A Population-Based Study to Evaluate the Associations of Nodal Stage, Lymph Node Ratio and Log Odds of Positive Lymph Nodes with Survival in Patients with Small Bowel Adenocarcinoma
Abstract
1. Introduction
2. Methods
2.1. Study Design and Data Sources
2.2. Study Population
2.3. Clinical Variables and Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Lymph Node Burden
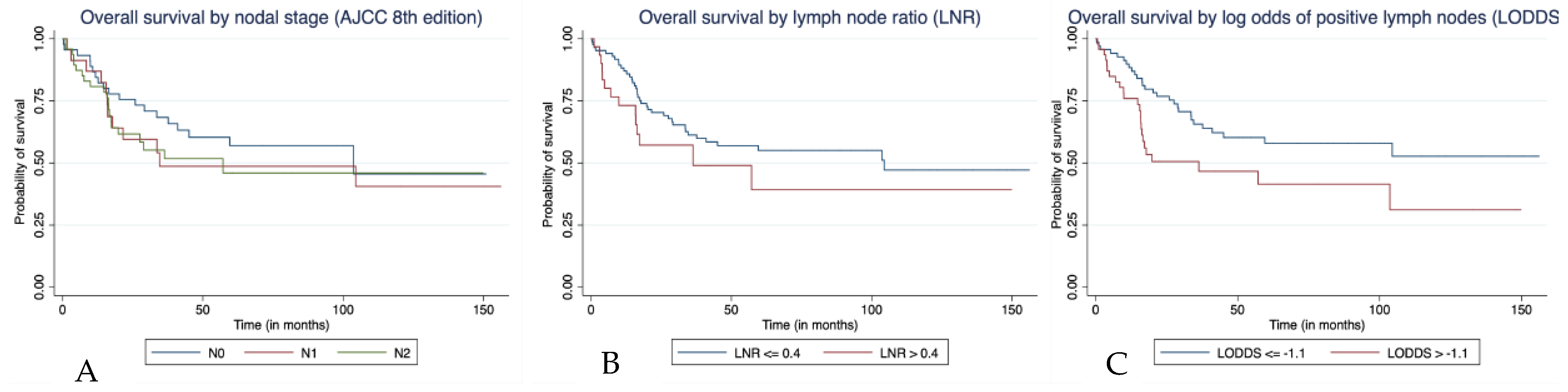
3.3. Survival Outcomes in Patients Who Underwent Lymph Node Dissection
3.4. Factors Predicting Survival in Patients with Positive Lymph Nodes
3.5. Factors Predicting Survival in Patients without Lymph Node Involvement
3.6. Survival Outcomes of Patients without Lymph Node Dissection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee on Cancer |
| LNR | Lymph Node Ratio |
| LOPLN | Log Odds Of Positive Lymph Nodes |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| AIC | Akaike’s Information Criterion |
| HR | Hazard Ratio |
| CI | Confidence Interval |
References
- Cancer of the Small Intestine—Cancer Stat Facts [Internet]. SEER. Available online: https://seer.cancer.gov/statfacts/html/smint.html (accessed on 5 May 2020).
- Aparicio, T.; Zaanan, A.; Svrcek, M.; Laurent-Puig, P.; Carrere, N.; Manfredi, S.; Locher, C.; Afchain, P. Small bowel adenocarcinoma: Epidemiology, risk factors, diagnosis and treatment. Dig. Liver Dis. 2014, 46, 97–104. [Google Scholar] [CrossRef]
- Pan, S.Y.; Morrison, H. Epidemiology of cancer of the small intestine. World J. Gastrointest. Oncol. 2011, 3, 33–42. [Google Scholar] [CrossRef]
- Bilimoria, K.Y.; Bentrem, D.J.; Wayne, J.D.; Ko, C.Y.; Bennett, C.L.; Talamonti, M.S. Small bowel cancer in the United States: Changes in epidemiology, treatment, and survival over the last 20 years. Ann. Surg. 2009, 249, 63–71. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics, 2009. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; McWilliams, R.R.; Donohue, J.H.; Quevedo, J.F. A single-institution experience with 491 cases of small bowel adenocarcinoma. Am. J. Surg. 2010, 199, 797–803. [Google Scholar] [CrossRef]
- Dabaja, B.S.; Suki, D.; Pro, B.; Bonnen, M.; Ajani, J. Adenocarcinoma of the small bowel: Presentation, prognostic factors, and outcome of 217 patients. Cancer 2004, 101, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.W.; Rha, S.Y.; Shin, S.J.; Chang, H.; Shim, H.S.; Roh, J.K. Adenocarcinoma of the small bowel at a single Korean institute: Management and prognosticators. J. Cancer Res. Clin. Oncol. 2010, 136, 387–394. [Google Scholar] [CrossRef]
- Shen, S.S.; Haupt, B.X.; Ro, J.Y.; Zhu, J.; Bailey, H.R.; Schwartz, M.R. Number of Lymph Nodes Examined and Associated Clinicopathologic Factors in Colorectal Carcinoma. Arch. Pathol. Lab. Med. 2009, 133, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhou, Z.; Song, Y.; Dang, C.; Zhang, H. Surgical Management and Prognostic Prediction of Adenocarcinoma of Jejunum and Ileum. Sci. Rep. 2017, 7, 15163. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5680303/ (accessed on 5 May 2020). [CrossRef] [PubMed]
- Overman, M.J.; Hu, C.-Y.; Wolff, R.A.; Chang, G.J. Prognostic value of lymph node evaluation in small bowel adenocarcinoma. Cancer 2010, 116, 5374–5382. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-40617-6. Available online: https://www.springer.com/gp/book/9783319406176 (accessed on 5 May 2020).
- Pei, J.-P.; Zhang, C.-D.; Fan, Y.-C.; Dai, D.-Q. Comparison of Different Lymph Node Staging Systems in Patients With Resectable Colorectal Cancer. Front. Oncol. 2019, 8, 671. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6340930/ (accessed on 5 May 2020). [CrossRef] [PubMed]
- Sabbagh, C.; Mauvais, F.; Cosse, C.; Rebibo, L.; Joly, J.-P.; Dromer, D.; Aubert, C.; Carton, S.; Dron, B.; Dadamessi, I.; et al. A Lymph Node Ratio of 10% Is Predictive of Survival in Stage III Colon Cancer: A French Regional Study. Int. Surg. 2014, 99, 344–353. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhong, S.; Li, Z.; Zhu, X.; Wu, F.; Li, Y. Pathologic lymph node ratio is a predictor of esophageal carcinoma patient survival: A literature-based pooled analysis. Oncotarget 2017, 8, 62231–62239. [Google Scholar] [CrossRef] [PubMed]
- You, M.S.; Lee, S.H.; Choi, Y.H.; Shin, B.; Paik, W.H.; Ryu, J.K.; Kim, Y.-T.; Jang, D.K.; Lee, J.K.; Kwon, W.; et al. Lymph node ratio as valuable predictor in pancreatic cancer treated with R0 resection and adjuvant treatment. BMC Cancer 2019, 19, 952. [Google Scholar] [CrossRef] [PubMed]
- Bouliaris, K.; Rachiotis, G.; Diamantis, A.; Christodoulidis, G.; Polychronopoulou, E.; Tepetes, K. Lymph node ratio as a prognostic factor in gastric cancer patients following D1 resection. Comparison with the current TNM staging system. Eur. J. Surg. Oncol. 2017, 43, 1350–1356. [Google Scholar] [CrossRef]
- Persiani, R.; Cananzi, F.C.M.; Biondi, A.; Paliani, G.; Tufo, A.; Ferrara, F.; Vigorita, V.; D’Ugo, D. Log odds of positive lymph nodes in colon cancer: A meaningful ratio-based lymph node classification system. World J. Surg. 2012, 36, 667–674. [Google Scholar] [CrossRef]
- Wang, J.; Hassett, J.M.; Dayton, M.T.; Kulaylat, M.N. The prognostic superiority of log odds of positive lymph nodes in stage III colon cancer. J. Gastrointest. Surg. 2008, 12, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet Lond. Engl. 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Compton, C.C.; Fielding, L.P.; Burgart, L.J.; Conley, B.; Cooper, H.S.; Hamilton, S.R.; Hammond, M.E.; Henson, D.E.; Hutter, R.V.; Nagle, R.B.; et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch. Pathol. Lab. Med. 2000, 124, 979–994. [Google Scholar] [CrossRef]
- Nelson, H.; Petrelli, N.; Carlin, A.; Couture, J.; Fleshman, J.; Guillem, J.; Miedema, B.; Ota, D.; Sargent, D. National Cancer Institute Expert Panel Guidelines 2000 for colon and rectal cancer surgery. J. Natl. Cancer Inst. 2001, 93, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Sobin, L.H.; Greene, F.L. TNM classification: Clarification of number of regional lymph nodes for pNo. Cancer 2001, 92, 452. [Google Scholar] [CrossRef]
- De Ridder, M.; Vinh-Hung, V.; Van Nieuwenhove, Y.; Hoorens, A.; Sermeus, A.; Storme, G. Prognostic value of the lymph node ratio in node positive colon cancer. Gut 2006, 55, 1681. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, C.-H.; Li, Y.-Y.; Zhang, Q.-W.; Biondi, A.; Fico, V.; Persiani, R.; Ni, X.-C.; Luo, M. The Prognostic Impact of the Metastatic Lymph Nodes Ratio in Colorectal Cancer. Front. Oncol. 2018, 8, 628. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6305371/ (accessed on 13 May 2020). [CrossRef]
- Fortea-Sanchis, C.; Martínez-Ramos, D.; Escrig-Sos, J. The lymph node status as a prognostic factor in colon cancer: Comparative population study of classifications using the logarithm of the ratio between metastatic and nonmetastatic nodes (LODDS) versus the pN-TNM classification and ganglion ratio systems. BMC Cancer 2018, 18, 1208. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.C.; Sigurdson, E.R.; LeVoyer, T.; Hanlon, A.; Mayer, R.J.; Macdonald, J.S.; Catalano, P.J.; Haller, D.G. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J. Clin. Oncol. 2005, 23, 8706–8712. [Google Scholar] [CrossRef]
- Harrell, F.E.; Califf, R.M.; Pryor, D.B.; Lee, K.L.; Rosati, R.A. Evaluating the yield of medical tests. JAMA 1982, 247, 2543–2546. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Occhionorelli, S.; Andreotti, D.; Vallese, P.; Morganti, L.; Lacavalla, D.; Forini, E.; Pascale, G. Evaluation on prognostic efficacy of lymph nodes ratio (LNR) and log odds of positive lymph nodes (LODDS) in complicated colon cancer: The first study in emergency surgery. World J. Surg. Oncol. 2018, 16, 186. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6137917/ (accessed on 6 May 2020). [CrossRef] [PubMed]
- Zhou, Y.-Y.; Du, X.-J.; Zhang, C.-H.; Aparicio, T.; Zaanan, A.; Afchain, P.; Chen, L.-P.; Hu, S.-K.; Zhang, P.-C.; Wu, M.; et al. Comparison of three lymph node staging schemes for predicting the outcome in patients with small bowel adenocarcinoma: A population-based cohort and international multicentre cohort study. EBioMedicine 2019, 41, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Howe, J.R.; Karnell, L.H.; Menck, H.R.; Scott-Conner, C. The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: Review of the National Cancer Data Base, 1985–1995. Cancer 1999, 86, 2693–2706. [Google Scholar] [CrossRef]

| Variable | N (%) |
|---|---|
| Age at diagnosis, in years | |
| Median | 67 |
| Interquartile range | 30–89 |
| Sex | |
| Females | 64 (45.4%) |
| Males | 77 (54.6%) |
| Site | |
| Duodenum | 78 (55.3%) |
| Jejunum | 37 (26.2%) |
| Ileum | 26 (18.4%) |
| Histopathological subtype | |
| Adenocarcinoma | 103 (73.0%) |
| Adenocarcinoma in tubulovillous adenoma | 11 (7.8%) |
| Adenocarcinoma in adenomatous polyp | 9 (6.4%) |
| Adenocarcinoma in villous adenoma | 4 (2.8%) |
| Mucinous | 9 (6.4%) |
| Intestinal | 3 (2.1%) |
| Adenocarcinoma with mixed subtypes | 2(1.4%) |
| Lymph nodes resected | |
| 0 | 25 (17.7%) * |
| 1–11 | 45 (31.9%) |
| ≥12 | 71 (50.4%) |
| AJCC (8th edition) Stage (n = 115) | |
| I | 6 (5.2%) |
| II | 38 (33.0%) |
| III | 71 (61.7%) |
| AJCC (8th edition) T stage (n = 115) | |
| T1 | 4 (3.5%) |
| T2 | 4 (3.5%) |
| T3 | 49 (42.6%) |
| T4 | 58 (50.4%) |
| Histological grade (n = 115) | |
| Grade I/II | 88 (76.5%) |
| Grade III/IV | 27 (23.5%) |
| Lymphovascular invasion (n = 87) | |
| Yes | 42 (48.3%) |
| No | 45 (51.7%) |
| Zone of residence | |
| Calgary | 51 |
| Central | 39 |
| Edmonton | 21 |
| North | 19 |
| South | 6 |
| Unknown | 5 |
| Variable | Parameter | Duodenal | Jejunal | Ileal |
|---|---|---|---|---|
| (n = 56) | (n = 35) | (n = 25) | ||
| AJCC N stage (8th edition) | ||||
| N0 | 45 (38.8%) | 15 (26.8%) | 16 (45.7%) | 14 (56.0%) |
| N1 | 23 (19.8%) | 13 (23.2%) | 7 (20.0%) | 3 (12.0%) |
| N2 | 48 (41.4%) | 28 (50.0%) | 12 (34.3%) | 8 (32.0%) |
| Lymph Node ratio (LNR) | ||||
| <0.4 | 85 (73.3%) | 38 (67.9%) | 28 (80.0%) | 19 (76.0%) |
| >0.4 | 31 (26.7%) | 18 (32.1%) | 7 (20.0%) | 6 (24.0%) |
| Log odds of positive lymph nodes | ||||
| (LOPLN) | ||||
| <−1.1 | 69 (59.5%) | 27 (48.2%) | 23 (65.7%) | 19 (76.0%) |
| >−1.1 | 47 (40.5%) | 29 (51.8%) | 12 (34.3%) | 6 (24.0%) |
| Variable | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| Age at diagnosis | 1.03 (0.99–1.06) | 0.111 | 1.02 (0.99–1.06) | 0.123 | 1.02 (0.99–1.05) | 0.129 |
| Sex | ||||||
| Female | ||||||
| Male | 1.33 (0.56–3.15) | 0.511 | 1.21 (0.52–2.84) | 0.655 | 1.68 (0.7–4.07) | 0.246 |
| Primary site | ||||||
| Duodenum | ||||||
| Jejunum | 0.16 (0.04–0.56) | 0.004 | 0.20 (0.06–0.72) | 0.014 | 0.15 (0.04–0.56) | 0.005 |
| Ileum | 1.73 (0.69–4.36) | 0.242 | 1.49 (0.60–3.74) | 0.390 | 1.70 (0.66–4.38) | 0.273 |
| AJCC tumour stage | ||||||
| T1 | ||||||
| T2 | 0.74 (0.04–14.93) | 0.842 | 0.88 (0.04–17.58) | 0.933 | 1.08 (0.05–21.98) | 0.96 |
| T3 | 0.64 (0.07–5.69) | 0.689 | 0.75 (0.09–6.43) | 0.795 | 0.63 (0.07–5.50) | 0.677 |
| T4 | 1.24 (0.14–11.06) | 0.845 | 1.83 (0.22–15.44) | 0.577 | 1.65 (0.19–13.94) | 0.647 |
| Grade | ||||||
| 1–2 | ||||||
| 3–4 | 0.78 (0.25–2.46) | 0.673 | 0.68 (0.19–2.36) | 0.541 | 0.42 (0.11–1.53) | 0.187 |
| Lymphovascular invasion | ||||||
| No | ||||||
| Yes | 0.97 (0.36–2.60) | 0.952 | 1.05 (0.41–2.71) | 0.917 | 1.19 (0.47–3.01) | 0.707 |
| Lymph nodes resected | ||||||
| <12 | ||||||
| ≥12 | 0.86 (0.37–1.99) | 0.724 | 1.50 (0.59–3.85) | 0.392 | 1.61 (0.66–3.92) | 0.299 |
| AJCC Nodal stage | ||||||
| 0 | ||||||
| 1 | 1.83 (0.61–5.45) | 0.280 | ||||
| 2 | 3.71 (1.19–11.57) | 0.024 | ||||
| Lymph node ratio (LNR) ≤0.4 | ||||||
| >0.4 | 4.39 (1.33–14.05) | |||||
| Log odds of positive lymph nodes (LOPLN) | ||||||
| ≤−1.1 | 5.97 (1.92–18.57) | 0.002 | ||||
| >−1.1 | ||||||
| Variable | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| Age at diagnosis | 0.99 (0.95–1.04) | 0.833 | 1.01 (0.96–1.07) | 0.732 | 1.03 (0.97–1.09) | 0.276 |
| Sex | ||||||
| Female | ||||||
| Male | 1.22 (0.32–4.62) | 0.765 | 1.72 (0.36–8.34) | 0.500 | 8.54 (1.40–54.63) | 0.020 |
| Primary site | ||||||
| Duodenum | ||||||
| Jejunum | 0.06 (0.01–0.47) | 0.008 | 0.08 (0.01–0.69) | 0.021 | 0.03 (0.03–0.30) | 0.003 |
| Ileum | 1.15 (0.27–4.90) | 0.850 | 1.13 (0.24–5.39) | 0.879 | 2.99 (0.63–14.24) | 0.170 |
| AJCC T stage | ||||||
| T1/2 | - | |||||
| T3 | Ref | Ref | Ref | |||
| T4 | 2.44 (0.73–8.11) | 0.146 | 3.81 (1.13–12.87) | 0.031 | 8.80 (2.26–34.32) | 0.002 |
| Grade | ||||||
| 1–2 | ||||||
| 3–4 | 0.78 (0.22–2.81) | 0.707 | 0.41 (0.07–2.39) | 0.320 | 0.06 (0.01–0.56) | 0.014 |
| Lymphovascular invasion | ||||||
| No | ||||||
| Yes | 1.00 (0.23–4.25) | 0.997 | 0.75 (0.17–3.38) | 0.709 | 1.11 (0.30–4.09) | 0.876 |
| Lymph nodes resected | ||||||
| <12 | ||||||
| ≥12 | 0.86 (0.25–3.00) | 0.815 | 2.28 (0.43–12.07) | 0.333 | 4.48 (0.86–23.50) | 0.076 |
| AJCC Nodal stage | ||||||
| 1 | Ref | |||||
| 2 | 1.82 (0.56–5.93) | 0.317 | ||||
| Lymph node ratio (LNR) ≤0.4 | ||||||
| >0.4 | 5.64 (1.02–30.90) | 0.046 | ||||
| Log odds of positive lymph nodes (LOPLN) | ||||||
| ≤−1.1 | 31.75 (4.30–234.16) | 0.001 | ||||
| >−1.1 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batra, A.; Kong, S.; Hannouf, M.B.; Cheung, W.Y. A Population-Based Study to Evaluate the Associations of Nodal Stage, Lymph Node Ratio and Log Odds of Positive Lymph Nodes with Survival in Patients with Small Bowel Adenocarcinoma. Curr. Oncol. 2022, 29, 1298-1308. https://doi.org/10.3390/curroncol29030110
Batra A, Kong S, Hannouf MB, Cheung WY. A Population-Based Study to Evaluate the Associations of Nodal Stage, Lymph Node Ratio and Log Odds of Positive Lymph Nodes with Survival in Patients with Small Bowel Adenocarcinoma. Current Oncology. 2022; 29(3):1298-1308. https://doi.org/10.3390/curroncol29030110
Chicago/Turabian StyleBatra, Atul, Shiying Kong, Malek B. Hannouf, and Winson Y. Cheung. 2022. "A Population-Based Study to Evaluate the Associations of Nodal Stage, Lymph Node Ratio and Log Odds of Positive Lymph Nodes with Survival in Patients with Small Bowel Adenocarcinoma" Current Oncology 29, no. 3: 1298-1308. https://doi.org/10.3390/curroncol29030110
APA StyleBatra, A., Kong, S., Hannouf, M. B., & Cheung, W. Y. (2022). A Population-Based Study to Evaluate the Associations of Nodal Stage, Lymph Node Ratio and Log Odds of Positive Lymph Nodes with Survival in Patients with Small Bowel Adenocarcinoma. Current Oncology, 29(3), 1298-1308. https://doi.org/10.3390/curroncol29030110




