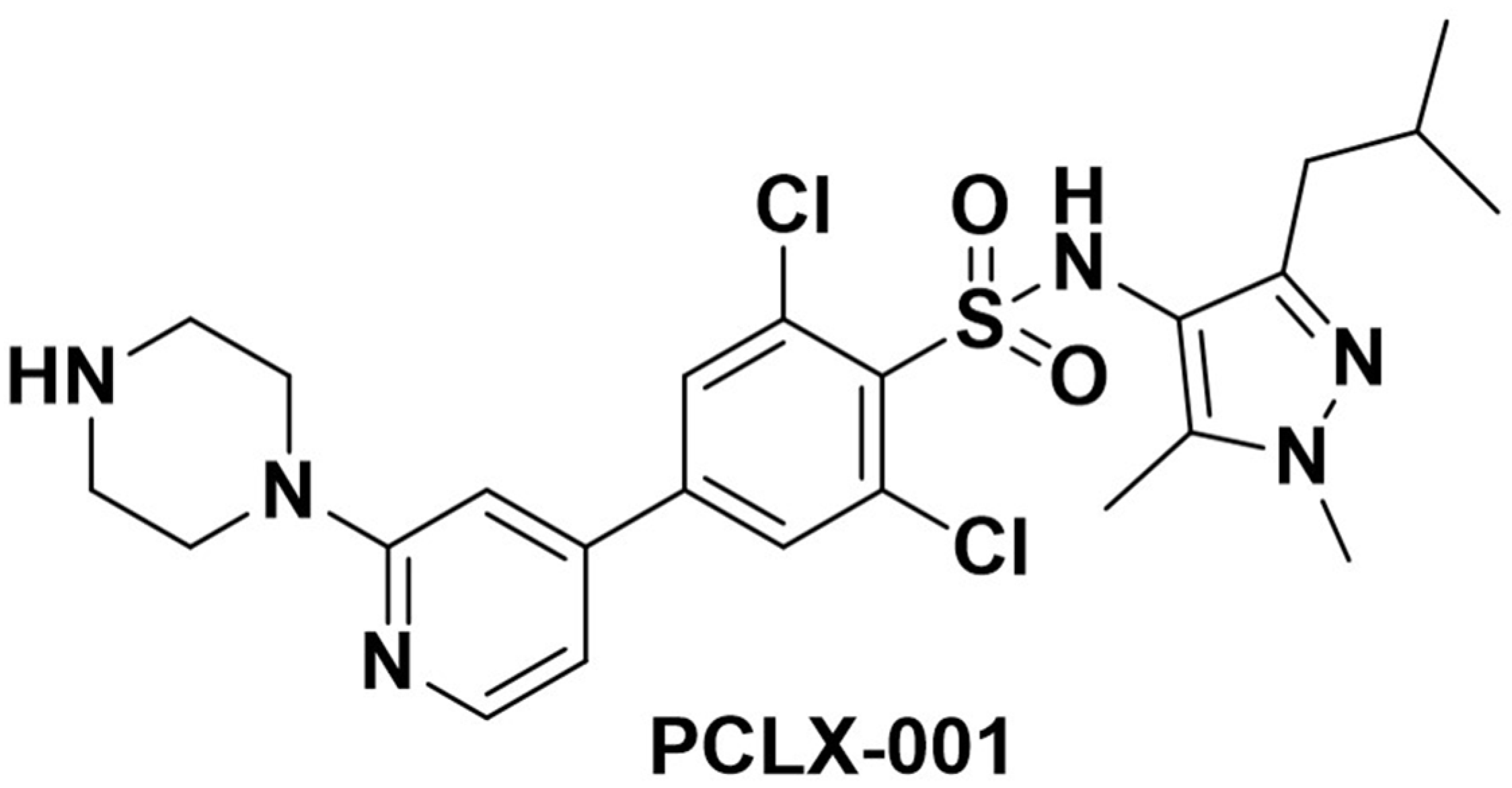
2.1. PCLX-001—The Investigational Agent
2.2. Clinical Trial
2.3. Pharmacokinetic Analysis
2.4. Pharmacodynamic Analysis
2.5. Toxicity Assessment
2.6. Efficacy Assessment
3. Results
3.1. Patient Demographics
3.2. PCLX-001 Administration
3.3. Pharmacokinetics
3.4. Pharmacodynamics
3.5. PCLX-001 Toxicities
3.6. Efficacy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castrec, B.; Dian, C.; Ciccone, S.; Ebert, C.L.; Bienvenut, W.V.; Le Caer, J.P.; Steyaert, J.M.; Giglione, C.; Meinnel, T. Structural and genomic decoding of human and plant myristoylomes reveals a definitive recognition pattern. Nat. Chem. Biol. 2018, 14, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, P.; Lakshmikuttyamma, A.; Shrivastav, A.; Das, S.B.; Dimmock, J.R.; Sharma, R.K. Potential role of N-myristoyltransferase in cancer. Prog. Lipid Res. 2007, 46, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, E.; Yap, M.C.; Iyer, A.; Perinpanayagam, M.A.; Gamma, J.M.; Vincent, K.M.; Lakshmanan, M.; Raju, A.; Tergaonkar, V.; Tan, S.Y.; et al. Targeting N-myristoylation for therapy of B-cell lymphomas. Nat. Commun. 2020, 11, 5348. [Google Scholar] [CrossRef] [PubMed]
- Mackey, J.R.; Lai, J.; Chauhan, U.; Beauchamp, E.; Dong, W.F.; Glubrecht, D.; Sim, Y.W.; Ghosh, S.; Bigras, G.; Lai, R.; et al. N-myristoyltransferase proteins in breast cancer: Prognostic relevance and validation as a new drug target. Breast Cancer Res. Treat. 2021, 186, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.; Dillberger, J.; Mackey, J.R.; Wyatt, P.; Gray, D.; Read, K.; Li, C.; Parenteau, A.; Berthiaume, L.G. Initial Characterization and Toxicology of an Nmt Inhibitor in Development for Hematologic Malignancies. Blood 2019, 134, 3362. [Google Scholar] [CrossRef]
- Sangha, R.; Mackey, J.R.; Sehn, L.H.; Kuruvilla, J.; Weickert, M.J.; Berthiaume, L.G. An Open-Label, First-in-Human, Phase I Trial of Daily Pclx-001. Blood 2021, 138, 1364. [Google Scholar] [CrossRef]
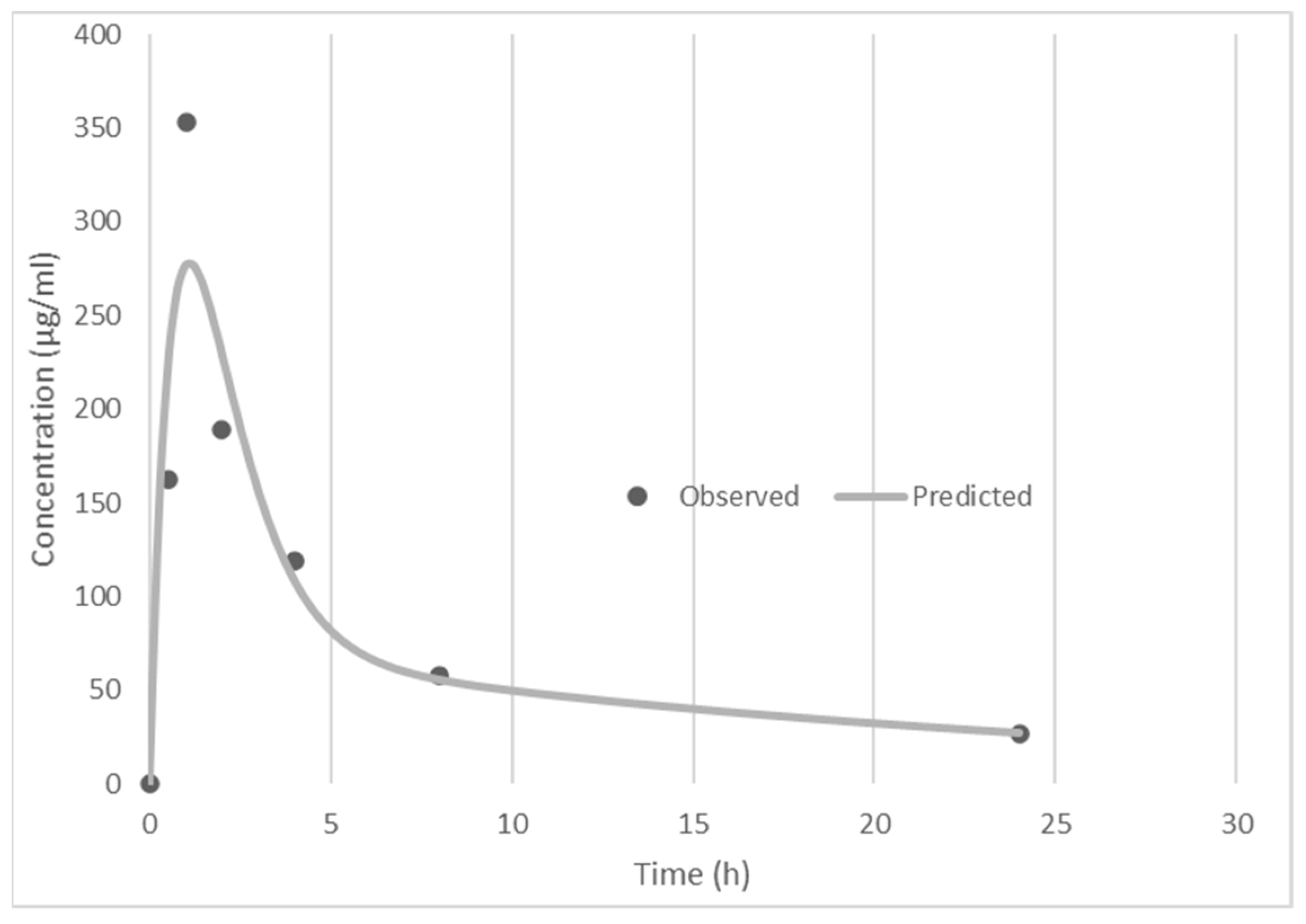
| Pharmacokinetic Parameters | Data |
|---|---|
| Cmax (ng/mL) | 278.23 |
| Tmax (h) | 1.1 |
| AUC0-tlast (ng/Ml.h) | 1701.33 |
| AUC0–∞ (ng/mL.h)\ | 2355.81 |
| Day 15 AUC0–∞ (ng/mL.h) | 2635.63 |
| MRT (h) | 18.63 |
| T ½ (h) | 16.42 |
| AUMC (ng/mL.h2) | 43,883.76 |
| CLoral L/h | 8.4 |
| Vdss (L) | 156.49 |
| Day 22 pre-dose (ng/mL) | 51.4 |
| Cell Populations | Screening Sample (%) | Day 1 (%) | Day 28 (%) |
|---|---|---|---|
| Total T cells | 66.6 ± 0.52 | 66.7 ± 0.51 | 70.1 ± 0.50 |
| CD4+ T cells | 40.2 ± 0.24 | 42.5 ± 0.24 | 46.8 ± 0.22 |
| CD8+ T cells | 25.1 ± 0.33 | 23.1 ± 0.34 | 22.5 ± 0.31 |
| CD19+ B cells | 0.71 ± 0.66 | 1.18 ± 0.51 | 0.59 ± 0.50 |
| CD14+ monocytes | 5.01 ± 0.4 | 14.2 ± 0.40 | 4.51 ± 0.42 |
| Cell Populations | Screening Sample (%) | Day 1 (%) | Day 28 (%) |
|---|---|---|---|
| CD4+ T cells | |||
| HGAL | 12.7 ± 0.57 | 22.1 ± 0.73 | 8.6 ± 0.58 |
| Lyn | 13.7 ± 0.28 | 20 ± 0.28 | 28.7 ± 0.37 |
| NMT1 | 2.99 ± 0.69 | 4.87 ± 0.63 | 2.22 ± 0.63 |
| NMT2 | 22.8 ± 0.33 | 25.7 ± 0.31 | 35.3 ± 0.36 |
| CD8+ T cells | |||
| HGAL | 25 ± 0.61 | 30.3 ± 0.58 | 17 ± 0.63 |
| Lyn | 17.6 ± 0.17 | 25.8 ± 0.28 | 33.3 ± 0.31 |
| NMT1 | 0.94 ± 0.55 | 0.85 ± 0.64 | 0.77 ± 0.38 |
| NMT2 | 11 ± 0.11 | 12.7 ± 0.27 | 18.7 ± 0.33 |
| CD19+ B cells | |||
| HGAL | 2.44 ± 0.41 | 11.2 ± 0.51 | 8.33 ± 0.39 |
| Lyn | 8.33 ± 0.29 | 12.4 ± 0.27 | 12.1 ± 0.15 |
| NMT1 | 0.98 ± 0.41 | 2.65 ± 0.71 | 2.42 ± 0.42 |
| NMT2 | 31.8 ± 0.29 | 29 ± 0.3 | 33.3 ± 0.24 |
| CD14+ Monocytes | |||
| HGAL | 83.9 ± 0.64 | 87.5 ± 0.65 | 88.3 ± 0.64 |
| Lyn | 51.4 ± 0.50 | 40.5 ± 0.39 | 66.6 ± 0.59 |
| NMT1 | 1.59 ± 0.19 | 1.37 ± 0.33 | 4.55 ± 0.30 |
| NMT2 | 6.21 ± 0.45 | 6.7 ± 1.13 | 11.7 ± 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangha, R.; Davies, N.M.; Namdar, A.; Chu, M.; Spratlin, J.; Beauchamp, E.; Berthiaume, L.G.; Mackey, J.R. Novel, First-in-Human, Oral PCLX-001 Treatment in a Patient with Relapsed Diffuse Large B-Cell Lymphoma. Curr. Oncol. 2022, 29, 1939-1946. https://doi.org/10.3390/curroncol29030158
Sangha R, Davies NM, Namdar A, Chu M, Spratlin J, Beauchamp E, Berthiaume LG, Mackey JR. Novel, First-in-Human, Oral PCLX-001 Treatment in a Patient with Relapsed Diffuse Large B-Cell Lymphoma. Current Oncology. 2022; 29(3):1939-1946. https://doi.org/10.3390/curroncol29030158
Chicago/Turabian StyleSangha, Randeep, Neal M. Davies, Afshin Namdar, Michael Chu, Jennifer Spratlin, Erwan Beauchamp, Luc G. Berthiaume, and John R. Mackey. 2022. "Novel, First-in-Human, Oral PCLX-001 Treatment in a Patient with Relapsed Diffuse Large B-Cell Lymphoma" Current Oncology 29, no. 3: 1939-1946. https://doi.org/10.3390/curroncol29030158
APA StyleSangha, R., Davies, N. M., Namdar, A., Chu, M., Spratlin, J., Beauchamp, E., Berthiaume, L. G., & Mackey, J. R. (2022). Novel, First-in-Human, Oral PCLX-001 Treatment in a Patient with Relapsed Diffuse Large B-Cell Lymphoma. Current Oncology, 29(3), 1939-1946. https://doi.org/10.3390/curroncol29030158






