Impact of COVID-19 on Radiation Oncology, an Austrian Experience
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; Lopes, G.D.L.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Yang, F.; Shi, S.; Zhu, J.; Shi, J.; Dai, K.; Chen, X. Clinical characteristics and outcomes of cancer patients with COVID-19. J. Med. Virol. 2020, 92, 2067–2073. [Google Scholar] [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Miyashita, H.; Mikami, T.; Chopra, N.; Yamada, T.; Chernyavsky, S.; Rizk, D.; Cruz, C. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann. Oncol. 2020, 31, 1088–1089. [Google Scholar] [CrossRef] [PubMed]
- Garassino, M.C.; Whisenant, J.G.; Huang, L.-C.; Trama, A.; Torri, V.; Agustoni, F.; Baena, J.; Banna, G.; Berardi, R.; Bettini, A.C.; et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): First results of an international, registry-based, cohort study. Lancet Oncol. 2020, 21, 914–922. [Google Scholar] [CrossRef]
- Nagar, H.; Formenti, S.C. Cancer and COVID-19—potentially deleterious effects of delaying radiotherapy. Nat. Rev. Clin. Oncol. 2020, 17, 332–334. [Google Scholar] [CrossRef]
- Sud, A.; Jones, M.E.; Broggio, J.; Loveday, C.; Torr, B.; Garrett, A.; Nicol, D.L.; Jhanji, S.; Boyce, S.A.; Gronthoud, F.; et al. Collateral damage: The impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann. Oncol. 2020, 31, 1065–1074. [Google Scholar] [CrossRef]
- Maringe, C.; Spicer, J.; Morris, M.; Purushotham, A.; Nolte, E.; Sullivan, R.; Nicol, D.L.; Jhanji, S.; Boyce, S.A.; Gronthoud, F.; et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. Lancet Oncol. 2020, 21, 1023–1034. [Google Scholar] [CrossRef]
- Sud, A.; Torr, B.; Jones, M.E.; Broggio, J.; Scott, S.; Loveday, C.; Garrett, A.; Gronthoud, F.; Nicol, D.L.; Jhanji, S.; et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: A modelling study. Lancet Oncol. 2020, 21, 1035–1044. [Google Scholar] [CrossRef]
- Combs, S.E.; Belka, C.; Niyazi, M.; Corradini, S.; Pigorsch, S.; Wilkens, J.; Grosu, A.L.; Guckenberger, M.; Ganswindt, U.; Bernhardt, D. First statement on preparation for the COVID-19 pandemic in large German Speaking University-based radiation oncology departments. Radiat. Oncol. 2020, 15, 74. [Google Scholar] [CrossRef] [Green Version]
- Slotman, B.J.; Lievens, Y.; Poortmans, P.; Cremades, V.; Eichler, T.; Wakefield, D.V.; Ricardi, U. Effect of COVID-19 pandemic on practice in European radiation oncology centers. Radiother. Oncol. 2020, 150, 40–42. [Google Scholar] [CrossRef]
- Tey, J.; Ho, S.; Choo, B.A.; Ho, F.; Yap, S.P.; Tuan, J.K.L.; Leong, C.N.; Cheo, T.; Sommat, K.; Wang, M.L.C. Navigating the challenges of the COVID-19 outbreak: Perspectives from the radiation oncology service in Singapore. Radiother. Oncol. 2020, 148, 189–193. [Google Scholar] [CrossRef]
- Zaorsky, N.G.; Yu, J.B.; McBride, S.M.; Dess, R.T.; Jackson, W.C.; Mahal, B.A.; Chen, R.; Choudhury, A.; Henry, A.; Syndikus, I.; et al. Prostate Cancer Radiation Therapy Recommendations in Response to COVID-19. Adv. Radiat. Oncol. 2020, 5, 659–665. [Google Scholar] [CrossRef]
- Guckenberger, M.; Belka, C.; Bezjak, A.; Bradley, J.; Daly, M.E.; DeRuysscher, D.; Dziadziuszko, R.; Faivre-Finn, C.; Flentje, M.; Gore, E.; et al. Practice recommendations for lung cancer radiotherapy during the COVID-19 pandemic: An ESTRO-ASTRO consensus statement. Radiother. Oncol. 2020, 146, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Coles, C.E.; Aristei, C.; Bliss, J.; Boersma, L.; Brunt, A.M.; Chatterjee, S.; Hanna, G.; Jagsi, R.; Person, O.K.; Kirby, A.; et al. International Guidelines on Radiation Therapy for Breast Cancer During the COVID-19 Pandemic. Clin. Oncol. 2020, 32, 279–281. [Google Scholar] [CrossRef] [PubMed]
- NICE. COVID-19 Rapid Guideline: Delivery of Radiotherapy; The National Institute for Health and Care Excellence (NICE): London, UK, 2020. [Google Scholar]
- Thomson, D.J.; Palma, D.; Guckenberger, M.; Balermpas, P.; Beitler, J.J.; Blanchard, P.; Brizel, D.; Budach, W.; Caudell, J.; Corry, J.; et al. Practice Recommendations for Risk-Adapted Head and Neck Cancer Radiation Therapy During the COVID-19 Pandemic: An ASTRO-ESTRO Consensus Statement. Int. J. Radiat. Oncol. Biol. Physics. 2020, 107, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Moshammer, H.; Poteser, M.; Lemmerer, K.; Wallner, P.; Hutter, H.-P. Time Course of COVID-19 Cases in Austria. Int. J. Environ. Res. Public Health 2020, 17, 3270. [Google Scholar] [CrossRef]
- Seitz, T.; Hoepler, W.; Weseslindtner, L.; Aberle, J.H.; Aberle, S.W.; Puchhammer-Stoeckl, E.; Baumgartner, S.; Traugott, M.; Karolyi, M.; Pawelka, E.; et al. Successful management of the first reported case in Austria of COVID-19 with ARDS. Infection 2020, 48, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Jazieh, A.R.; Akbulut, H.; Curigliano, G.; Rogado, A.; Alsharm, A.A.; Razis, E.D.; Mula-Hussain, L.; Errihani, H.; Khattak, A.; De Guzman, R.B.; et al. Impact of the COVID-19 Pandemic on Cancer Care: A Global Collaborative Study. JCO Glob. Oncol. 2020, 6, 1428–1438. [Google Scholar] [CrossRef]
- Gathani, T.; Clayton, G.; MacInnes, E.; Horgan, K. The COVID-19 pandemic and impact on breast cancer diagnoses: What happened in England in the first half of 2020. Br. J. Cancer 2021, 124, 710–712. [Google Scholar] [CrossRef]
- Hamilton, A.C.; Donnelly, D.W.; Loughrey, M.B.; Turkington, R.C.; Fox, C.; Fitzpatrick, D.; O’Neill, C.E.; Gavin, A.T.; Coleman, H.G. Inequalities in the decline and recovery of pathological cancer diagnoses during the first six months of the COVID-19 pandemic: A population-based study. Br. J. Cancer 2021, 125, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, E.; Swanton, C. Consequences of COVID-19 for cancer care—A CRUK perspective. Nat. Rev. Clin. Oncol. 2021, 18, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.L.; Howe, J.R.; Chang, G.; Crago, A.; Hogg, M.; Karakousis, G.; Oncology, F.T.S.O.S.; Levine, E.; Maker, A.; Mamounas, E.; et al. Management of Cancer Surgery Cases During the COVID-19 Pandemic: Considerations. Ann. Surg. Oncol. 2020, 27, 1717–1720. [Google Scholar] [CrossRef]
- Spencer, K.; Jones, C.M.; Girdler, R.; Roe, C.; Sharpe, M.; Lawton, S.; Miller, L.; Lewis, P.; Evans, M.; Sebag-Montefiore, D.; et al. The impact of the COVID-19 pandemic on radiotherapy services in England, UK: A population-based study. Lancet Oncol. 2021, 22, 309–320. [Google Scholar] [CrossRef]
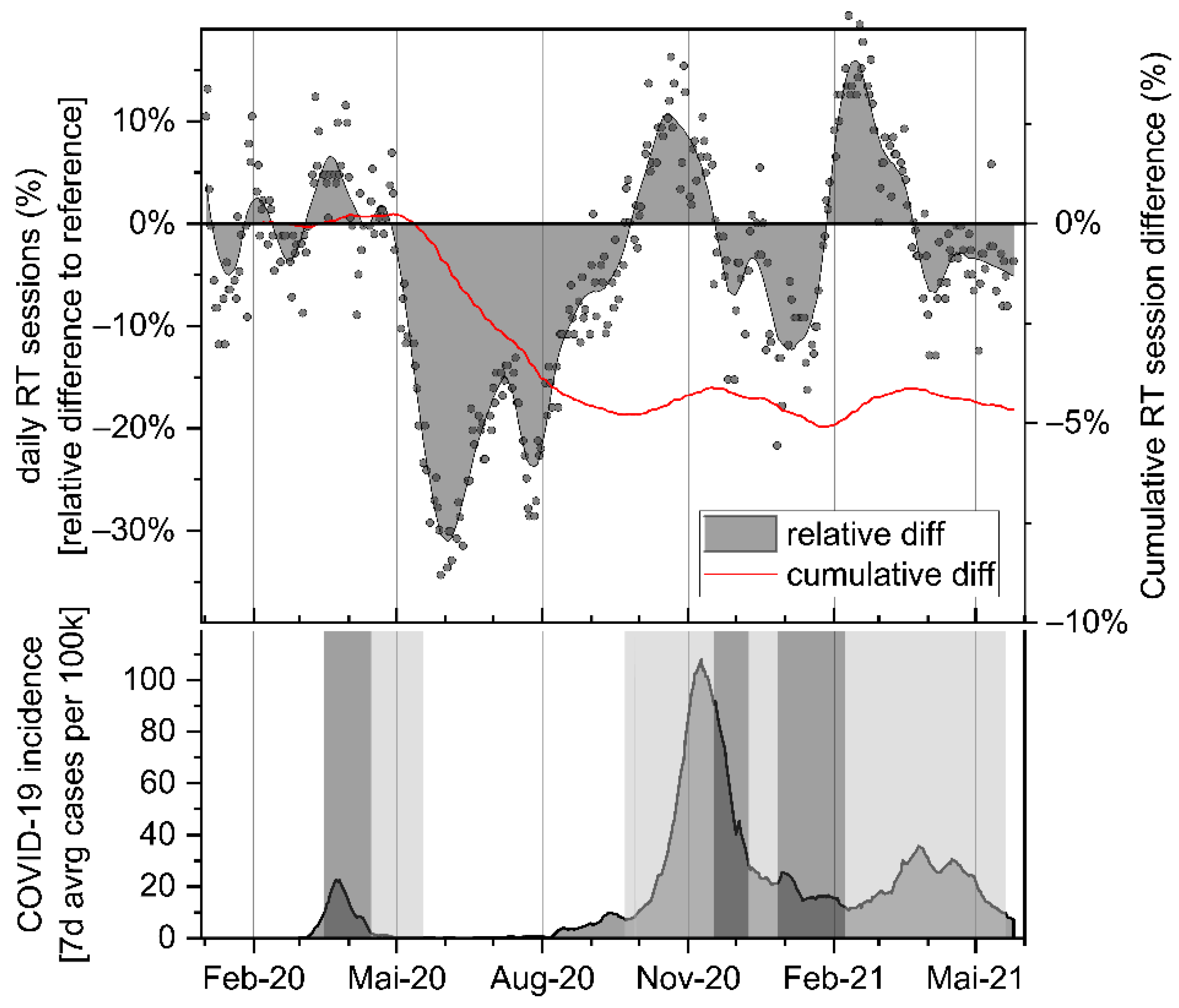
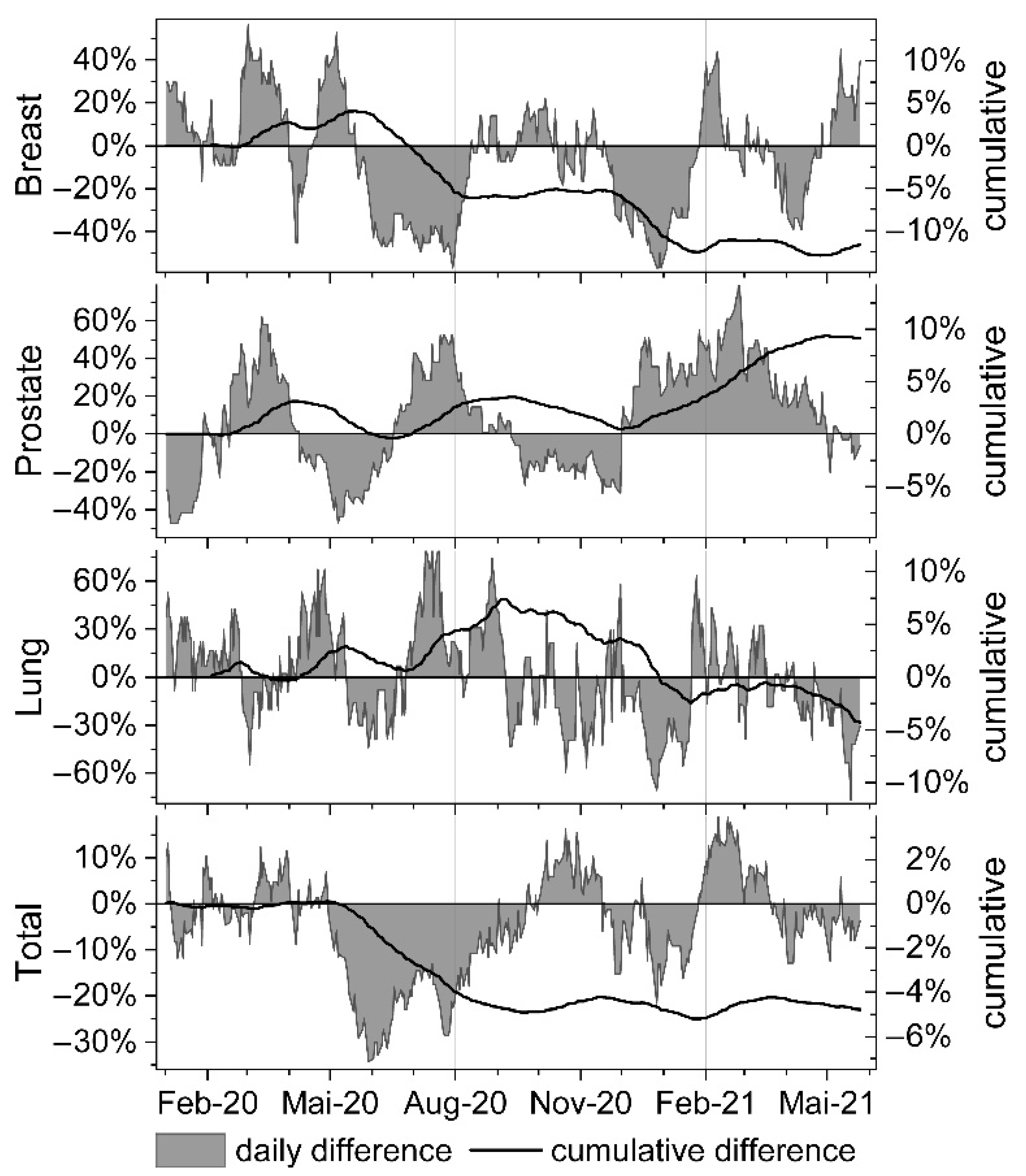
| Month | N Days | Mean Session Difference [%] | p-Value | CI 95% Lower | CI 95% Upper |
|---|---|---|---|---|---|
| February 20 | 20 | −1.94% | 0.133 | −4.53% | 0.65% |
| March 20 | 22 | 4.11% | 0.001 | 1.88% | 6.33% |
| April 20 | 21 | 0.54% | 0.491 | −1.07% | 2.16% |
| May 20 | 19 | −18.93% | <0.001 | −23.06% | −14.79% |
| June 20 | 20 | −24.92% | <0.001 | −27.38% | −22.45% |
| July 20 | 23 | −19.48% | <0.001 | −21.72% | −17.24% |
| August 20 | 21 | −11.64% | <0.001 | −13.39% | −9.88% |
| September 20 | 22 | −3.53% | 0.001 | −5.39% | −1.66% |
| October 20 | 21 | 6.90% | 0.003 | 2.64% | 11.15% |
| November 20 | 21 | −1.63% | 0.578 | −7.63% | 4.38% |
| December 20 | 21 | −6.75% | <0.001 | −9.82% | −3.67% |
| January 21 | 20 | −7.38% | <0.001 | −9.98% | −4.78% |
| February 21 | 20 | 13.40% | <0.001 | 11.81% | 14.98% |
| March 21 | 23 | 2.78% | 0.008 | 0.80% | 4.76% |
| April 21 | 21 | −4.59% | <0.001 | −6.30% | −2.87% |
| May 21 | 15 | −4.36% | 0.001 | −6.55% | −2.17% |
| Month | Breast | Prostate | Lung | Total |
|---|---|---|---|---|
| February 20 | −0.34% | 0.54% | −0.08% | −0.11% |
| March 20 | 1.12% | 3.01% | −1.68% | 0.26% |
| April 20 | 1.14% | 2.49% | −0.21% | 0.45% |
| May 20 | 1.21% | −0.59% | −1.03% | −1.06% |
| June 20 | −1.75% | −0.57% | −1.59% | −2.61% |
| July 20 | −4.81% | 2.53% | 1.21% | −3.42% |
| August 20 | −5.20% | 3.02% | 2.25% | −4.27% |
| September 20 | −4.43% | 3.11% | 1.76% | −4.09% |
| October 20 | −4.80% | 1.33% | 0.23% | −4.10% |
| November 20 | −5.00% | 0.04% | −0.42% | −3.89% |
| December 20 | −7.80% | 1.95% | −2.69% | −4.07% |
| January 21 | −10.34% | 2.99% | −4.34% | −5.01% |
| February 21 | −9.98% | 5.38% | −4.77% | −4.26% |
| March 21 | −10.27% | 8.13% | −5.06% | −3.63% |
| April 21 | −11.30% | 9.24% | −6.09% | −3.76% |
| May 21 | −11.65% | 8.66% | −6.53% | −4.41% |
| Month | Relative Difference in Daily Session Counts [%] | |||||
|---|---|---|---|---|---|---|
| Breast | Prostate | Lung | ||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| February 20 | 2.51% | −4.72%/9.75% | 15.08% | 5.27%/24.89% ** | 13.49% | 5.4%/21.58% ** |
| March 20 | 32.12% | 26.9%/37.34% ** | 36.69% | 30.52%/42.85% ** | −14.28% | −21.18%/−7.37% ** |
| April 20 | 1.89% | −9.83%/13.6% | −8.12% | −13.05%/−3.18% ** | 31.32% | 22.01%/40.63% ** |
| May 20 | 12.90% | 1.79%/24.02% * | −35.21% | −37.87%/−32.54% ** | −7.15% | −18.21%/3.92% |
| June 20 | −39.23% | −41.87%/−36.59% ** | −0.88% | −7.51%/5.75% | −13.60% | −22.06%/−5.14% ** |
| July 20 | −45.33% | −47.61%/−43.06% ** | 41.76% | 38.14%/45.38% ** | 42.14% | 31.71%/52.57% ** |
| August 20 | −5.44% | −13.1%/2.23% | 13.00% | 8.58%/17.43% ** | 28.16% | 17.98%/38.34% ** |
| September 20 | 3.45% | −0.73%/7.63% | −5.86% | −11.03%/−0.69% * | −6.68% | −18.63%/5.26% |
| October 20 | 0.94% | −6.01%/7.89% | −18.68% | −23.78%/−13.58% ** | −13.05% | −25.37%/−0.73% * |
| November 20 | −8.17% | −16.76%/0.41% | −24.24% | −30.88%/−17.6% ** | −15.09% | −28.32%/−1.85% * |
| December 20 | −39.98% | −44.79%/−35.18% ** | 28.68% | 21.5%/35.86% ** | −31.57% | −41.52%/−21.62% ** |
| January 21 | −21.23% | −29.5%/−12.97% ** | 36.61% | 32.67%/40.54% ** | −13.12% | −30.33%/4.1% |
| February 21 | 15.40% | 6.83%/23.96% ** | 54.61% | 47.75%/61.48% ** | 7.57% | −0.94%/16.08% |
| March 21 | −4.26% | −8.69%/0.16% | 34.99% | 29.94%/40.05% ** | −0.14% | −8.05%/7.77% |
| April 21 | −17.30% | −24.45%/−10.16% ** | 17.03% | 13.5%/20.57% ** | −13.38% | −19.5%/−7.26% ** |
| May 21 | 22.39% | 15.94%/28.84% ** | −4.48% | −8.17%/−0.79% * | −34.39% | −44.28%/−24.49% ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangesius, J.; Arnold, C.R.; Seppi, T.; Mangesius, S.; Brüggl, M.; Eichberger, P.; Ganswindt, U. Impact of COVID-19 on Radiation Oncology, an Austrian Experience. Curr. Oncol. 2021, 28, 4776-4785. https://doi.org/10.3390/curroncol28060404
Mangesius J, Arnold CR, Seppi T, Mangesius S, Brüggl M, Eichberger P, Ganswindt U. Impact of COVID-19 on Radiation Oncology, an Austrian Experience. Current Oncology. 2021; 28(6):4776-4785. https://doi.org/10.3390/curroncol28060404
Chicago/Turabian StyleMangesius, Julian, Christoph Reinhold Arnold, Thomas Seppi, Stephanie Mangesius, Mario Brüggl, Paul Eichberger, and Ute Ganswindt. 2021. "Impact of COVID-19 on Radiation Oncology, an Austrian Experience" Current Oncology 28, no. 6: 4776-4785. https://doi.org/10.3390/curroncol28060404
APA StyleMangesius, J., Arnold, C. R., Seppi, T., Mangesius, S., Brüggl, M., Eichberger, P., & Ganswindt, U. (2021). Impact of COVID-19 on Radiation Oncology, an Austrian Experience. Current Oncology, 28(6), 4776-4785. https://doi.org/10.3390/curroncol28060404





