Patterns of Pretreatment Diagnostic Assessment in Patients Treated with Stereotactic Body Radiation Therapy (SBRT) for Non-Small Cell Lung Cancer (NSCLC): Special Characteristics in the COVID Pandemic and Influence on Outcomes
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients and Study Design
2.2. Stereotactic Body Radiation Therapy
2.3. Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. Comparison of Pretreatment Assessment in the pre-COVID Era and in the COVID Era
3.3. Influence of Pretreatment Diagnostic Assessment on Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zentrum für Krebsregisterdaten. Krebs in Deutschland für 2015/2016. Available online: https://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/kid_2019/krebs_in_deutschland_2019.pdf;jsessionid=B371F720965F4D3A2D70BBF503024020.2_cid290?__blob=publicationFile (accessed on 19 July 2021).
- McPhail, S.; Johnson, S.; Greenberg, D.; Peake, M.; Rous, B. Stage at diagnosis and early mortality from cancer in England. Br. J. Cancer 2015, 112 (Suppl. S1), S108–S115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebastian, N.T.; Xu-Welliver, M.; Williams, T.M. Stereotactic body radiation therapy (SBRT) for early stage non-small cell lung cancer (NSCLC): Contemporary insights and advances. J. Thorac. Dis. 2018, 10, S2451–S2464. [Google Scholar] [CrossRef] [PubMed]
- Ball, D.; Mai, G.T.; Vinod, S.; Babington, S.; Ruben, J.; Kron, T.; Chesson, B.; Herschtal, A.; Vanevski, M.; Rezo, A.; et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): A phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019, 20, 494–503. [Google Scholar] [CrossRef]
- Jacobs, C.D.; Mehta, K.; Gao, J.; Wang, X.; Salama, J.K.; Kelsey, C.R.; Torok, J.A. Nomogram Predicting Overall Survival Benefit of Stereotactic Ablative Radiotherapy for Early-Stage Non-Small Cell Lung Cancer. Clin. Lung Cancer 2021, in press. [Google Scholar] [CrossRef]
- Timmerman, R.; Heinzerling, J.; Abdulrahman, R.; Choy, H.; Meyer, J.L. Stereotactic body radiation therapy for thoracic cancers: Recommendations for patient selection, setup and therapy. Front. Radiat. Ther. Oncol. 2011, 43, 395–411. [Google Scholar] [CrossRef]
- van Tinteren, H.; Hoekstra, O.S.; Smit, E.F.; van den Bergh, J.H.; Schreurs, A.J.M.; Stallaert, R.A.; van Velthoven, P.C.M.; Comans, E.F.I.; Diepenhorst, F.W.; Verboom, P.; et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: The PLUS multicentre randomised trial. Lancet 2002, 359, 1388–1392. [Google Scholar] [CrossRef]
- Mohammed, N.; Kestin, L.L.; Grills, I.S.; Battu, M.; Fitch, D.L.; Wong, C.-Y.O.; Margolis, J.H.; Chmielewski, G.W.; Welsh, R.J. Rapid disease progression with delay in treatment of non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 466–472. [Google Scholar] [CrossRef]
- Videtic, G.M.M.; Donington, J.; Giuliani, M.; Heinzerling, J.; Karas, T.Z.; Kelsey, C.R.; Lally, B.E.; Latzka, K.; Lo, S.S.; Moghanaki, D.; et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract. Radiat. Oncol. 2017, 7, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Cornwell, L.D.; Korb, M.L.; Burt, B.M. Guidelines for stereotactic body radiation therapy treatment of lung cancer highlight important research questions: What is the next step? J. Thorac. Dis. 2018, 10, 1339–1342. [Google Scholar] [CrossRef] [Green Version]
- Schneider, B.J.; Daly, M.E.; Kennedy, E.B.; Antonoff, M.B.; Broderick, S.; Feldman, J.; Jolly, S.; Meyers, B.; Rocco, G.; Rusthoven, C.; et al. Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J. Clin. Oncol. 2018, 36, 710–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guckenberger, M.; Andratschke, N.; Dieckmann, K.; Hoogeman, M.S.; Hoyer, M.; Hurkmans, C.; Tanadini-Lang, S.; Lartigau, E.; Méndez Romero, A.; Senan, S.; et al. ESTRO ACROP consensus guideline on implementation and practice of stereotactic body radiotherapy for peripherally located early stage non-small cell lung cancer. Radiother. Oncol. 2017, 124, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Combs, S.E.; Belka, C.; Niyazi, M.; Corradini, S.; Pigorsch, S.; Wilkens, J.; Grosu, A.L.; Guckenberger, M.; Ganswindt, U.; Bernhardt, D. First statement on preparation for the COVID-19 pandemic in large German Speaking University-based radiation oncology departments. Radiat. Oncol. 2020, 15, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guckenberger, M.; Belka, C.; Bezjak, A.; Bradley, J.; Daly, M.E.; DeRuysscher, D.; Dziadziuszko, R.; Faivre-Finn, C.; Flentje, M.; Gore, E.; et al. Practice recommendations for lung cancer radiotherapy during the COVID-19 pandemic: An ESTRO-ASTRO consensus statement. Radiother. Oncol. 2020, 146, 223–229. [Google Scholar] [CrossRef]
- Jacob, L.; Loosen, S.H.; Kalder, M.; Luedde, T.; Roderburg, C.; Kostev, K. Impact of the COVID-19 Pandemic on Cancer Diagnoses in General and Specialized Practices in Germany. Cancers 2021, 13, 408. [Google Scholar] [CrossRef]
- AWMF. S3-Leitlinie: Prävention, Diagnostik, Therapie und Nachsorge des Lungenkarzinoms. Available online: https://www.awmf.org/uploads/tx_szleitlinien/020-007OL_l_S3_Lungenkarzinom_2018-03.pdf (accessed on 19 July 2021).
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Gross, A.; Ziepert, M.; Scholz, M. KMWin—a convenient tool for graphical presentation of results from Kaplan-Meier survival time analysis. PLoS ONE 2012, 7, e38960. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Schmitt, D.; Blanck, O.; Gauer, T.; Fix, M.K.; Brunner, T.B.; Fleckenstein, J.; Loutfi-Krauss, B.; Manser, P.; Werner, R.; Wilhelm, M.-L.; et al. Technological quality requirements for stereotactic radiotherapy: Expert review group consensus from the DGMP Working Group for Physics and Technology in Stereotactic Radiotherapy. Strahlenther Onkol. 2020, 196, 421–443. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.gov. Durvalumab vs. Placebo with Stereotactic Body Radiation Therapy in Early Stage Unresected Non-Small Cell Lung Cancer Patients (PACIFIC-4, NCT03833154). Available online: https://clinicaltrials.gov/ct2/show/NCT03833154 (accessed on 20 July 2021).
- Boilève, A.; Stoclin, A.; Barlesi, F.; Varin, F.; Suria, S.; Rieutord, A.; Blot, F.; Netzer, F.; Scotté, F. COVID-19 management in a cancer center: The ICU storm. Support. Care Cancer 2020, 28, 5037–5044. [Google Scholar] [CrossRef]
- Donovan, E.K.; Swaminath, A. Stereotactic body radiation therapy (SBRT) in the management of non-small-cell lung cancer: Clinical impact and patient perspectives. Lung Cancer 2018, 9, 13–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Febbo, J.A.; Gaddikeri, R.S.; Shah, P.N. Stereotactic Body Radiation Therapy for Early-Stage Non-Small Cell Lung Cancer: A Primer for Radiologists. Radiographics 2018, 38, 1312–1336. [Google Scholar] [CrossRef] [PubMed]
- Couñago, F.; Navarro-Martin, A.; Luna, J.; Rodríguez de Dios, N.; Rodríguez, A.; Casas, F.; García, R.; Gómez-Caamaño, A.; Contreras, J.; Serrano, J. GOECP/SEOR clinical recommendations for lung cancer radiotherapy during the COVID-19 pandemic. World J. Clin. Oncol. 2020, 11, 510–527. [Google Scholar] [CrossRef]
- Jazieh, A.R.; Akbulut, H.; Curigliano, G.; Rogado, A.; Alsharm, A.A.; Razis, E.D.; Mula-Hussain, L.; Errihani, H.; Khattak, A.; de Guzman, R.B.; et al. Impact of the COVID-19 Pandemic on Cancer Care: A Global Collaborative Study. JCO Glob. Oncol. 2020, 6, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Chinazzi, M.; Davis, J.T.; Ajelli, M.; Gioannini, C.; Litvinova, M.; Merler, S.; Pastore, Y.; Piontti, A.; Mu, K.; Rossi, L.; et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science 2020, 368, 395–400. [Google Scholar] [CrossRef] [Green Version]
- The Lancet. COVID-19: Protecting health-care workers. Lancet 2020, 395, 922. [Google Scholar] [CrossRef]
- Pieterman, R.M.; van Putten, J.W.; Meuzelaar, J.J.; Mooyaart, E.L.; Vaalburg, W.; Koëter, G.H.; Fidler, V.; Pruim, J.; Groen, H.J. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N. Engl. J. Med. 2000, 343, 254–261. [Google Scholar] [CrossRef]
- Wang, J.; Welch, K.; Wang, L.; Kong, F.-M.S. Negative predictive value of positron emission tomography and computed tomography for stage T1-2N0 non-small-cell lung cancer: A meta-analysis. Clin. Lung Cancer 2012, 13, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Hochhegger, B.; Alves, G.R.T.; Irion, K.L.; Fritscher, C.C.; Fritscher, L.G.; Concatto, N.H.; Marchiori, E. PET/CT imaging in lung cancer: Indications and findings. J. Bras. Pneumol. 2015, 41, 264–274. [Google Scholar] [CrossRef]
- Everitt, S.; Herschtal, A.; Callahan, J.; Plumridge, N.; Ball, D.; Kron, T.; Schneider-Kolsky, M.; Binns, D.; Hicks, R.J.; MacManus, M. High rates of tumor growth and disease progression detected on serial pretreatment fluorodeoxyglucose-positron emission tomography/computed tomography scans in radical radiotherapy candidates with nonsmall cell lung cancer. Cancer 2010, 116, 5030–5037. [Google Scholar] [CrossRef]
- Nagar, H.; Formenti, S.C. Cancer and COVID-19—Potentially deleterious effects of delaying radiotherapy. Nat. Rev. Clin. Oncol. 2020, 17, 332–334. [Google Scholar] [CrossRef] [PubMed]




| Parameter | |
|---|---|
| Age, years, median (min–max) | 73.0 (57.2–89.8) |
| ECOG, median (min–max) | 1 (0–4) |
| Gender | |
| Female | 32 (33.3) |
| Male | 64 (66.7) |
| Histology | |
| Adenocarcinoma | 37 (38.5) |
| Squamous cell cancer | 36 (37.5) 2 |
| Large cell carcinoma | 1 (1.0) |
| Carcinoma, not otherwise specified | 4 (4.2) |
| Without histological confirmation | 18 (18.8) |
| Tumor stage 1 | |
| IA | 58 (60.4) 2 |
| IB | 17 (17.7) |
| IIA | 5 (5.2) |
| IIB | 10 (10.4) |
| IIIA | 6 (6.3) |
| cT category 1,4 | |
| cT1 | 58 (60.4) 2 |
| cT2 | 22 (22.9) |
| cT3 | 10 (10.4) |
| cT4 | 6 (6.3) |
| SBRT, total dose [Gy]/number of fractions | |
| 60/8 | 46 (47.9) |
| 55/5 | 37 (38.5) 2 |
| 54/3 | 6 (6.3) |
| 54/18 3 | 4 (4.2) |
| 50/10 | 2 (2.1) |
| 60/12 | 1 (1.0) |
| SBRT, technique | |
| 3DCRT | 10 (10.4) |
| IMRT | 3 (3.1) |
| VMAT | 83 (86.5) 2 |
| Parameter | Pre-COVID Era (2012–2019), n = 77 Patients | COVID Era (2020–2021), n = 19 Patients | p-Value |
|---|---|---|---|
| Treated patients per month 1 | 0.9 (0–5) | 1.5 (0–3) | 0.04 5 |
| Multidisciplinary tumor board decision to SBRT [weeks] 2,7 | 4.0 (0–13.7) | 6.4 (2.0–59.7) | 0.005 5 |
| Bronchoscopy for staging 3 | 69 (89.6) | 19 (100) | 0.14 4 |
| Bronchoscopy to SBRT [weeks] 2 | 7.6 (3.3–23.1) | 9.0 (5.9–62.7) | 0.04 5 |
| Histological confirmation of diagnosis 3 | 63 (81.8) | 15 (78.9) | 0.77 4 |
| PET/CT for tumor Staging 3 | 68 (88.3) | 15 (78.9) | 0.29 4 |
| PET/CT or chest CT scan 6 to SBRT [weeks] 2 | 7.1 (2.3–49.1) | 7.6 (4.3–60.0) | 0.27 5 |
| PET/CT to SBRT [weeks] 2 | 7.0 (2.3–49.1) | 7.4 (4.3–60.0) | 0.76 5 |
| cMRI/CCT for staging 3 | 62 (80.5) | 16 (84.2) | 0.71 4 |
| Planning CT to SBRT [weeks] 2 | 1.7 (0.3–4.1) | 3.0 (1.4–4.9) | <0.001 5 |
| OS | PFS | LRPFS | LRC | DPFS | DC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter (Numbers of Patients) | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Multidisciplinary tumor board decision to SBRT, weeks, median = 4.1, ≥median (n = 50) vs. <median (n = 44) 1 | 1.11 (0.63–1.98) | 0.72 | 1.25 (0.72–2.19) | 0.43 | 1.24 (0.71–2.19) | 0.45 | 2.4 (0.6–9.65) | 0.22 | 1.22 (0.69–2.14) | 0.49 | 0.77 (0.22–2.64) | 0.67 |
| Bronchoscopy for staging, yes (n = 88) vs. no (n = 8) | 0.96 (0.38–2.44) | 0.93 | 1.08 (0.42–2.72) | 0.88 | 1.05 (0.41–2.66) | 0.92 | 23.36 (0–0.46 × 106) | 0.53 | 1.03 (0.41–2.62) | 0.95 | 23.3 (0–0.19*106) | 0.49 |
| Histological confirmation of diagnosis, yes (n = 78) vs. no (n = 18) | 0.73 (0.37–1.45) | 0.37 | 0.67 (0.35–1.28) | 0.23 | 0.65 (0.34–1.24) | 0.19 | 0.45 (0.11–1.79) | 0.26 | 0.78 (0.4–1.53) | 0.47 | 2.19 (0.28–17.12) | 0.46 |
| PET/CT for tumor staging, yes (n = 83) vs. no (n = 13) | 0.39 (0.18–0.85) | 0.02 | 0.34 (0.16–0.71) | <0.01 | 0.38 (0.18–0.82) | 0.01 | 0.32 (0.07–1.58) | 0.16 | 0.37 (0.18–0.77) | 0.01 | 0.34 (0.07–1.63) | 0.18 |
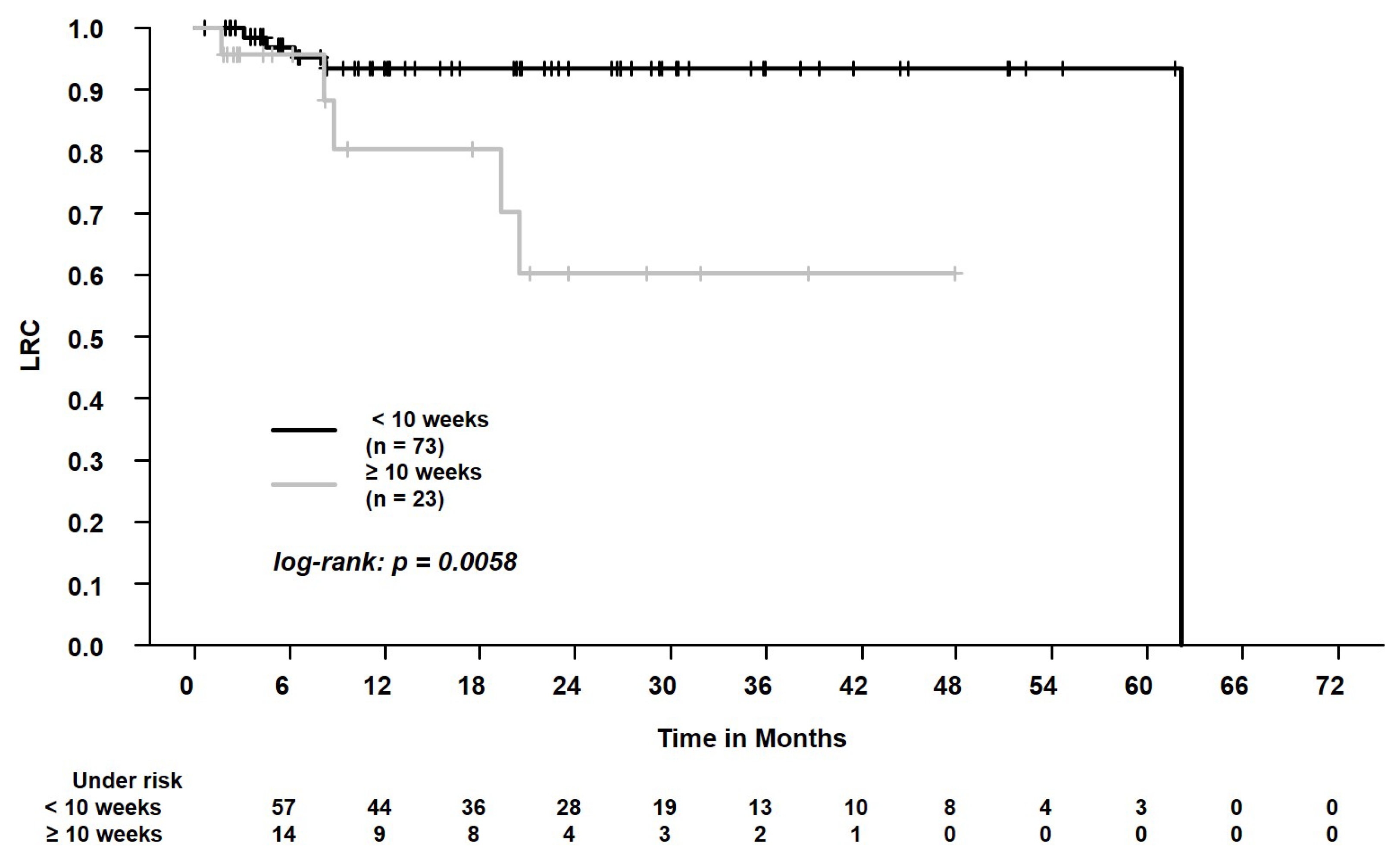
| PET/CT or chest CT scan 2 to SBRT, weeks, ≥10 (n = 23) vs. <10 (n = 73) 3 | 1.41 (0.73–2.72) | 0.31 | 1.59 (0.84–3.01) | 0.15 | 1.69 (0.89–3.2) | 0.11 | 5.26 (1.41–19.63) | 0.01 | 1.27 (0.71–2.28) | 0.42 | 0.81 (0.21–3.12) | 0.76 |
| PET/CT to SBRT, weeks, ≥10 (n = 17) vs. <10 (n = 66) 3 | 1.56 (0.76–3.2) | 0.23 | 1.82 (0.91–3.64) | 0.09 | 1.86 (0.93–3.72) | 0.08 | 6.44 (1.44–28.78) | 0.01 | 1.83 (0.91–3.68) | 0.09 | 1.52 (0.31–7.33) | 0.60 |
| cMRI/CCT for staging, yes (n = 78) vs. no (n = 18) | 0.8 (0.4–1.62) | 0.54 | 0.77 (0.39–1.52) | 0.46 | 0.75 (0.38–1.47) | 0.40 | 0.84 (0.17–4.04) | 0.83 | 0.71 (0.36–1.39) | 0.32 | 0.53 (0.14–2) | 0.35 |
| Planning CT to SBRT, weeks, median = 1.86, ≥median (n = 53) vs. <median (n = 43) | 0.62 (0.35–1.09) | 0.10 | 0.72 (0.42–1.24) | 0.23 | 0.71 (0.41–1.24) | 0.23 | 1.58 (0.39–6.3) | 0.52 | 0.67 (0.38–1.15) | 0.15 | 0.43 (0.13–1.47) | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habermann, F.-N.O.J.; Schmitt, D.; Failing, T.; Fischer, J.; Ziegler, D.A.; Fischer, L.A.; Alt, N.J.; Muster, J.; Donath, S.; Hille, A.; et al. Patterns of Pretreatment Diagnostic Assessment in Patients Treated with Stereotactic Body Radiation Therapy (SBRT) for Non-Small Cell Lung Cancer (NSCLC): Special Characteristics in the COVID Pandemic and Influence on Outcomes. Curr. Oncol. 2022, 29, 1080-1092. https://doi.org/10.3390/curroncol29020092
Habermann F-NOJ, Schmitt D, Failing T, Fischer J, Ziegler DA, Fischer LA, Alt NJ, Muster J, Donath S, Hille A, et al. Patterns of Pretreatment Diagnostic Assessment in Patients Treated with Stereotactic Body Radiation Therapy (SBRT) for Non-Small Cell Lung Cancer (NSCLC): Special Characteristics in the COVID Pandemic and Influence on Outcomes. Current Oncology. 2022; 29(2):1080-1092. https://doi.org/10.3390/curroncol29020092
Chicago/Turabian StyleHabermann, Felix-Nikolai Oschinka Jegor, Daniela Schmitt, Thomas Failing, Jann Fischer, David Alexander Ziegler, Laura Anna Fischer, Niklas Josua Alt, Julian Muster, Sandra Donath, Andrea Hille, and et al. 2022. "Patterns of Pretreatment Diagnostic Assessment in Patients Treated with Stereotactic Body Radiation Therapy (SBRT) for Non-Small Cell Lung Cancer (NSCLC): Special Characteristics in the COVID Pandemic and Influence on Outcomes" Current Oncology 29, no. 2: 1080-1092. https://doi.org/10.3390/curroncol29020092
APA StyleHabermann, F.-N. O. J., Schmitt, D., Failing, T., Fischer, J., Ziegler, D. A., Fischer, L. A., Alt, N. J., Muster, J., Donath, S., Hille, A., Schirmer, M. A., Guhlich, M., El Shafie, R. A., Rieken, S., Leu, M., & Dröge, L. H. (2022). Patterns of Pretreatment Diagnostic Assessment in Patients Treated with Stereotactic Body Radiation Therapy (SBRT) for Non-Small Cell Lung Cancer (NSCLC): Special Characteristics in the COVID Pandemic and Influence on Outcomes. Current Oncology, 29(2), 1080-1092. https://doi.org/10.3390/curroncol29020092







