Prognostic Relevance of Type 2 Diabetes and Metformin Treatment in Head and Neck Melanoma: Results from a Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Collection
2.2. Statistical Analysis
3. Results
3.1. Characterization of the Patient Cohort
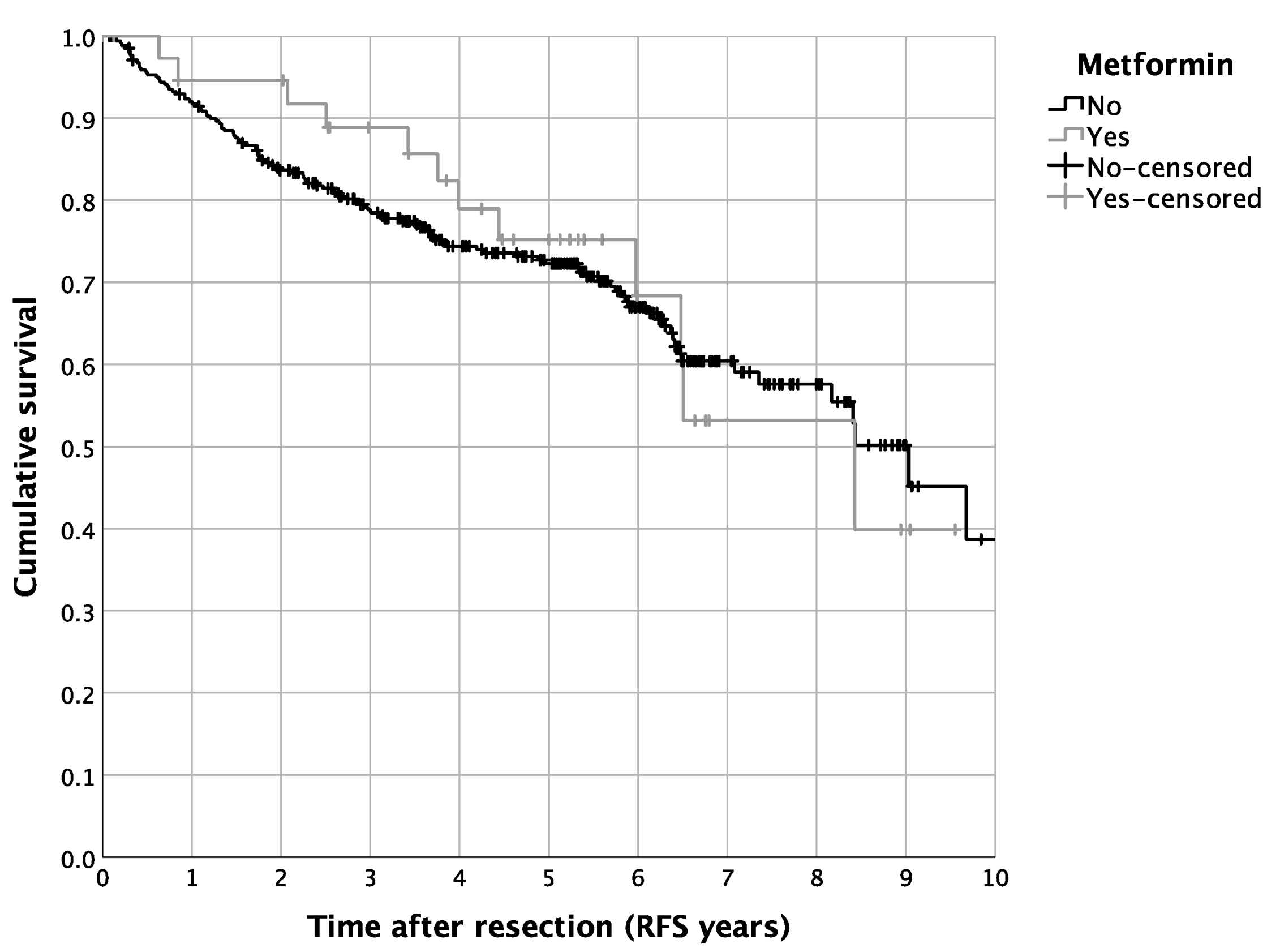
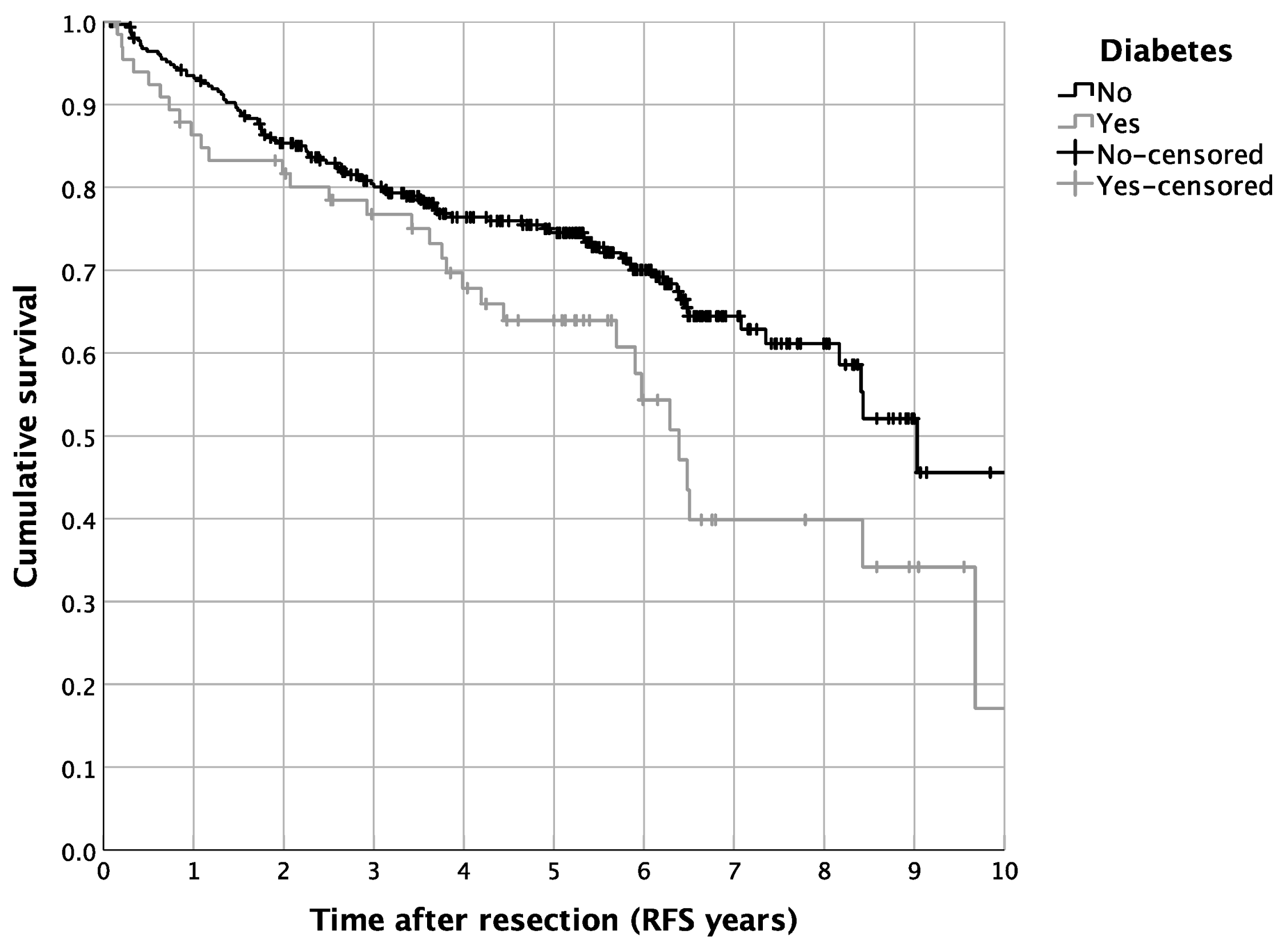
3.2. Impact of DM2 and Metformin Intake on Patient Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forouhi, N.G.; Wareham, N.J. Epidemiology of diabetes. Medicine 2014, 42, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Adami, H.O.; Chow, W.H.; Nyren, O.; Berne, C.; Linet, M.S.; Ekbom, A.; Wolk, A.; McLaughlin, J.K.; Fraumeni, J.F., Jr. Excess risk of primary liver cancer in patients with diabetes mellitus. J. Natl. Cancer Inst. 1996, 88, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.; Ansary-Moghaddam, A.; Berrington de González, A.; Barzi, F.; Woodward, M. Type-II diabetes and pancreatic cancer: A meta-analysis of 36 studies. Br. J. Cancer 2005, 92, 2076–2083. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.-H.; Gridley, G.; Nyrén, O.; Linet, M.S.; Ekbom, A.; Fraumeni, J.F., Jr.; Adami, H.-O. Risk of Pancreatic Cancer Following Diabetes Mellitus: A Nationwide Cohort Study in Sweden. J. Natl. Cancer Inst. 1995, 87, 930–931. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, M.; Lucisano, G.; Lapice, E.; Strippoli, G.F.; Pellegrini, F.; Nicolucci, A. Metformin therapy and risk of cancer in patients with type 2 diabetes: Systematic review. PLoS ONE 2013, 8, e71583. [Google Scholar] [CrossRef]
- Gunter, M.J.; Hoover, D.R.; Yu, H.; Wassertheil-Smoller, S.; Rohan, T.E.; Manson, J.E.; Li, J.; Ho, G.Y.; Xue, X.; Anderson, G.L.; et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J. Natl. Cancer Inst. 2009, 101, 48–60. [Google Scholar] [CrossRef]
- Nicolucci, A. Epidemiological aspects of neoplasms in diabetes. Acta Diabetol. 2010, 47, 87–95. [Google Scholar] [CrossRef]
- Saydah, S.H.; Loria, C.M.; Eberhardt, M.S.; Brancati, F.L. Abnormal glucose tolerance and the risk of cancer death in the United States. Am. J. Epidemiol. 2003, 157, 1092–1100. [Google Scholar] [CrossRef]
- Meyerhardt, J.A.; Catalano, P.J.; Haller, D.G.; Mayer, R.J.; Macdonald, J.S.; Benson, A.B., 3rd; Fuchs, C.S. Impact of diabetes mellitus on outcomes in patients with colon cancer. J. Clin. Oncol. 2003, 21, 433–440. [Google Scholar] [CrossRef]
- Karlin, N.; Dueck, A.; Nagi Reddy, S.; Verona, P.; Cook, C. Implications of breast cancer with diabetes mellitus on patient outcomes and care. Diabetes Manag. 2014, 4, 411–419. [Google Scholar] [CrossRef]
- Jullumstrø, E.; Kollind, M.; Lydersen, S.; Edna, T.-H. Diabetes mellitus and outcomes of colorectal cancer. Acta Oncol. 2009, 48, 361–367. [Google Scholar] [CrossRef]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Ferrannini, E.; Holman, R.R.; Sherwin, R.; Zinman, B. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009, 32, 193–203. [Google Scholar] [CrossRef]
- Libby, G.; Donnelly, L.A.; Donnan, P.T.; Alessi, D.R.; Morris, A.D.; Evans, J.M. New users of metformin are at low risk of incident cancer: A cohort study among people with type 2 diabetes. Diabetes Care 2009, 32, 1620–1625. [Google Scholar] [CrossRef]
- Landman, G.W.D.; Kleefstra, N.; van Hateren, K.J.J.; Groenier, K.H.; Gans, R.O.B.; Bilo, H.J.G. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care 2010, 33, 322–326. [Google Scholar] [CrossRef]
- Evans, J.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. Bmj 2005, 330, 1304–1305. [Google Scholar] [CrossRef]
- Spoerl, S.; Spanier, G.; Reiter, E.; Gerken, M.; Haferkamp, S.; Grosse, J.; Drexler, K.; Ettl, T.; Klinkhammer-Schalke, M.; Fischer, R.; et al. Head and neck melanoma: Outcome and predictors in a population-based cohort study. Head Face Med. 2021, 17, 45. [Google Scholar] [CrossRef]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Coyle, C.; Cafferty, F.H.; Vale, C.; Langley, R.E. Metformin as an adjuvant treatment for cancer: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 2184–2195. [Google Scholar] [CrossRef]
- Montaudié, H.; Cerezo, M.; Bahadoran, P.; Roger, C.; Passeron, T.; Machet, L.; Arnault, J.P.; Verneuil, L.; Maubec, E.; Aubin, F.; et al. Metformin monotherapy in melanoma: A pilot, open-label, prospective, and multicentric study indicates no benefit. Pigment Cell Melanoma Res. 2017, 30, 378–380. [Google Scholar] [CrossRef]
- Jaune, E.; Rocchi, S. Metformin: Focus on Melanoma. Front. Endocrinol. 2018, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Niraula, S.; Dowling, R.J.; Ennis, M.; Chang, M.C.; Done, S.J.; Hood, N.; Escallon, J.; Leong, W.L.; McCready, D.R.; Reedijk, M.; et al. Metformin in early breast cancer: A prospective window of opportunity neoadjuvant study. Breast Cancer Res. Treat. 2012, 135, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, T.; Brinks, R.; Hoyer, A.; Kuss, O.S.; Rathmann, W. The Prevalence and Incidence of Diabetes in Germany. Dtsch. Arztebl. Int. 2016, 113, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.G.; Plas, D.R.; Kubek, S.; Buzzai, M.; Mu, J.; Xu, Y.; Birnbaum, M.J.; Thompson, C.B. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell 2005, 18, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Tomic, T.; Botton, T.; Cerezo, M.; Robert, G.; Luciano, F.; Puissant, A.; Gounon, P.; Allegra, M.; Bertolotto, C.; Bereder, J.M.; et al. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis. 2011, 2, e199. [Google Scholar] [CrossRef] [PubMed]
- Heald, A.H.; Stedman, M.; Davies, M.; Livingston, M.; Alshames, R.; Lunt, M.; Rayman, G.; Gadsby, R. Estimating life years lost to diabetes: Outcomes from analysis of National Diabetes Audit and Office of National Statistics data. Cardiovasc. Endocrinol. Metab. 2020, 9, 183–185. [Google Scholar] [CrossRef]
- Tabish, S.A. Is Diabetes Becoming the Biggest Epidemic of the Twenty-first Century? Int. J. Health Sci. 2007, 1, v–viii. [Google Scholar]
- De Bruijn, K.M.; Arends, L.R.; Hansen, B.E.; Leeflang, S.; Ruiter, R.; van Eijck, C.H. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br. J. Surg. 2013, 100, 1421–1429. [Google Scholar] [CrossRef]
- Pandey, A.; Forte, V.; Abdallah, M.; Alickaj, A.; Mahmud, S.; Asad, S.; McFarlane, S.I. Diabetes mellitus and the risk of cancer. Minerva Endocrinol. 2011, 36, 187–209. [Google Scholar]
- Stein, K.B.; Snyder, C.F.; Barone, B.B.; Yeh, H.C.; Peairs, K.S.; Derr, R.L.; Wolff, A.C.; Brancati, F.L. Colorectal cancer outcomes, recurrence, and complications in persons with and without diabetes mellitus: A systematic review and meta-analysis. Dig. Dis. Sci. 2010, 55, 1839–1851. [Google Scholar] [CrossRef]
- Barone, B.B.; Yeh, H.C.; Snyder, C.F.; Peairs, K.S.; Stein, K.B.; Derr, R.L.; Wolff, A.C.; Brancati, F.L. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analysis. Jama 2008, 300, 2754–2764. [Google Scholar] [CrossRef]
- Qi, L.; Qi, X.; Xiong, H.; Liu, Q.; Li, J.; Zhang, Y.; Ma, X.; Wu, N.; Liu, Q.; Feng, L. Type 2 diabetes mellitus and risk of malignant melanoma: A systematic review and meta-analysis of cohort studies. Iran. J. Public Health 2014, 43, 857–866. [Google Scholar]
| Variable | Diabetes | |||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | Total | χ2 | |||||
| N | Column % | N | Column % | N | Column % | p | ||
| Sex | Male | 188 | 59.9% | 34 | 50.0% | 222 | 58.1% | 0.135 |
| Female | 126 | 40.1% | 34 | 50.0% | 160 | 41.9% | ||
| Age at diagnosis | <50 | 66 | 21.0% | 1 | 1.5% | 67 | 17.5% | <0.001 |
| 50–59 | 52 | 16.6% | 3 | 4.4% | 55 | 14.4% | ||
| 60–69 | 59 | 18.8% | 17 | 25.0% | 76 | 19.9% | ||
| 70–79 | 91 | 29.0% | 32 | 47.1% | 123 | 32.2% | ||
| ≥80.0 | 46 | 14.6% | 15 | 22.1% | 61 | 16.0% | ||
| Charlson comorbidity index (without diabetes) | 0 | 260 | 82.8% | 47 | 69.1% | 307 | 80.4% | 0.013 |
| 1 | 39 | 12.4% | 14 | 20.6% | 53 | 13.9% | ||
| 2 | 10 | 3.2% | 2 | 2.9% | 12 | 3.1% | ||
| 3 | 4 | 1.3% | 4 | 5.9% | 8 | 2.1% | ||
| 4 | 0 | 0.0% | 1 | 1.5% | 1 | 0.3% | ||
| 5 | 1 | 0.3% | 0 | 0.0% | 1 | 0.3% | ||
| Charlson comorbidity index (without diabetes) | 0 | 260 | 82.8% | 47 | 69.1% | 307 | 80.4% | 0.010 |
| ≥1 | 54 | 17.2% | 21 | 30.9% | 75 | 19.6% | ||
| BMI | Underweight | 1 | 0.3% | 0 | 0.0% | 1 | 0.3% | <0.001 |
| Normal weight | 95 | 30.3% | 4 | 5.9% | 99 | 25.9% | ||
| Overweight | 113 | 36.0% | 25 | 36.8% | 138 | 36.1% | ||
| Obesity | 62 | 19.7% | 32 | 47.1% | 94 | 24.6% | ||
| N/A. | 43 | 13.7% | 7 | 10.3% | 50 | 13.1% | ||
| BMI | Under-normal weight | 96 | 30.6% | 4 | 5.9% | 100 | 26.2% | <0.001 |
| Overweight–obese | 175 | 55.7% | 57 | 83.8% | 232 | 60.7% | ||
| N/A | 43 | 13.7% | 7 | 10.3% | 50 | 13.1% | ||
| Metformin | No | 314 | 100.0% | 29 | 42.6% | 343 | 89.8% | <0.001 |
| Yes | 0 | 0.0% | 39 | 57.4% | 39 | 10.2% | ||
| Statin | No | 269 | 85.7% | 42 | 61.8% | 311 | 81.4% | <0.001 |
| Yes | 45 | 14.3% | 26 | 38.2% | 71 | 18.6% | ||
| ASA | No | 256 | 81.5% | 43 | 63.2% | 299 | 78.3% | 0.001 |
| Yes | 58 | 18.5% | 25 | 36.8% | 83 | 21.7% | ||
| Localization ICDO-3 | C44.0 Lip skin | 1 | 0.3% | 1 | 1.5% | 2 | 0.5% | 0.216 |
| C44.1 Eyelid | 15 | 4.8% | 3 | 4.4% | 18 | 4.7% | ||
| C44.2 Outer ear | 53 | 16.9% | 6 | 8.8% | 59 | 15.4% | ||
| C44.3 Other parts of the face | 143 | 45.5% | 39 | 57.4% | 182 | 47.6% | ||
| C44.4 Scalp and neck | 102 | 32.5% | 19 | 27.9% | 121 | 31.7% | ||
| Localization | Scalp and neck | 102 | 32.5% | 19 | 27.9% | 121 | 31.7% | 0.465 |
| Face | 212 | 67.5% | 49 | 72.1% | 261 | 68.3% | ||
| AJCC stage | IA | 172 | 54.8% | 33 | 48.5% | 205 | 53.7% | 0.595 |
| IB | 53 | 16.9% | 16 | 23.5% | 69 | 18.1% | ||
| II | 72 | 22.9% | 16 | 23.5% | 88 | 23.0% | ||
| III | 17 | 5.4% | 3 | 4.4% | 20 | 5.2% | ||
| AJCC stage | I | 225 | 71.7% | 49 | 72.1% | 274 | 71.7% | 0.947 |
| II + III | 89 | 28.3% | 19 | 27.9% | 108 | 28.3% | ||
| Tumor thickness (mm) | <1 | 190 | 60.5% | 38 | 55.9% | 228 | 59.7% | |
| 1–2 | 50 | 15.9% | 13 | 19.1% | 63 | 16.5% | ||
| 2–4 | 35 | 11.1% | 7 | 10.3% | 42 | 11.0% | ||
| >4 | 39 | 12.4% | 10 | 14.7% | 49 | 12.8% | ||
| Tumor size (pT) | 1 | 195 | 62.1% | 39 | 57.4% | 234 | 61.3% | 0.908 |
| 2 | 53 | 16.9% | 13 | 19.1% | 66 | 17.3% | ||
| 3 | 30 | 9.6% | 7 | 10.3% | 37 | 9.7% | ||
| 4 | 36 | 11.5% | 9 | 13.2% | 45 | 11.8% | ||
| Nodal status (pN) | 0 | 297 | 94.6% | 65 | 95.6% | 362 | 94.8% | 0.737 |
| 1 | 17 | 5.4% | 3 | 4.4% | 20 | 5.2% | ||
| Ulceration | no | 249 | 79.3% | 54 | 79.4% | 303 | 79.3% | 0.715 |
| yes | 62 | 19.7% | 14 | 20.6% | 76 | 19.9% | ||
| N/A | 3 | 1.0% | 0 | 0.0% | 3 | 0.8% | ||
| Histological subgroup | Lentigo-maligna melanoma | 125 | 39.8% | 29 | 42.6% | 154 | 40.3% | 0.678 |
| Nodular melanoma | 46 | 14.6% | 13 | 19.1% | 59 | 15.4% | ||
| Superficial spreading melanoma | 103 | 32.8% | 19 | 27.9% | 122 | 31.9% | ||
| Melanoma n. o. s. and other | 40 | 12.7% | 7 | 10.3% | 47 | 12.3% | ||
| Surgical margin (mm) | <5 | 75 | 23.9% | 18 | 26.5% | 93 | 24.3% | 0.018 |
| 5–9 | 122 | 38.9% | 22 | 32.4% | 144 | 37.7% | ||
| ≥10 | 117 | 37.3% | 26 | 38.2% | 143 | 37.4% | ||
| N/A | 0 | 0.0% | 2 | 2.9% | 2 | 0.5% | ||
| Neck dissection | No | 294 | 93.6% | 58 | 85.3% | 352 | 92.1% | 0.021 |
| Yes | 20 | 6.4% | 10 | 14.7% | 30 | 7.9% | ||
| Adjuvant radiotherapy | No | 310 | 98.7% | 68 | 100.0% | 378 | 99.0% | 0.349 |
| Yes | 4 | 1.3% | 0 | 0.0% | 4 | 1.0% | ||
| Adjuvant chemo/immunotherapy | No | 299 | 95.2% | 65 | 95.6% | 364 | 95.3% | 0.897 |
| Yes | 15 | 4.8% | 3 | 4.4% | 18 | 4.7% | ||
| Total | 314 | 100.0% | 68 | 100.0% | 382 | 100.0% | ||
| Variable | Metformin | |||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | Total | χ2 | |||||
| N | Column % | N | Column % | N | Column % | p | ||
| Sex | Male | 207 | 60.3% | 15 | 38.5% | 222 | 58.1% | 0.009 |
| Female | 136 | 39.7% | 24 | 61.5% | 160 | 41.9% | ||
| Age at diagnosis | <50 | 66 | 19.2% | 1 | 2.6% | 67 | 17.5% | 0.017 |
| 50–59 | 53 | 15.5% | 2 | 5.1% | 55 | 14.4% | ||
| 60–69 | 66 | 19.2% | 10 | 25.6% | 76 | 19.9% | ||
| 70–79 | 106 | 30.9% | 17 | 43.6% | 123 | 32.2% | ||
| ≥80 | 52 | 15.2% | 9 | 23.1% | 61 | 16.0% | ||
| Charlson comorbidity index (without diabetes) | 0 | 277 | 80.8% | 30 | 76.9% | 307 | 80.4% | 0.537 |
| 1 | 46 | 13.4% | 7 | 17.9% | 53 | 13.9% | ||
| 2 | 12 | 3.5% | 0 | 0.0% | 12 | 3.1% | ||
| 3 | 6 | 1.7% | 2 | 5.1% | 8 | 2.1% | ||
| 4 | 1 | 0.3% | 0 | 0.0% | 1 | 0.3% | ||
| 5 | 1 | 0.3% | 0 | 0.0% | 1 | 0.3% | ||
| Charlson comorbidity index (without diabetes) | 0 | 277 | 80.8% | 30 | 76.9% | 307 | 80.4% | 0.568 |
| ≥1 | 66 | 19.2% | 9 | 23.1% | 75 | 19.6% | ||
| BMI | Underweight | 1 | 0.3% | 0 | 0.0% | 1 | 0.3% | <0.001 |
| Normal weight | 96 | 28.0% | 3 | 7.7% | 99 | 25.9% | ||
| Overweight | 124 | 36.2% | 14 | 35.9% | 138 | 36.1% | ||
| Obesity | 74 | 21.6% | 20 | 51.3% | 94 | 24.6% | ||
| N/A | 48 | 14.0% | 2 | 5.1% | 50 | 13.1% | ||
| BMI | Under-normal weight | 97 | 28.3% | 3 | 7.7% | 100 | 26.2% | 0.002 |
| Overweight–obese | 198 | 57.7% | 34 | 87.2% | 232 | 60.7% | ||
| N/A | 48 | 14.0% | 2 | 5.1% | 50 | 13.1% | ||
| Statin | No | 282 | 82.2% | 29 | 74.4% | 311 | 81.4% | 0.232 |
| Yes | 61 | 17.8% | 10 | 25.6% | 71 | 18.6% | ||
| ASA | No | 274 | 79.9% | 25 | 64.1% | 299 | 78.3% | 0.024 |
| Yes | 69 | 20.1% | 14 | 35.9% | 83 | 21.7% | ||
| Localization ICDO-3 | C44.0 Lip skin | 1 | 0.3% | 1 | 2.6% | 2 | 0.5% | 0.445 |
| C44.1 Eyelid | 16 | 4.7% | 2 | 5.1% | 18 | 4.7% | ||
| C44.2 Outer ear | 54 | 15.7% | 5 | 12.8% | 59 | 15.4% | ||
| C44.3 Other parts of the face | 164 | 47.8% | 18 | 46.2% | 182 | 47.6% | ||
| C44.4 Scalp and neck | 108 | 31.5% | 13 | 33.3% | 121 | 31.7% | ||
| Localization | Scalp and neck | 108 | 31.5% | 13 | 33.3% | 121 | 31.7% | 0.814 |
| Face | 235 | 68.5% | 26 | 66.7% | 261 | 68.3% | ||
| AJCC stage | IA | 185 | 53.9% | 20 | 51.3% | 205 | 53.7% | 0.590 |
| IB | 59 | 17.2% | 10 | 25.6% | 69 | 18.1% | ||
| II | 81 | 23.6% | 7 | 17.9% | 88 | 23.0% | ||
| III | 18 | 5.2% | 2 | 5.1% | 20 | 5.2% | ||
| AJCC stage | I | 244 | 71.1% | 30 | 76.9% | 274 | 71.7% | 0.447 |
| II + III | 99 | 28.9% | 9 | 23.1% | 108 | 28.3% | ||
| Tumor thickness (mm) | <1 | 204 | 59.5% | 24 | 61.5% | 228 | 59.7% | 0.938 |
| 1–2 | 57 | 16.6% | 6 | 15.4% | 63 | 16.5% | ||
| 2–4 | 37 | 10.8% | 5 | 12.8% | 42 | 11.0% | ||
| >4 | 45 | 13.1% | 4 | 10.3% | 49 | 12.8% | ||
| Tumor size (TNM) | 1 | 209 | 60.9% | 25 | 64.1% | 234 | 61.3% | 0.752 |
| 2 | 60 | 17.5% | 6 | 15.4% | 66 | 17.3% | ||
| 3 | 32 | 9.3% | 5 | 12.8% | 37 | 9.7% | ||
| 4 | 42 | 12.2% | 3 | 7.7% | 45 | 11.8% | ||
| Nodal status (TNM) | 0 | 325 | 94.8% | 37 | 94.9% | 362 | 94.8% | 0.975 |
| 1 | 18 | 5.2% | 2 | 5.1% | 20 | 5.2% | ||
| Ulceration | No | 270 | 78.7% | 33 | 84.6% | 303 | 79.3% | 0.625 |
| Yes | 70 | 20.4% | 6 | 15.4% | 76 | 19.9% | ||
| N/A | 3 | 0.9% | 0 | 0.0% | 3 | 0.8% | ||
| Histological subgroup | Lentigo-maligna melanoma | 140 | 40.8% | 14 | 35.9% | 154 | 40.3% | 0.555 |
| Nodular melanoma | 53 | 15.5% | 6 | 15.4% | 59 | 15.4% | ||
| Superficial spreading melanoma | 106 | 30.9% | 16 | 41.0% | 122 | 31.9% | ||
| Melanoma n. o. s. and other | 44 | 12.8% | 3 | 7.7% | 47 | 12.3% | ||
| Surgical margin (mm) | <5 | 86 | 25.1% | 7 | 17.9% | 93 | 24.3% | 0.152 |
| 5–9 | 131 | 38.2% | 13 | 33.3% | 144 | 37.7% | ||
| ≥10 | 125 | 36.4% | 18 | 46.2% | 143 | 37.4% | ||
| N/A | 1 | 0.3% | 1 | 2.6% | 2 | 0.5% | ||
| Neck dissection | No | 316 | 92.1% | 36 | 92.3% | 352 | 92.1% | 0.969 |
| Yes | 27 | 7.9% | 3 | 7.7% | 30 | 7.9% | ||
| Adjuvant radiotherapy | No | 339 | 98.8% | 39 | 100.0% | 378 | 99.0% | 0.498 |
| Yes | 4 | 1.2% | 0 | 0.0% | 4 | 1.0% | ||
| Adjuvant chemo/immunotherapy | No | 326 | 95.0% | 38 | 97.4% | 364 | 95.3% | |
| Yes | 17 | 5.0% | 1 | 2.6% | 18 | 4.7% | 0.504 | |
| Total | 343 | 100.0% | 39 | 100.0% | 382 | 100.0% | ||
| Multivariable Cox Regression * | ||||
|---|---|---|---|---|
| Complete Cohort | p | Hazard Ratio | Lower 95%-CI | Upper 95%-CI |
| Recurrence-free survival | ||||
| Metformin yes vs. no | 0.024 | 0.396 | 0.177 | 0.884 |
| Diabetes yes vs. no | 0.021 | 1.980 | 1.108 | 3.538 |
| Cumulative recurrence rate | ||||
| Metformin yes vs. no | 0.061 | 0.325 | 0.100 | 1.054 |
| Diabetes yes vs. no | 0.141 | 1.915 | 0.807 | 4.547 |
| Cumulative locoregional recurrence rate | ||||
| Metformin yes vs. no | 0.008 | 0.135 | 0.031 | 0.589 |
| Diabetes yes vs. no | 0.003 | 4.173 | 1.628 | 10.697 |
| Cumulative distant recurrence rate | ||||
| Metformin yes vs. no | 0.702 | 1.356 | 0.286 | 6.426 |
| Diabetes yes vs. no | 0.124 | 0.370 | 0.104 | 1.312 |
| Multivariable Cox Regression * | ||||
|---|---|---|---|---|
| Diabetes Cohort | p | Hazard Ratio | Lower 95%-CI | Upper 95%-CI |
| Recurrence-free survival | ||||
| Metformin yes vs. no | 0.032 | 0.352 | 0.135 | 0.913 |
| Cumulative recurrence rate | ||||
| Metformin yes vs. no | 0.869 | 0.855 | 0.133 | 5.479 |
| Cumulative locoregional recurrence rate | ||||
| Metformin yes vs. no | 0.964 | 0.952 | 0.109 | 8.305 |
| Cumulative distant recurrence rate | ||||
| Metformin yes vs. no | - & | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spoerl, S.; Gerken, M.; Schimnitz, S.; Taxis, J.; Fischer, R.; Lindner, S.R.; Ettl, T.; Ludwig, N.; Spoerl, S.; Reichert, T.E.; et al. Prognostic Relevance of Type 2 Diabetes and Metformin Treatment in Head and Neck Melanoma: Results from a Population-Based Cohort Study. Curr. Oncol. 2022, 29, 9660-9670. https://doi.org/10.3390/curroncol29120758
Spoerl S, Gerken M, Schimnitz S, Taxis J, Fischer R, Lindner SR, Ettl T, Ludwig N, Spoerl S, Reichert TE, et al. Prognostic Relevance of Type 2 Diabetes and Metformin Treatment in Head and Neck Melanoma: Results from a Population-Based Cohort Study. Current Oncology. 2022; 29(12):9660-9670. https://doi.org/10.3390/curroncol29120758
Chicago/Turabian StyleSpoerl, Steffen, Michael Gerken, Susanne Schimnitz, Juergen Taxis, René Fischer, Sophia R. Lindner, Tobias Ettl, Nils Ludwig, Silvia Spoerl, Torsten E. Reichert, and et al. 2022. "Prognostic Relevance of Type 2 Diabetes and Metformin Treatment in Head and Neck Melanoma: Results from a Population-Based Cohort Study" Current Oncology 29, no. 12: 9660-9670. https://doi.org/10.3390/curroncol29120758
APA StyleSpoerl, S., Gerken, M., Schimnitz, S., Taxis, J., Fischer, R., Lindner, S. R., Ettl, T., Ludwig, N., Spoerl, S., Reichert, T. E., & Spanier, G. (2022). Prognostic Relevance of Type 2 Diabetes and Metformin Treatment in Head and Neck Melanoma: Results from a Population-Based Cohort Study. Current Oncology, 29(12), 9660-9670. https://doi.org/10.3390/curroncol29120758








