CT and 3 Tesla MRI in the TN Staging of Colon Cancer: A Prospective, Blind Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
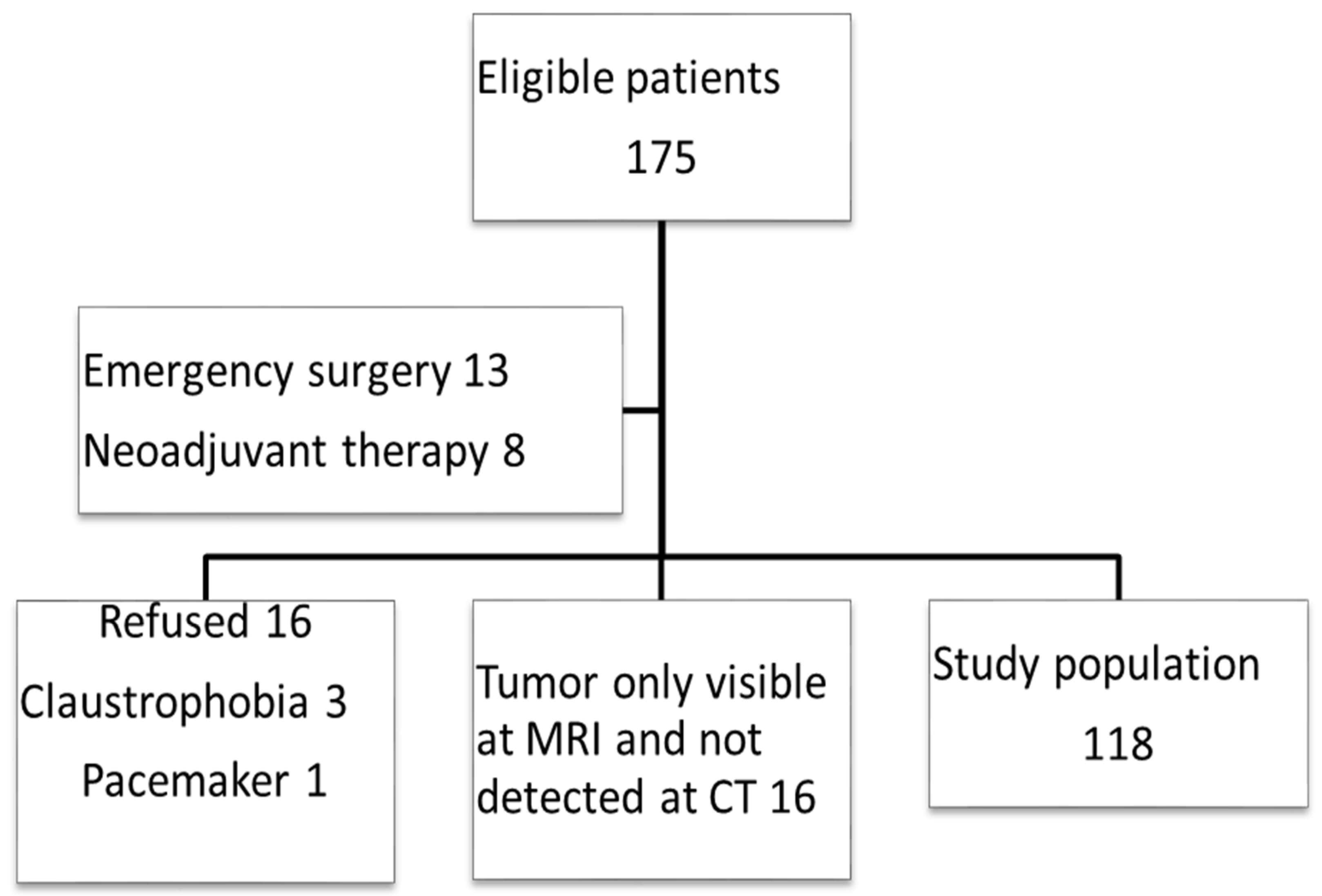
2.2. Inclusion of Trial Participants
2.3. Data
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
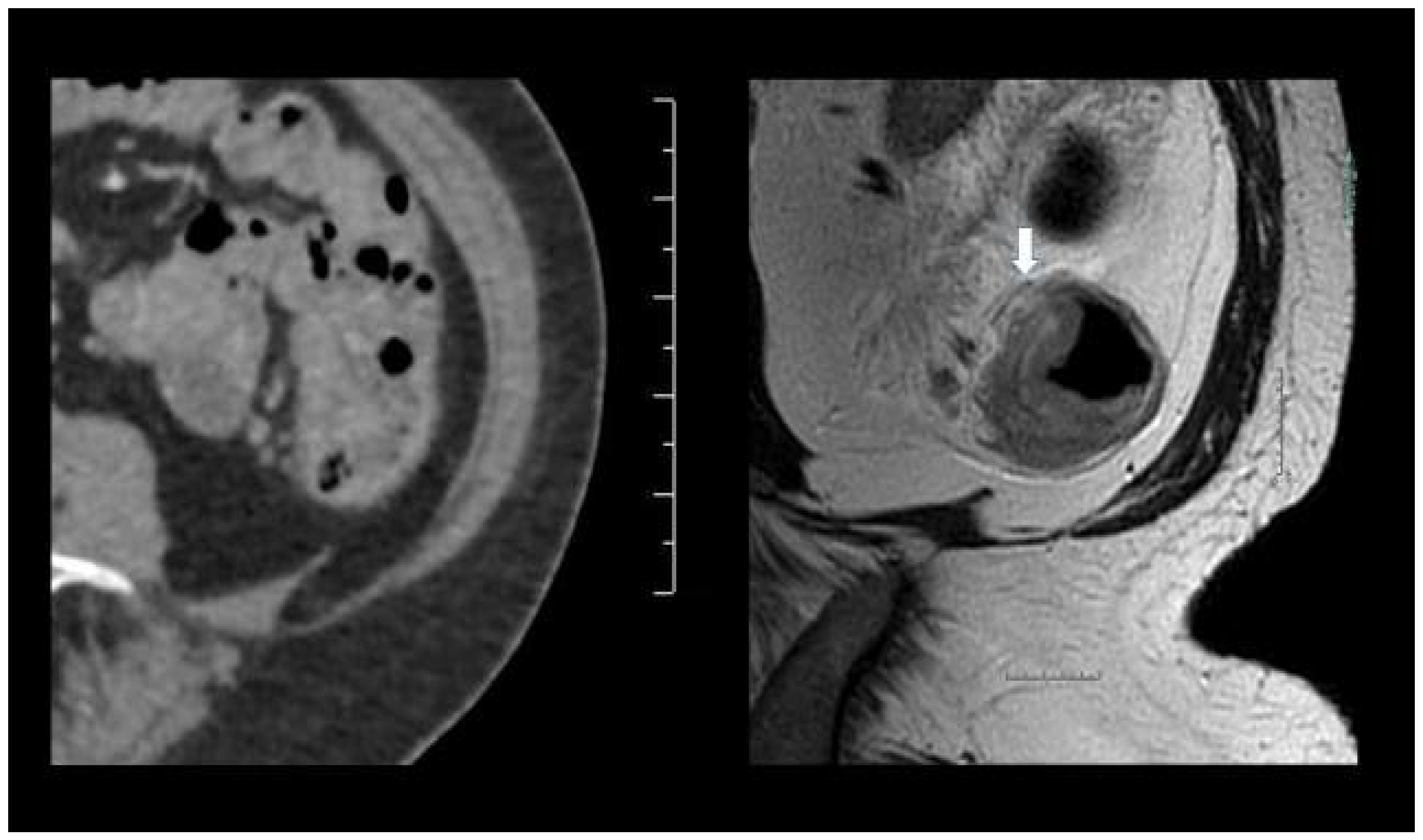
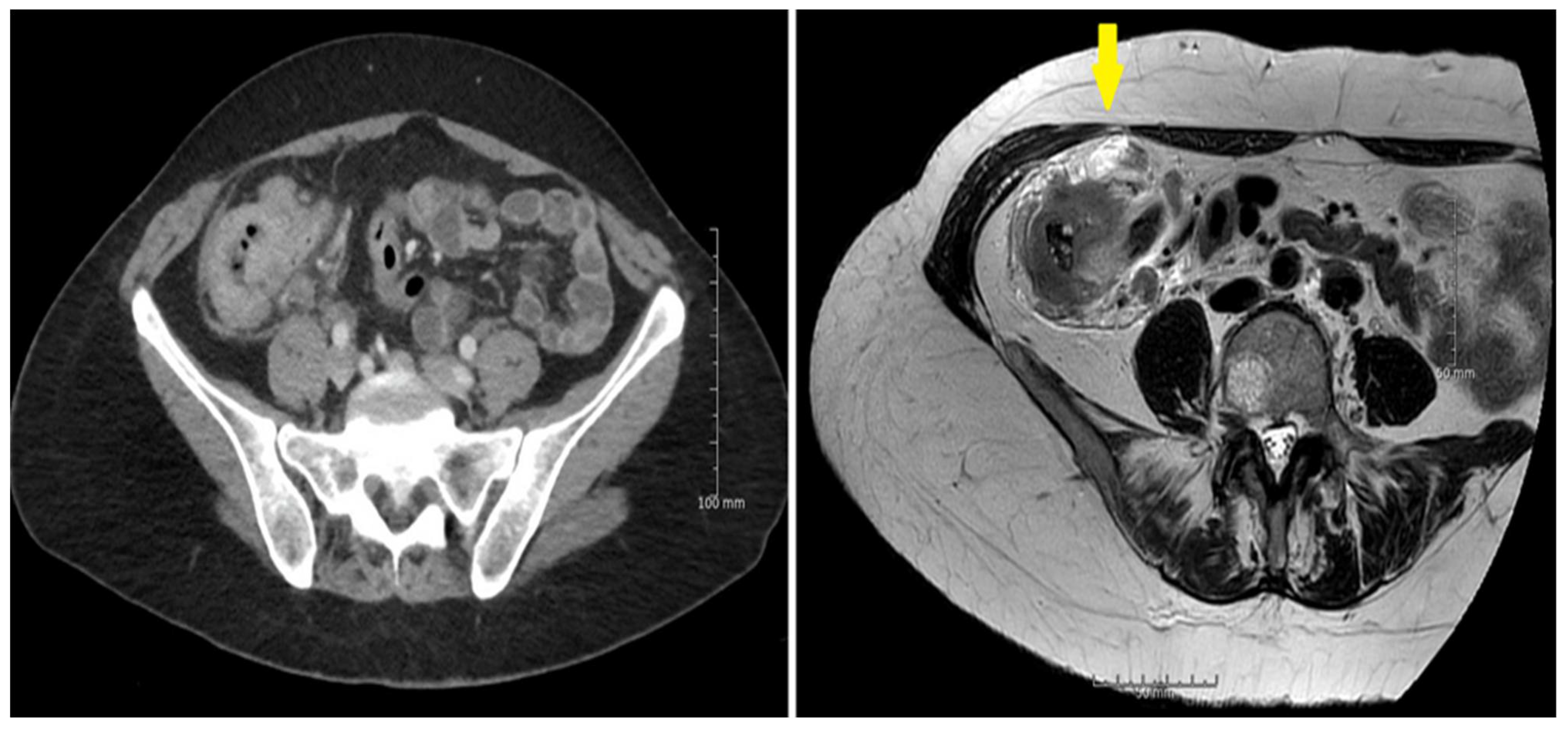
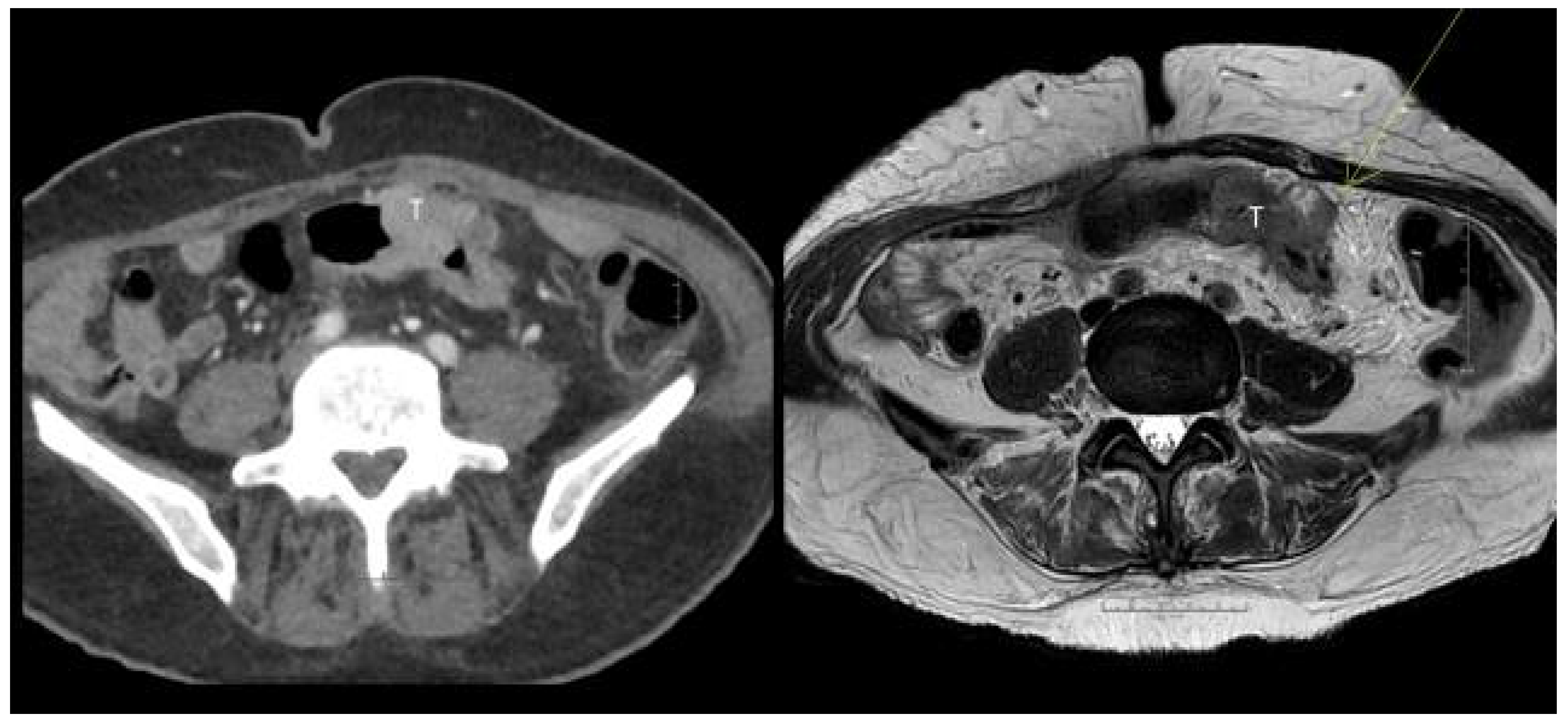
3.2. Sensitivity and Specificity
3.3. Diffusion Weighted MRI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyers, B.; Cosby, R.; Quereshy, F.; Jonker, D. Adjuvant Chemotherapy for Stage II and III Colon Cancer Following Complete Resection: A Cancer Care Ontario Systematic Review. Clin. Oncol. 2017, 29, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Appelt, A.L.; Pløen, J.; Harling, H.; Jensen, F.S.; Jensen, L.H.; Jørgensen, J.C.R.; Lindebjerg, J.; Rafaelsen, S.R.; Jakobsen, A. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: A prospective observational study. Lancet Oncol. 2015, 16, 919–927. [Google Scholar] [CrossRef]
- Fernandez, L.M.; Julião, G.P.S.; Figueiredo, N.L.; Beets, G.L.; van der Valk, M.J.M.; Bahadoer, R.R.; Hilling, D.E.; Kranenbarg, E.M.-K.; Roodvoets, A.G.H.; Renehan, A.G.; et al. Conditional recurrence-free survival of clinical complete responders managed by watch and wait after neoadjuvant chemoradiotherapy for rectal cancer in the International Watch & Wait Database: A retrospective, international, multicentre registry study. Lancet Oncol. 2021, 22, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, A.; Andersen, F.; Fischer, A.; Jensen, L.H.; Jørgensen, J.C.R.; Larsen, O.; Lindebjerg, J.; Pløen, J.; Rafaelsen, S.; Vilandt, J. Neoadjuvant chemotherapy in locally advanced colon cancer.A phase II trial. Acta Oncol. 2015, 54, 1747–1753. [Google Scholar] [CrossRef]
- Gosavi, R.; Chia, C.; Michael, M.; Heriot, A.G.; Warrier, S.K.; Kong, J.C. Neoadjuvant chemotherapy in locally advanced colon cancer: A systematic review and meta-analysis. Int. J. Color. Dis. 2021, 36, 2063–2070. [Google Scholar] [CrossRef]
- Chang, H.; Yu, X.; Xiao, W.; Wang, Q.-X.; Zhou, W.-H.; Zeng, Z.-F.; Ding, P.-R.; Li, L.-R.; Gao, Y.-H. Neoadjuvant chemoradiotherapy followed by surgery in patients with unresectable locally advanced colon cancer: A prospective observational study. OncoTargets Ther. 2018, 11, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Malmstrøm, M.L.; Brisling, S.; Klausen, T.W.; Saftoiu, A.; Perner, T.; Vilmann, P.; Gögenur, I. Staging with computed tomography of patients with colon cancer. Int. J. Color. Dis. 2017, 33, 9–17. [Google Scholar] [CrossRef]
- Nørgaard, A.; Dam, C.; Jakobsen, A.; Pløen, J.; Lindebjerg, J.; Rafaelsen, S. Selection of colon cancer patients for neoadjuvant chemotherapy by preoperative CT scan. Scand. J. Gastroenterol. 2013, 49, 202–208. [Google Scholar] [CrossRef]
- Dam, C.; Lindebjerg, J.; Jakobsen, A.; Jensen, L.H.; Rahr, H.; Rafaelsen, S.R. Local staging of sigmoid colon cancer using MRI. Acta Radiol. Open 2017, 6, 2058460117720957. [Google Scholar] [CrossRef]
- Beets-Tan, R.G.H.; Lambregts, D.M.J.; Maas, M.; Bipat, S.; Barbaro, B.; Curvo-Semedo, L.; Fenlon, H.M.; Gollub, M.J.; Gourtsoyianni, S.; Halligan, S.; et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur. Radiol. 2017, 28, 1465–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollvén, E.; Holm, T.; Glimelius, B.; Lörinc, E.; Blomqvist, L. Potentials of high resolution magnetic resonance imaging versus computed tomography for preoperative local staging of colon cancer. Acta Radiol. 2013, 54, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.; Blake, H.; Jeyadevan, N.; Abulafi, M.; Swift, I.; Toomey, P.; Brown, G. Local staging and assessment of colon cancer with 1.5-T magnetic resonance imaging. Br. J. Radiol. 2016, 89, 20160257. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Oh, S.N.; Yeo, D.-M.; Kang, W.K.; Jung, C.-K.; Kim, S.W.; Park, M.Y. Computed tomography and magnetic resonance imaging evaluation of lymph node metastasis in early colorectal cancer. World J. Gastroenterol. 2015, 21, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Koh, F.H.X.; Tan, K.-K.; Teo, L.L.S.; Ang, B.W.L.; Thian, Y.-L. Prospective comparison between magnetic resonance imaging and computed tomography in colorectal cancer staging. ANZ J. Surg. 2017, 88, E498–E502. [Google Scholar] [CrossRef] [PubMed]
- Nerad, E.; Lambregts, D.; Kersten, E.L.J.; Maas, M.; Bakers, F.C.H.; Bosch, H.C.M.V.D.; Grabsch, H.I.; Beets-Tan, R.G.H.; Lahaye, M.J. MRI for Local Staging of Colon Cancer: Can MRI Become the Optimal Staging Modality for Patients with Colon Cancer? Dis. Colon Rectum 2017, 60, 385–392. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Y.; An, J.; Fu, P. Local Staging of Colon Cancer: A Cross-Sectional Analysis for Diagnostic Performance of Magnetic Resonance Imaging and by Experience. Cancer Investig. 2021, 39, 379–389. [Google Scholar] [CrossRef]
- Liu, L.; Lv, H.; Wang, Z.-C.; Rao, S.-X.; Zeng, M.-S. Performance comparison between MRI and CT for local staging of sigmoid and descending colon cancer. Eur. J. Radiol. 2019, 121, 108741. [Google Scholar] [CrossRef]
- Park, S.Y.; Cho, S.H.; Lee, M.A.; Yoon, G.; Kim, H.J.; Park, J.S.; Kim, W.H.; Lee, S.M.; Shin, K.-M.; Kim, G.C.; et al. Diagnostic performance of MRI- versus MDCT-categorized T3cd/T4 for identifying high-risk stage II or stage III colon cancers: A pilot study. Abdom. Radiol. 2019, 44, 1675–1685. [Google Scholar] [CrossRef]
- Hunter, C.; Siddiqui, M.; Delisle, T.G.; Blake, H.; Jeyadevan, N.; Abulafi, M.; Swift, I.; Toomey, P.; Brown, G. CT and 3-T MRI accurately identify T3c disease in colon cancer, which strongly predicts disease-free survival. Clin. Radiol. 2017, 72, 307–315. [Google Scholar] [CrossRef]
- Hong, E.K.; Castagnoli, F.; Gennaro, N.; Landolfi, F.; Pérez-Serrano, C.; Kurilova, I.; Roberti, S.; Beets-Tan, R. Locoregional CT staging of colon cancer: Does a learning curve exist? Abdom. Radiol. 2021, 46, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, W. Does McNemar’s test compare the sensitivities and specificities of two diagnostic tests? Stat. Methods Med. Res. 2014, 26, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Klang, E.; Eifer, M.; Kopylov, U.; Belsky, V.; Raskin, S.; Konen, E.; Amitai, M. Pitfalls in diagnosing colon cancer on abdominal CT. Clin. Radiol. 2017, 72, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Ohta, S.; Nitta, N.; Yoshimura, M.; Shimizu, T.; Tani, M.; Kushima, R.; Murata, K. MRI can be used to assess advanced T-stage colon carcinoma as well as rectal carcinoma. Jpn. J. Radiol. 2016, 34, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.K.; Landolfi, F.; Castagnoli, F.; Park, S.J.; Boot, J.; Berg, J.V.D.; Lee, J.M.; Beets-Tan, R. CT for lymph node staging of Colon cancer: Not only size but also location and number of lymph node count. Abdom. Radiol. 2021, 46, 4096–4105. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Wang, X.; Zhou, M.; Chen, X.; Luan, K. An MRI-based multi-objective radiomics model predicts lymph node status in patients with rectal cancer. Abdom. Radiol. 2021, 46, 1816–1824. [Google Scholar] [CrossRef]
- Nerad, E.; Pizzi, A.D.; Lambregts, D.M.J.; Maas, M.; Wadhwani, S.; Bakers, F.C.H.; Bosch, H.C.M.V.D.; Beets-Tan, R.G.H.; Lahaye, M.J. The Apparent Diffusion Coefficient (ADC) is a useful biomarker in predicting metastatic colon cancer using the ADC-value of the primary tumor. PLoS ONE 2019, 14, e0211830. [Google Scholar] [CrossRef]
- Takahashi, Y.; Hayano, K.; Ohira, G.; Imanishi, S.; Hanaoka, T.; Watanabe, H.; Hirata, A.; Kawasaki, Y.; Miyauchi, H.; Matsubara, H. Histogram Analysis of Diffusion-Weighted MR Imaging as a Biomarker to Predict Survival of Surgically Treated Colorectal Cancer Patients. Am. J. Dig. Dis. 2021, 66, 1227–1232. [Google Scholar] [CrossRef]
- Er, H.; Erden, A. Mean ADC values discriminate rectal mucinous carcinomafrom rectal nonmucinous adenocarcinoma. Turk. J. Med. Sci. 2017, 47, 1520–1525. [Google Scholar] [CrossRef]
- Hosseini, S.; Nguyen, N.; Mohammadianpanah, M.; Mirzaei, S.; Bananzadeh, A.M. Predictive Significance of Mucinous Histology on Pathologic Complete Response Rate Following Capecitabine-Based Neoadjuvant Chemoradiation in Rectal Cancer: A Comparative Study. J. Gastrointest. Cancer 2019, 50, 716–722. [Google Scholar] [CrossRef]
- Zeng, Z.; Yan, Q.; Chen, G.; Zhang, X.; Huang, J.; Fu, K.; Peng, X.; Xiao, S. Characteristics of colorectal carcinoma patients with PMS2 defects detected by immunohistochemistry. Eur. J. Cancer Prev. 2020, 30, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Alpert, L.; Pai, R.K.; Srivastava, A.; McKinnon, W.; Wilcox, R.; Yantiss, R.K.; Arcega, R.; Wang, H.L.; Robert, M.E.; Liu, X.; et al. Colorectal Carcinomas With Isolated Loss of PMS2 Staining by Immunohistochemistry. Arch. Pathol. Lab. Med. 2018, 142, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.S.; Bertelsen, C.A.; Henriksen, R.; Campos, A.H.; Kristensen, B.; Ingeholm, P.; Gögenur, I. The pathological phenotype of colon cancer with microsatellite instability. Therapy 2016, 8, 9. [Google Scholar]
- Chalabi, M.; Cardona, A.; Nagarkar, D.; Scala, A.D.; Gandara, D.; Rittmeyer, A.; Albert, M.; Powles, T.; Kok, M.; Herrera, F. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: Pooled post hoc analyses of the OAK and POPLAR trials. Ann. Oncol. 2020, 31, 525–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, S.A.; Mallett, S.; Beare, S.; Bhatnagar, G.; Blunt, D.; Boavida, P.; Bridgewater, J.; Clarke, C.S.; Duggan, M.; Ellis, S.; et al. Diagnostic accuracy of whole-body MRI versus standard imaging pathways for metastatic disease in newly diagnosed colorectal cancer: The prospective Streamline C trial. Lancet Gastroenterol. Hepatol. 2019, 4, 529–537. [Google Scholar] [CrossRef] [Green Version]
| N = 118 (%) | |
|---|---|
| Gender | |
| Female | 56 (47.5) |
| Male | 62 (52.5) |
| Age | 70.6 years [range 39–91] |
| Mean tumor length | 4.5 cm [range 1.2–11.0] |
| Location of colon tumor | |
| Cecum | 18 (15.3) |
| Ascending | 36 (30.5) |
| Transverse | 14 (11.9) |
| Descending | 10 (8.5) |
| Sigmoid | 40 (33.8) |
| Pathology | |
| pT1 | 3 (2.5) |
| pT2 | 25 (21.1) |
| pT3 | 62 (52.5) |
| pT4 | 28 (23.7) |
| pN1-2 | 55 (46.7) |
| pV2 | 40 (33.9) |
| Tumor Stage | CT Sensitivity % (95% CI) | CT Specificity % (95% CI) | MRI Sensitivity % (95% CI) | MRI Specificity % (95% CI) | p |
|---|---|---|---|---|---|
| T1-2 vs. T3-4 | 61.8 (50.8–71.7) | 85.7 (66.4–95.5) | 77.8 (67.5–85.6) | 60.9 (38.9–79.5) | ns |
| T1-3b vs. ≥T3c | 51.1 (36.0–66.1) | 80.8 (69.6–88.8) | 80.0 (65.0–89.9) | 91.8 (82.4–96.6) | p = 0.02 * |
| T1-3d vs. T4 | 21.4 (9.0–41.5) | 94.3 (86.8–97.9) | 46.4 (28.0–65.8) | 94.4 (86.9–97.9) | ns |
| Nodal stage ± | 65.5 (51.3–77.4) | 50.0 (37.2–62.8) | 58.2 (44.1–71.1) | 50.0 (37.2–62.8) | ns |
| EMVI ± | 35.0 (21.1–51.7) | 82.0 (71.4–89.5) | 50.0 (34.1–65.9) | 81.8 (71.0–89.4) | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafaelsen, S.R.; Dam, C.; Vagn-Hansen, C.; Møller, J.; Rahr, H.B.; Sjöström, M.; Lindebjerg, J.; Hansen, T.F.; Pedersen, M.R.V. CT and 3 Tesla MRI in the TN Staging of Colon Cancer: A Prospective, Blind Study. Curr. Oncol. 2022, 29, 1069-1079. https://doi.org/10.3390/curroncol29020091
Rafaelsen SR, Dam C, Vagn-Hansen C, Møller J, Rahr HB, Sjöström M, Lindebjerg J, Hansen TF, Pedersen MRV. CT and 3 Tesla MRI in the TN Staging of Colon Cancer: A Prospective, Blind Study. Current Oncology. 2022; 29(2):1069-1079. https://doi.org/10.3390/curroncol29020091
Chicago/Turabian StyleRafaelsen, Søren R., Claus Dam, Chris Vagn-Hansen, Jakob Møller, Hans B. Rahr, Mikkel Sjöström, Jan Lindebjerg, Torben Frøstrup Hansen, and Malene Roland Vils Pedersen. 2022. "CT and 3 Tesla MRI in the TN Staging of Colon Cancer: A Prospective, Blind Study" Current Oncology 29, no. 2: 1069-1079. https://doi.org/10.3390/curroncol29020091
APA StyleRafaelsen, S. R., Dam, C., Vagn-Hansen, C., Møller, J., Rahr, H. B., Sjöström, M., Lindebjerg, J., Hansen, T. F., & Pedersen, M. R. V. (2022). CT and 3 Tesla MRI in the TN Staging of Colon Cancer: A Prospective, Blind Study. Current Oncology, 29(2), 1069-1079. https://doi.org/10.3390/curroncol29020091








