Assessing Choroidal Nevi, Melanomas and Indeterminate Melanocytic Lesions Using Multimodal Imaging—A Retrospective Chart Review
Abstract
1. Introduction
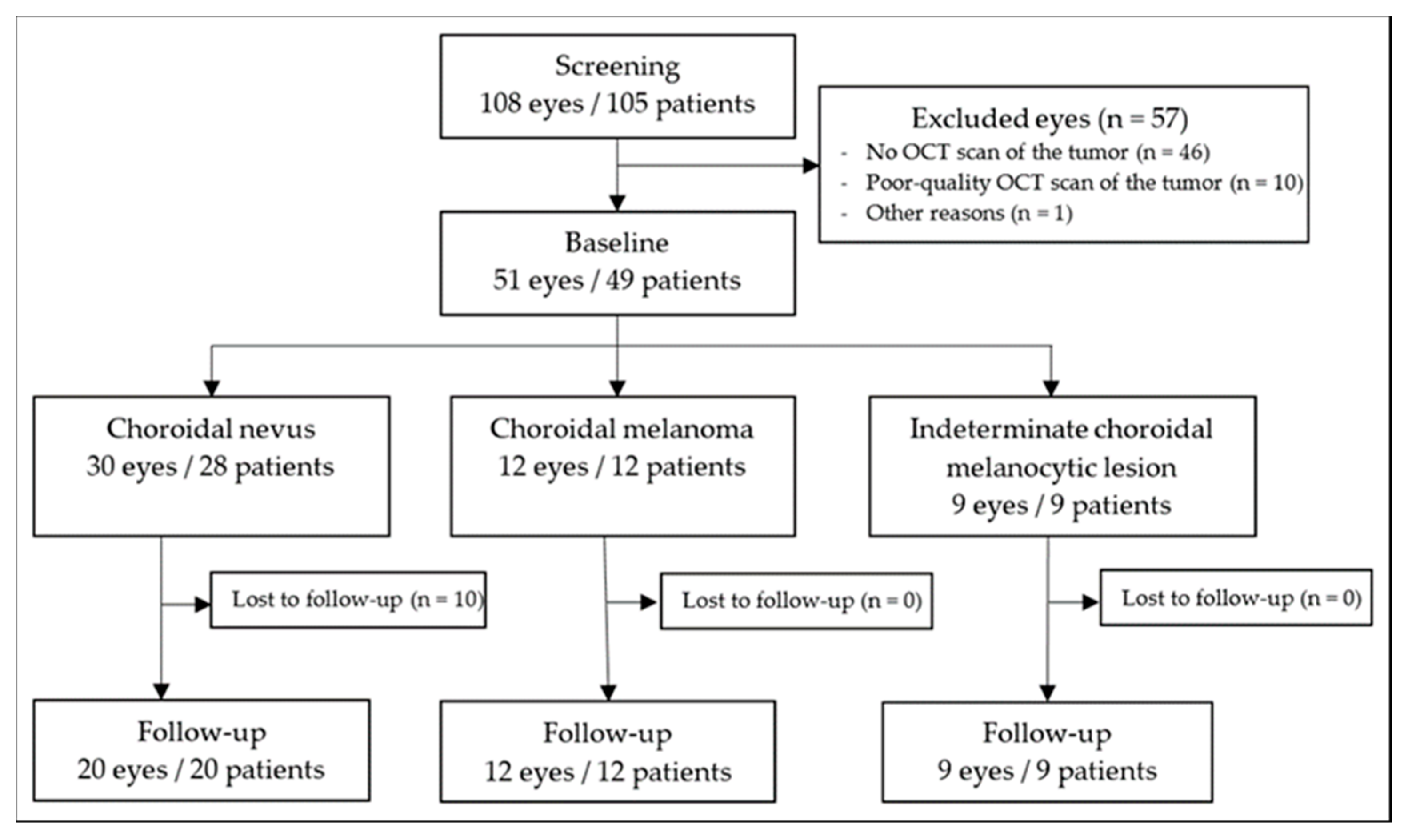
2. Materials and Methods
2.1. Data Collection
2.2. Baseline and Follow Up Measures
2.3. Statistical Analyses
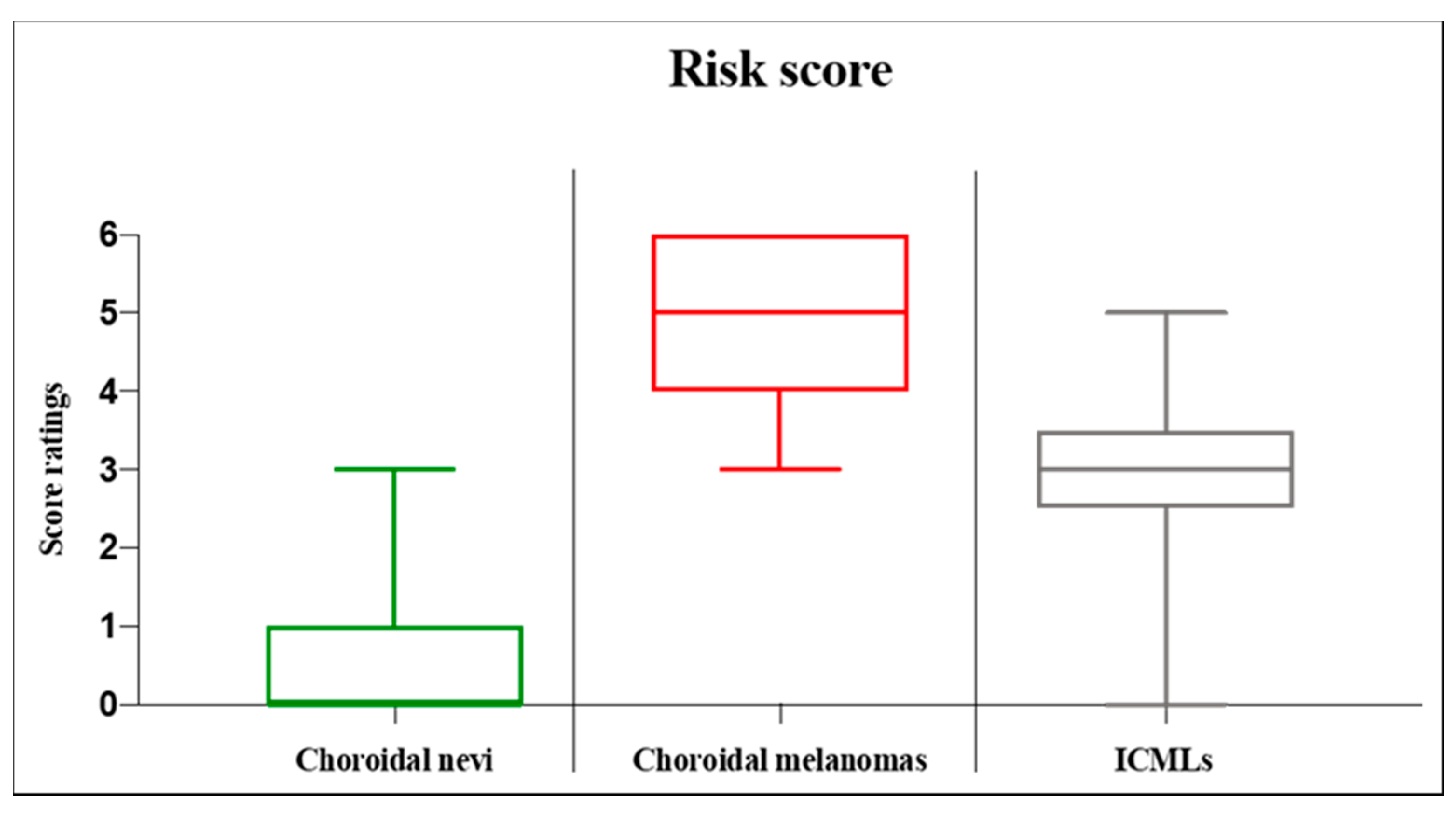
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yanoff, M.; Zimmerman, L.E. Histogenesis of malignant melanomas of the uvea. II. Relationship of uveal nevi to malignant melanomas. Cancer 1967, 20, 493–507. [Google Scholar] [CrossRef]
- Arnesen, K.; Nornes, M. Malignant melanoma of the choroid as related to coexistent benign nevus. Acta Ophthalmol. 1975, 53, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Prager, A.J.; Habib, L.A.; Busam, K.J.; Marr, B.P. Two Uveal Melanomas in One Eye: A Choroidal Nevus Giving Rise to a Melanoma in an Eye with a Separate Large Choroidal Melanoma. Ocul. Oncol. Pathol. 2018, 4, 355–358. [Google Scholar] [CrossRef] [PubMed]
- You, Q.S.; Xu, L.; Jonas, J.B.; Wang, S.; Yang, H. Change in choroidal nevi during a 5-year follow-up study: The Beijing Eye Study. Br. J. Ophthalmol. 2010, 94, 575–578. [Google Scholar] [CrossRef]
- Callaway, N.F.; Mruthyunjaya, P. Widefield imaging of retinal and choroidal tumors. Int. J. Retin. Vitr. 2019, 5, 49. [Google Scholar] [CrossRef]
- Espinoza, G.; Rosenblatt, B.; Harbour, J.W. Optical coherence tomography in the evaluation of retinal changes associated with suspicious choroidal melanocytic tumors. Am. J. Ophthalmol. 2004, 137, 90–95. [Google Scholar] [CrossRef]
- Shields, C.L.; Mashayekhi, A.; Materin, M.A.; Luo, C.K.; Marr, B.P.; Demirci, H.; Shields, J.A. Optical coherence tomography of choroidal nevus in 120 patients. Retina 2005, 25, 243–252. [Google Scholar] [CrossRef]
- Vazquez-Alfageme, C.; Papastefanou, V.P.; Patel, P.J.; Degli-Esposti, S.; Cohen, V.M.L.; Sagoo, M.S. Swept-Source OCT and Near-Infrared Reflectance Patterns in Choroidal Nevi. Ophthalmol. Retin. 2019, 3, 429–435. [Google Scholar] [CrossRef]
- Spaide, R. Autofluorescence from the outer retina and subretinal space: Hypothesis and review. Retina 2008, 28, 5–35. [Google Scholar] [CrossRef]
- Mithal, K.N.; Thakkar, H.H.; Tyagi, M.A.; Bharwada, R.M.; Billore, P.O. Role of echography in diagnostic dilemma in choroidal masses. Indian J. Ophthalmol. 2014, 62, 167–170. [Google Scholar] [CrossRef]
- Butler, P.; Char, D.H.; Zarbin, M.; Kroll, S. Natural history of indeterminate pigmented choroidal tumors. Ophthalmology 1994, 101, 710–716; discussion 717. [Google Scholar] [CrossRef]
- Singh, A.D.; Belfort, R.N.; Sayanagi, K.; Kaiser, P.K. Fourier domain optical coherence tomographic and auto-fluorescence findings in indeterminate choroidal melanocytic lesions. Br. J. Ophthalmol. 2010, 94, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Fallico, M.; Raciti, G.; Longo, A.; Reibaldi, M.; Bonfiglio, V.; Russo, A.; Caltabiano, R.; Gattuso, G.; Falzone, L.; Avitabile, T. Current molecular and clinical insights into uveal melanoma (Review). Int. J. Oncol. 2021, 58, 1. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Dalvin, L.A.; Ancona-Lezama, D.; Yu, M.D.; Di Nicola, M.; Williams, B.K., Jr.; Lucio-Alvarez, J.A.; Ang, S.M.; Maloney, S.; Welch, R.J.; et al. Choroidal nevus imaging features in 3806 cases and risk factors for transformation into melanoma in 2355 cases: The 2020 Taylor R. Smith and Victor T. Curtin lecture. Retina 2019, 39, 1840–1851. [Google Scholar] [CrossRef]
- Shields, C.L.; Furuta, M.; Mashayekhi, A.; Berman, E.L.; Zahler, J.D.; Hoberman, D.M.; Dinh, D.H.; Shields, J.A. Clinical spectrum of choroidal nevi based on age at presentation in 3422 consecutive eyes. Ophthalmology 2008, 115, 546–552.e2. [Google Scholar] [CrossRef]
- Mashayekhi, A.; Siu, S.; Shields, C.L.; Shields, J.A. Retinal pigment epithelial trough: A sign of chronicity of choroidal nevi. Eur. J. Ophthalmol. 2012, 22, 1019–1025. [Google Scholar] [CrossRef]
- Panwar, N.; Huang, P.; Lee, J.; Keane, P.A.; Chuan, T.S.; Richhariya, A.; Teoh, S.; Lim, T.H.; Agrawal, R. Fundus Photography in the 21st Century—A Review of Recent Technological Advances and Their Implications for Worldwide Healthcare. Telemed. J. e-Health 2016, 22, 198–208. [Google Scholar] [CrossRef]
- Patel, S.N.; Shi, A.; Wibbelsman, T.D.; Klufas, M.A. Ultra-widefield retinal imaging: An update on recent advances. Ther. Adv. Ophthalmol. 2020, 12, 2515841419899495. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Rojanaporn, D.; Ferenczy, S.R.; Shields, J.A. Enhanced depth imaging optical coherence tomography of small choroidal melanoma: Comparison with choroidal nevus. Arch. Ophthalmol. 2012, 130, 850–856. [Google Scholar] [CrossRef]
- Propst, S.L.; Kirschner, J.M.; Strachan, C.C.; Roumpf, S.K.; Menard, L.M.; Sarmiento, E.J.; Hunter, B.R. Ocular Point-of-Care Ultrasonography to Diagnose Posterior Chamber Abnormalities: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e1921460. [Google Scholar] [CrossRef]
- Al Harby, L.; Sagoo, M.S.; O’Day, R.; Hay, G.; Arora, A.K.; Keane, P.A.; Cohen, V.M.; Damato, B. Distinguishing Choroidal Nevi from Melanomas Using the MOLES Algorithm: Evaluation in an Ocular Nevus Clinic. Ocul. Oncol. Pathol. 2021, 7, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, M.T.; Mieler, W.F.; Leiderman, Y.I. Epidemiological trends in uveal melanoma. Br. J. Ophthalmol. 2015, 99, 1550–1553. [Google Scholar] [CrossRef] [PubMed]
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Sumich, P.; Mitchell, P.; Wang, J.J. Choroidal nevi in a white population: The Blue Mountains Eye Study. Arch. Ophthalmol. 1998, 116, 645–650. [Google Scholar] [CrossRef]
- Shields, C.L.; Furuta, M.; Berman, E.L.; Zahler, J.D.; Hoberman, D.M.; Dinh, D.H.; Mashayekhi, A.; Shields, J.A. Choroidal nevus transformation into melanoma: Analysis of 2514 consecutive cases. Arch. Ophthalmol. 2009, 127, 981–987. [Google Scholar] [CrossRef]
- Yaghy, A.; Yu, M.D.; Dalvin, L.A.; Mazloumi, M.; Ferenczy, S.R.; Shields, C.L. Photoreceptor morphology and correlation with subretinal fluid chronicity associated with choroidal nevus. Br. J. Ophthalmol. 2020, 104, 863–867. [Google Scholar] [CrossRef]
- Demirci, H.; Cullen, A.; Sundstrom, J.M. Enhanced depth imaging optical coherence tomography of choroidal metastasis. Retina 2014, 34, 1354–1359. [Google Scholar] [CrossRef]
- Matsumoto, H.; Kishi, S.; Otani, T.; Sato, T. Elongation of photoreceptor outer segment in central serous chorioretinopathy. Am. J. Ophthalmol. 2008, 145, 162–168. [Google Scholar] [CrossRef]
- Gupta, V.; Gupta, A.; Dogra, M.R.; Singh, I. Reversible retinal changes in the acute stage of sympathetic ophthalmia seen on spectral domain optical coherence tomography. Int. Ophthalmol. 2011, 31, 105–110. [Google Scholar] [CrossRef]
- Myakoshina, E.B.; Saakyan, S.V. Optical coherence tomography in diagnostics of small choroidal melanoma. Vestn. Oftalmol. 2020, 136, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, D.; Sznage, M.; Engelsberg, K.; Wittstrom, E. Clinical, optical coherence tomography, and fundus autofluorescence findings in patients with intraocular tumors. Clin. Ophthalmol. 2016, 10, 1953–1964. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weis, E.; Salopek, T.G.; McKinnon, J.G.; Larocque, M.P.; Temple-Oberle, C.; Cheng, T.; McWhae, J.; Sloboda, R.; Shea-Budgell, M. Management of uveal melanoma: A consensus-based provincial clinical practice guideline. Curr. Oncol. 2016, 23, e57–e64. [Google Scholar] [CrossRef] [PubMed]
- Weis, E.; Roelofs, K.; Larocque, M.; Murtha, A. Gene Expression Profiling as an Adjunctive Measure to Guide the Management of Indeterminate, High-Risk Choroidal Melanocytic Lesions: A Pilot Study. Ocul. Oncol. Pathol. 2019, 5, 102–109. [Google Scholar] [CrossRef] [PubMed]

| Choroidal Nevus | Choroidal Melanoma | ICML | Total | |
|---|---|---|---|---|
| Age; years | 65 (55.0–71.0 [27–87]) | 62 (57.0–65.5 [30–77]) | 65 (62.0–66.0 [35–81]) | 64 (56.0–70.5 [27–87]) |
| Sex; female | 16 of 28(57.1%) | 5 of 12 (41.7%) | 2 of 9 (22.2%) | 23 of 49 (46.9%) |
| Affected eye; right | 14 of 30 (46.7%) | 7 of 12 (58.3%) | 2 of 9 (22.2%) | 23 of 51 (45.2%) |
| Baseline diagnosis | 30 of 51(58.8%) | 12 of 51(23.5%) | 9 of 51(17.6%) | 51 |
| Follow-up diagnosis | 20 of 41 (48.8%) | 12 of 41 (29.3%) | 9 of 41(22.0%) | 41 |
| Follow-up time; months | 15.5 (10.0–39.0 [0.5–79.0]) | 30.0 (20.8–44.0 [2.0–134.0]) | 15.0 (14.0–37.0 [11.0–75.0]) | 25.0 (12.0–39.0 [0.5–134.0]) |
| Baseline visual acuity | 20/20 (20/20–20/25 [20/16–20/40]) | 20/32 (20/25–20/63 [20/20–20/400]) | 20/20 (20/20–20/25 [20/16–20/2000]) | 20/20 (20/20–20/25 [20/16–20/2000]) |
| Follow-up visual acuity | 20/22 (20/20–20/32 [20/20–20/50]) | 20/56 (20/30–20/400 [20/20-20/4000]) | 20/20 (20/20–20/32 [20/20–20/2000]) | 20/25 (20/20–20/36 [20/20–20/4000]) |
| Choroidal Nevus, N1 | Choroidal Melanoma, N2 | ICML, N3 | |
|---|---|---|---|
| Subretinal fluid Overlying tumor Within 3 mm from tumor margin Subfoveal | 3 (10.0%) 3 (10.0%) 2 (6.7%) | 12 (100.0%) 0 (0.0%) 8 (66.7%) | 7 (77.8%) 0 (0.0%) 2 (22.2%) |
| Retinal invasion | 1 (3.3%) | 0 (0.0%) | 0 (0.0%) |
| Retinal edema over tumor | 1 (3.3%) | 7 (58.3%) | 5 (55.6%) |
| Drusen above tumor | 14 (46.7%) | 1 (8.3%) | 3 (33.3%) |
| Shaggy photoreceptors overlying tumor | 0 (0.0%) | 6 (50.0%) | 2 (22.2%) |
| Loss of ellipsoid zone | 6 (20.0%) | 9 (75.0%) | 8 (88.9%) |
| Irregularity of ellipsoid zone | 8 (26.7%) | 12 (100.0%) | 7 (77.8%) |
| RPE atrophy | 6 (20.0%) | 11 (91.7%) | 8 (88.9%) |
| RPE hyperplasia | 4 (13.3%) | 8 (66.7%) | 5 (55.6%) |
| RPE fibrous metaplasia | 1 (3.3%) | 2 (16.7%) | 0 (0.0%) |
| RPE detachment | 5 (16.7%) | 8 (66.7%) | 2 (22.2%) |
| CNV | 3 (10.0%) | 1 (8.3%) | 1 (11.1%) |
| Choriocapillaris compression | 26 (86.7%) | 12 (100.0%) | 9 (100.0%) |
| Surface configuration Dome-shaped Lumpy bumpy Excavated Flat | 6 (20.0%) 1 (3.3%) 0 (0.0%) 23 (76.7%) | 12 (100.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) | 7 (77.8%) 0 (0.0%) 0 (0.0%) 2 (22.2%) |
| Tumor margin <3 mm to the optic disc | 7 (27.5%) | 4 (33.3%) | 3 (33.3%) |
| Choroidal Nevus | Choroidal Melanoma | ICML | ||
|---|---|---|---|---|
| Shape Flat configuration Dome configuration Not available | 4 of 9 (44.4%) 5 of 9 (55.6%) 21 of 30 (70.0%) | 0 of 12 (0.0%) 12 of 12 (100.0%) 0 of 12 (0.0%) | 3 of 9 (33.3%) 6 of 9 (66.7%) 0 of 9 (0.0%) | |
| Echogenicity Hollow Dense Not available | 2 of 9 (22.2%) 7 of 9 (77.8%) 21 of 30 (70.0%) | 10 of 12 (83.3%) 2 of 12 (16.7%) 0 of 12 (0.0%) | 3 of 9 (33.3%) 6 of 9 (66.7%) 0 of 9 (0.0%) | |
| Thickness; millimeter | 1.1 (1.0–1.5 [0.8–1.9]) | 2.5 (2.3–4.2 [1.4–6.0]) | 1.5 (1.5–1.7 [1.1– 2.1]) | |
| Largest basal diameter; millimeters | 3.5 (2.2–4.6 [1.0–8.0]) | 10.4 (5.2–12.7 [3.9–17.6]) | 6.1 (5.5–8.3 [3.3–11.4]) |
| Choroidal Nevus, N1 | Choroidal Melanoma, N2 | ICML, N3 | Total, N | |
|---|---|---|---|---|
| Secondary CNV | 3 (10.0%) | 1 (8.3%) | 1 (11.1%) | 5 (9.8%) |
| Retinal detachment | 0 (0.0%) | 5 (41.7%) | 1 (11.1%) | 6 (11.8%) |
| Toxic tumor syndrome | 0 (0.0%) | 1 (8.3%) | 0 (0.0%) | 1 (2.0%) |
| Metastasis | 0 (0.0%) | 1 (8.3%) | 0 (0.0%) | 1 (2.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geiger, F.; Said, S.; Bajka, A.; Toro, M.D.; Wiest, M.R.J.; Stahel, M.; Barthelmes, D.; Zweifel, S.A. Assessing Choroidal Nevi, Melanomas and Indeterminate Melanocytic Lesions Using Multimodal Imaging—A Retrospective Chart Review. Curr. Oncol. 2022, 29, 1018-1028. https://doi.org/10.3390/curroncol29020087
Geiger F, Said S, Bajka A, Toro MD, Wiest MRJ, Stahel M, Barthelmes D, Zweifel SA. Assessing Choroidal Nevi, Melanomas and Indeterminate Melanocytic Lesions Using Multimodal Imaging—A Retrospective Chart Review. Current Oncology. 2022; 29(2):1018-1028. https://doi.org/10.3390/curroncol29020087
Chicago/Turabian StyleGeiger, Fredy, Sadiq Said, Anahita Bajka, Mario Damiano Toro, Maximilian Robert Justus Wiest, Marc Stahel, Daniel Barthelmes, and Sandrine Anne Zweifel. 2022. "Assessing Choroidal Nevi, Melanomas and Indeterminate Melanocytic Lesions Using Multimodal Imaging—A Retrospective Chart Review" Current Oncology 29, no. 2: 1018-1028. https://doi.org/10.3390/curroncol29020087
APA StyleGeiger, F., Said, S., Bajka, A., Toro, M. D., Wiest, M. R. J., Stahel, M., Barthelmes, D., & Zweifel, S. A. (2022). Assessing Choroidal Nevi, Melanomas and Indeterminate Melanocytic Lesions Using Multimodal Imaging—A Retrospective Chart Review. Current Oncology, 29(2), 1018-1028. https://doi.org/10.3390/curroncol29020087






